 Found 55 hits with Last Name = 'li' and Initial = 'lt'
Found 55 hits with Last Name = 'li' and Initial = 'lt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific demethylase 4B
(Homo sapiens (Human)) | BDBM50047962
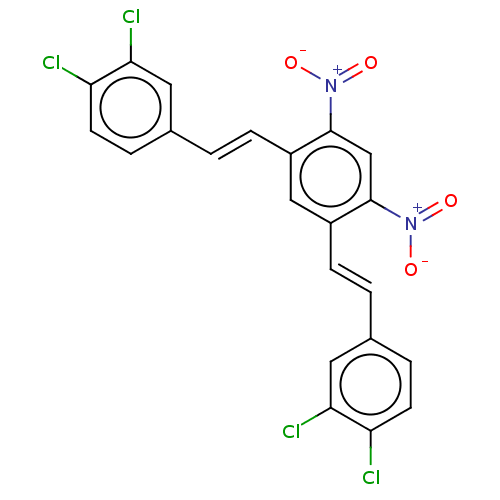
(CHEMBL1966136)Show SMILES [O-][N+](=O)c1cc(c(\C=C\c2ccc(Cl)c(Cl)c2)cc1\C=C\c1ccc(Cl)c(Cl)c1)[N+]([O-])=O Show InChI InChI=1S/C22H12Cl4N2O4/c23-17-7-3-13(9-19(17)25)1-5-15-11-16(22(28(31)32)12-21(15)27(29)30)6-2-14-4-8-18(24)20(26)10-14/h1-12H/b5-1+,6-2+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing-Hua University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human KDM4B (1 to 348 aa) expressed in Escherichia coli using H3K9me3 peptide as substrate after 30 mins by dou... |
J Med Chem 57: 5975-85 (2014)
Article DOI: 10.1021/jm500249n
BindingDB Entry DOI: 10.7270/Q2QC0557 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50047962

(CHEMBL1966136)Show SMILES [O-][N+](=O)c1cc(c(\C=C\c2ccc(Cl)c(Cl)c2)cc1\C=C\c1ccc(Cl)c(Cl)c1)[N+]([O-])=O Show InChI InChI=1S/C22H12Cl4N2O4/c23-17-7-3-13(9-19(17)25)1-5-15-11-16(22(28(31)32)12-21(15)27(29)30)6-2-14-4-8-18(24)20(26)10-14/h1-12H/b5-1+,6-2+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing-Hua University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human KDM4A (1 to 347 aa) expressed in Escherichia coli using H3K9me3 peptide as substrate after 30 mins by dou... |
J Med Chem 57: 5975-85 (2014)
Article DOI: 10.1021/jm500249n
BindingDB Entry DOI: 10.7270/Q2QC0557 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223090

(3-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...)Show InChI InChI=1S/C17H11ClFN3O/c18-14-9-12(4-5-15(14)19)22-17-13-8-11(2-1-7-23)3-6-16(13)20-10-21-17/h3-6,8-10,23H,7H2,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50095259

((6,7-Dimethoxy-quinazolin-4-yl)-(3-ethynyl-phenyl)...)Show InChI InChI=1S/C18H15N3O2/c1-4-12-6-5-7-13(8-12)21-18-14-9-16(22-2)17(23-3)10-15(14)19-11-20-18/h1,5-11H,2-3H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223084

(CHEMBL251498 | N-(3-chloro-4-fluorophenyl)-6-(3-mo...)Show InChI InChI=1S/C21H18ClFN4O/c22-18-13-16(4-5-19(18)23)26-21-17-12-15(3-6-20(17)24-14-25-21)2-1-7-27-8-10-28-11-9-27/h3-6,12-14H,7-11H2,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223082

(CHEMBL251700 | N-(3-chloro-4-fluorophenyl)-6-(3-(p...)Show InChI InChI=1S/C22H20ClFN4/c23-19-14-17(7-8-20(19)24)27-22-18-13-16(6-9-21(18)25-15-26-22)5-4-12-28-10-2-1-3-11-28/h6-9,13-15H,1-3,10-12H2,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223095

(4-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...)Show SMILES CC(O)C#Cc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 |w:1.1| Show InChI InChI=1S/C18H13ClFN3O/c1-11(24)2-3-12-4-7-17-14(8-12)18(22-10-21-17)23-13-5-6-16(20)15(19)9-13/h4-11,24H,1H3,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223092

