

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079508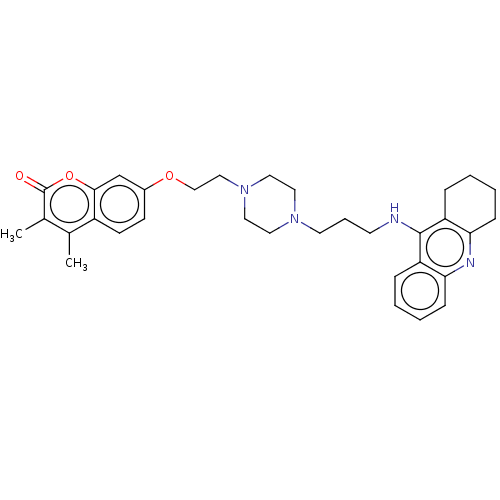 (CHEMBL3417300) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE by Lineweaver-Burk plot analysis | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016469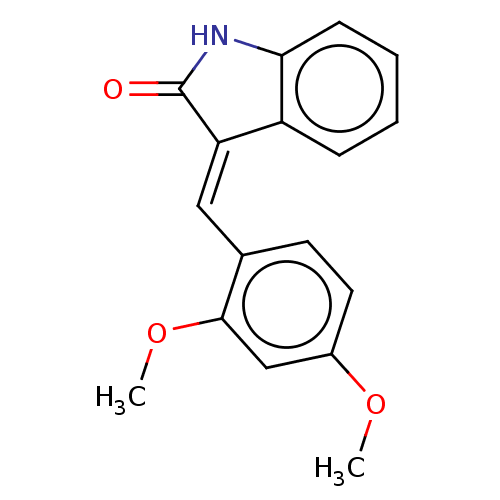 (CHEMBL514709) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016480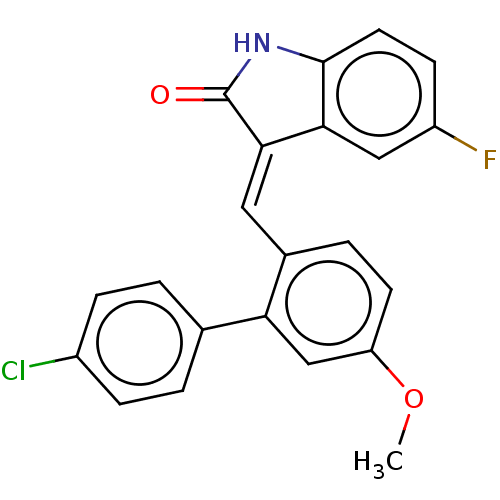 (CHEMBL3265104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016476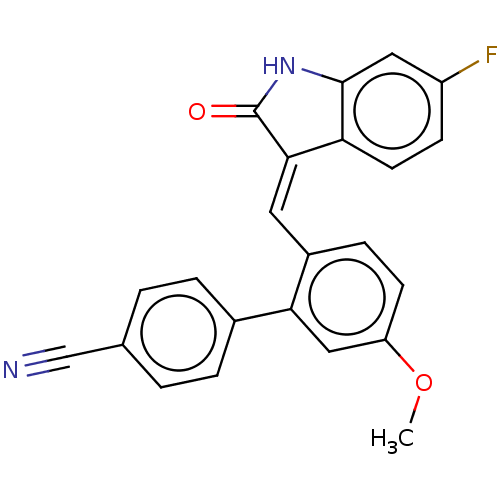 (CHEMBL3265101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016470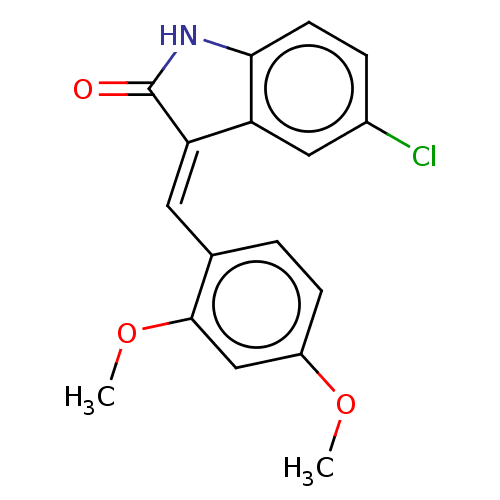 (CHEMBL3265094) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016478