(5-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...)Show InChI InChI=1S/C19H15ClFN3O/c20-16-11-14(6-7-17(16)21)24-19-15-10-13(4-2-1-3-9-25)5-8-18(15)22-12-23-19/h5-8,10-12,25H,1,3,9H2,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223085

(CHEMBL249509 | N-(3-chloro-4-fluorophenyl)-6-ethyn...)Show InChI InChI=1S/C16H9ClFN3/c1-2-10-3-6-15-12(7-10)16(20-9-19-15)21-11-4-5-14(18)13(17)8-11/h1,3-9H,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223093

(4-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...)Show SMILES CC(C)(O)C#Cc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C19H15ClFN3O/c1-19(2,25)8-7-12-3-6-17-14(9-12)18(23-11-22-17)24-13-4-5-16(21)15(20)10-13/h3-6,9-11,25H,1-2H3,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223088

(4-(4-(3-chloro-4-fluorophenylamino)quinazolin-6-yl...)Show InChI InChI=1S/C18H13ClFN3O/c19-15-10-13(5-6-16(15)20)23-18-14-9-12(3-1-2-8-24)4-7-17(14)21-11-22-18/h4-7,9-11,24H,2,8H2,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223086
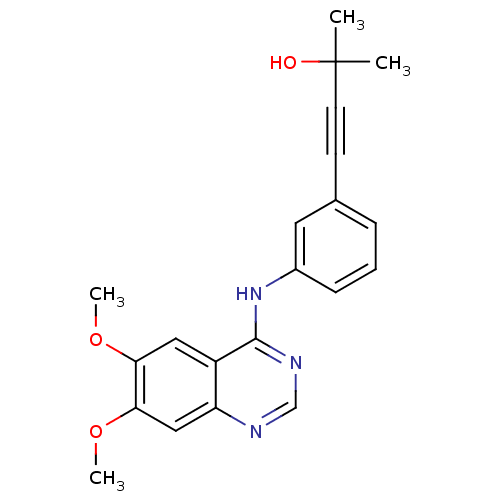
(4-(3-(6,7-dimethoxyquinazolin-4-ylamino)phenyl)-2-...)Show InChI InChI=1S/C21H21N3O3/c1-21(2,25)9-8-14-6-5-7-15(10-14)24-20-16-11-18(26-3)19(27-4)12-17(16)22-13-23-20/h5-7,10-13,25H,1-4H3,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223094

(CHEMBL249511 | N-(3-chloro-4-fluorophenyl)-6-(2-ph...)Show InChI InChI=1S/C22H13ClFN3/c23-19-13-17(9-10-20(19)24)27-22-18-12-16(8-11-21(18)25-14-26-22)7-6-15-4-2-1-3-5-15/h1-5,8-14H,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223091

(4-(3-(6,7-dimethoxyquinazolin-4-ylamino)phenyl)but...)Show InChI InChI=1S/C20H19N3O3/c1-25-18-11-16-17(12-19(18)26-2)21-13-22-20(16)23-15-8-5-7-14(10-15)6-3-4-9-24/h5,7-8,10-13,24H,4,9H2,1-2H3,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223087

(6,7-dimethoxy-N-(3-(2-phenylethynyl)phenyl)quinazo...)Show InChI InChI=1S/C24H19N3O2/c1-28-22-14-20-21(15-23(22)29-2)25-16-26-24(20)27-19-10-6-9-18(13-19)12-11-17-7-4-3-5-8-17/h3-10,13-16H,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 921 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167307

(1-Benzo[b]thiophen-2-ylmethyl-5-iodo-1H-indole-2,3...)Show InChI InChI=1S/C17H10INO2S/c18-11-5-6-14-13(8-11)16(20)17(21)19(14)9-12-7-10-3-1-2-4-15(10)22-12/h1-8H,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167309

(1-Benzo[b]thiophen-2-ylmethyl-7-bromo-1H-indole-2,...)Show InChI InChI=1S/C17H10BrNO2S/c18-13-6-3-5-12-15(13)19(17(21)16(12)20)9-11-8-10-4-1-2-7-14(10)22-11/h1-8H,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50167307

(1-Benzo[b]thiophen-2-ylmethyl-5-iodo-1H-indole-2,3...)Show InChI InChI=1S/C17H10INO2S/c18-11-5-6-14-13(8-11)16(20)17(21)19(14)9-12-7-10-3-1-2-4-15(10)22-12/h1-8H,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Chymotrypsin (serine protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223089

(5-(3-(6,7-dimethoxyquinazolin-4-ylamino)phenyl)pen...)Show InChI InChI=1S/C21H21N3O3/c1-26-19-12-17-18(13-20(19)27-2)22-14-23-21(17)24-16-9-6-8-15(11-16)7-4-3-5-10-25/h6,8-9,11-14,25H,3,5,10H2,1-2H3,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223096

(CHEMBL398963 | N-(3-chloro-4-fluorophenyl)-6-(2-(t...)Show InChI InChI=1S/C20H11ClFN3S/c21-17-11-14(5-7-18(17)22)25-20-16-10-13(3-6-15-2-1-9-26-15)4-8-19(16)23-12-24-20/h1-2,4-5,7-12H,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50223083
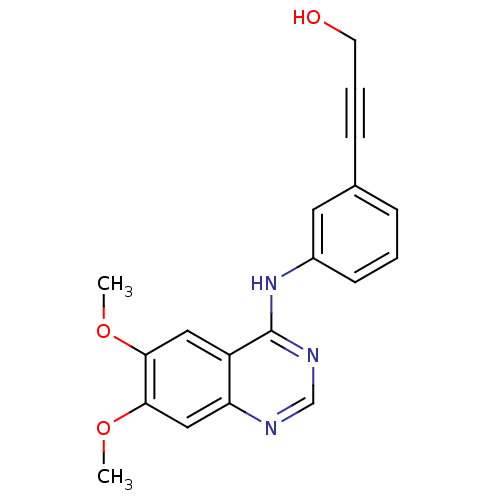
(3-(3-(6,7-dimethoxyquinazolin-4-ylamino)phenyl)pro...)Show InChI InChI=1S/C19H17N3O3/c1-24-17-10-15-16(11-18(17)25-2)20-12-21-19(15)22-14-7-3-5-13(9-14)6-4-8-23/h3,5,7,9-12,23H,8H2,1-2H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6373-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.061
BindingDB Entry DOI: 10.7270/Q25Q4VT1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167306
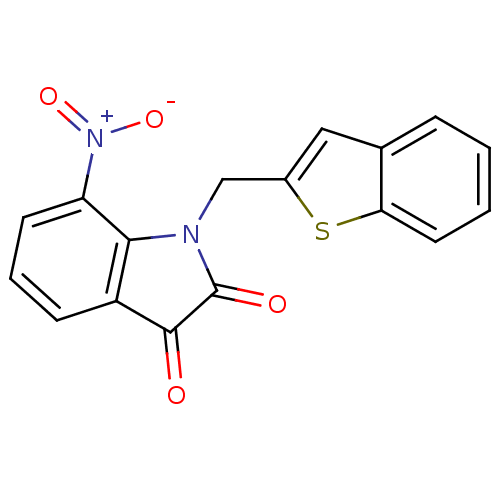
(1-Benzo[b]thiophen-2-ylmethyl-7-nitro-1H-indole-2,...)Show SMILES [O-][N+](=O)c1cccc2C(=O)C(=O)N(Cc3cc4ccccc4s3)c12 Show InChI InChI=1S/C17H10N2O4S/c20-16-12-5-3-6-13(19(22)23)15(12)18(17(16)21)9-11-8-10-4-1-2-7-14(10)24-11/h1-8H,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167311
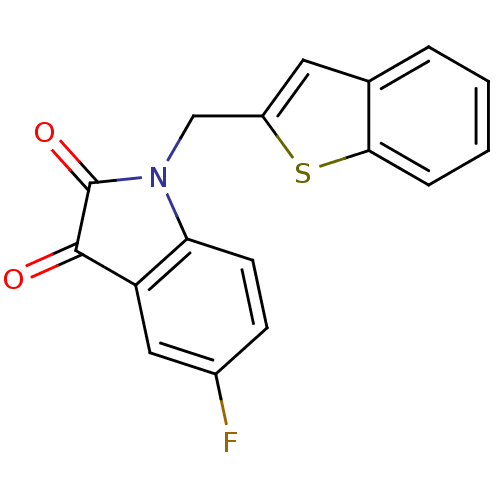
(1-Benzo[b]thiophen-2-ylmethyl-5-fluoro-1H-indole-2...)Show InChI InChI=1S/C17H10FNO2S/c18-11-5-6-14-13(8-11)16(20)17(21)19(14)9-12-7-10-3-1-2-4-15(10)22-12/h1-8H,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50047962

(CHEMBL1966136)Show SMILES [O-][N+](=O)c1cc(c(\C=C\c2ccc(Cl)c(Cl)c2)cc1\C=C\c1ccc(Cl)c(Cl)c1)[N+]([O-])=O Show InChI InChI=1S/C22H12Cl4N2O4/c23-17-7-3-13(9-19(17)25)1-5-15-11-16(22(28(31)32)12-21(15)27(29)30)6-2-14-4-8-18(24)20(26)10-14/h1-12H/b5-1+,6-2+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing-Hua University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human KDM4A (1 to 347 aa) expressed in Escherichia coli using H3K9me3 (3 to 17 aa) peptide as substrate after 3... |
J Med Chem 57: 5975-85 (2014)
Article DOI: 10.1021/jm500249n
BindingDB Entry DOI: 10.7270/Q2QC0557 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167314

(1-(3,5-Dimethyl-isoxazol-4-ylmethyl)-2,3-dioxo-2,3...)Show InChI InChI=1S/C15H11N3O3/c1-8-12(9(2)21-17-8)7-18-13-4-3-10(6-16)5-11(13)14(19)15(18)20/h3-5H,7H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4B
(Homo sapiens (Human)) | BDBM50047962

(CHEMBL1966136)Show SMILES [O-][N+](=O)c1cc(c(\C=C\c2ccc(Cl)c(Cl)c2)cc1\C=C\c1ccc(Cl)c(Cl)c1)[N+]([O-])=O Show InChI InChI=1S/C22H12Cl4N2O4/c23-17-7-3-13(9-19(17)25)1-5-15-11-16(22(28(31)32)12-21(15)27(29)30)6-2-14-4-8-18(24)20(26)10-14/h1-12H/b5-1+,6-2+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing-Hua University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human KDM4B (1 to 348 aa) expressed in Escherichia coli using H3K9me3 (3 to 17 aa) peptide as substrate after 3... |
J Med Chem 57: 5975-85 (2014)
Article DOI: 10.1021/jm500249n
BindingDB Entry DOI: 10.7270/Q2QC0557 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167317
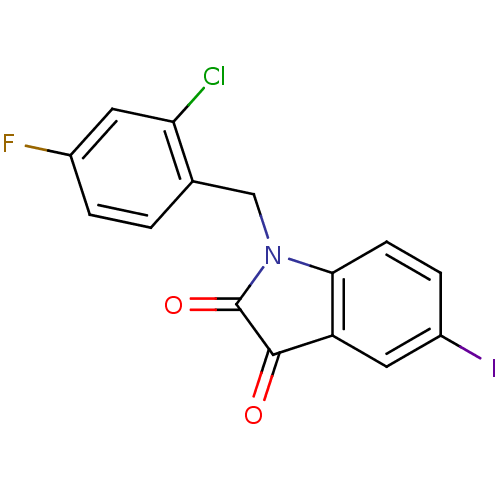
(1-(2-Chloro-4-fluoro-benzyl)-5-iodo-1H-indole-2,3-...)Show InChI InChI=1S/C15H8ClFINO2/c16-12-5-9(17)2-1-8(12)7-19-13-4-3-10(18)6-11(13)14(20)15(19)21/h1-6H,7H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50167309

(1-Benzo[b]thiophen-2-ylmethyl-7-bromo-1H-indole-2,...)Show InChI InChI=1S/C17H10BrNO2S/c18-13-6-3-5-12-15(13)19(17(21)16(12)20)9-11-8-10-4-1-2-7-14(10)22-11/h1-8H,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Chymotrypsin (serine protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
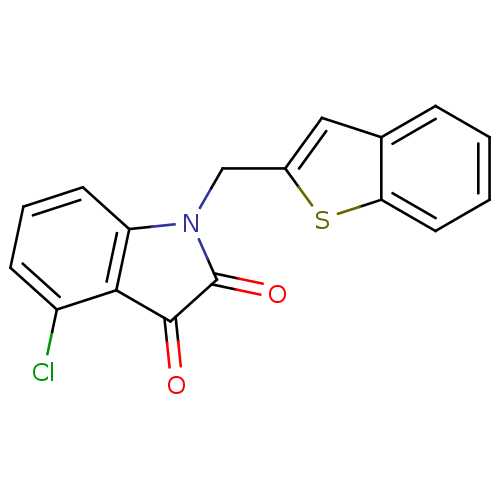
(Human SARS coronavirus (SARS-CoV)) | BDBM50167312
(1-Benzo[b]thiophen-2-ylmethyl-4-chloro-1H-indole-2...)Show InChI InChI=1S/C17H10ClNO2S/c18-12-5-3-6-13-15(12)16(20)17(21)19(13)9-11-8-10-4-1-2-7-14(10)22-11/h1-8H,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167308
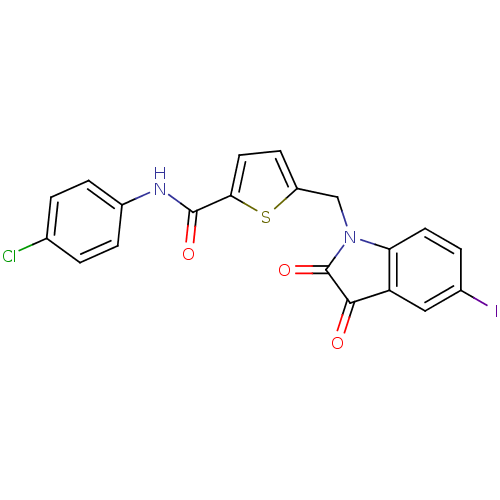
(5-(5-Iodo-2,3-dioxo-2,3-dihydro-indol-1-ylmethyl)-...)Show SMILES Clc1ccc(NC(=O)c2ccc(CN3C(=O)C(=O)c4cc(I)ccc34)s2)cc1 Show InChI InChI=1S/C20H12ClIN2O3S/c21-11-1-4-13(5-2-11)23-19(26)17-8-6-14(28-17)10-24-16-7-3-12(22)9-15(16)18(25)20(24)27/h1-9H,10H2,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167313

(1-Benzo[b]thiophen-2-ylmethyl-1H-indole-2,3-dione ...)Show InChI InChI=1S/C17H11NO2S/c19-16-13-6-2-3-7-14(13)18(17(16)20)10-12-9-11-5-1-4-8-15(11)21-12/h1-9H,10H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167316

(1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-5-iodo...)Show InChI InChI=1S/C17H12INO4/c18-10-5-6-13-12(7-10)16(20)17(21)19(13)8-11-9-22-14-3-1-2-4-15(14)23-11/h1-7,11H,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) at 20 uM |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167315

(5-Iodo-1-[5-(piperidine-1-carbonyl)-thiophen-2-ylm...)Show SMILES Ic1ccc2N(Cc3ccc(s3)C(=O)N3CCCCC3)C(=O)C(=O)c2c1 Show InChI InChI=1S/C19H17IN2O3S/c20-12-4-6-15-14(10-12)17(23)19(25)22(15)11-13-5-7-16(26-13)18(24)21-8-2-1-3-9-21/h4-7,10H,1-3,8-9,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50167310

(1-((E)-3-Benzo[b]thiophen-2-yl-allyl)-5-iodo-1H-in...)Show SMILES Ic1ccc2N(C\C=C\c3cc4ccccc4s3)C(=O)C(=O)c2c1 Show InChI InChI=1S/C19H12INO2S/c20-13-7-8-16-15(11-13)18(22)19(23)21(16)9-3-5-14-10-12-4-1-2-6-17(12)24-14/h1-8,10-11H,9H2/b5-3+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) |
Bioorg Med Chem Lett 15: 3058-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.027
BindingDB Entry DOI: 10.7270/Q2H131JB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2E1
(Homo sapiens (Human)) | BDBM50614333

(CHEMBL5267172) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data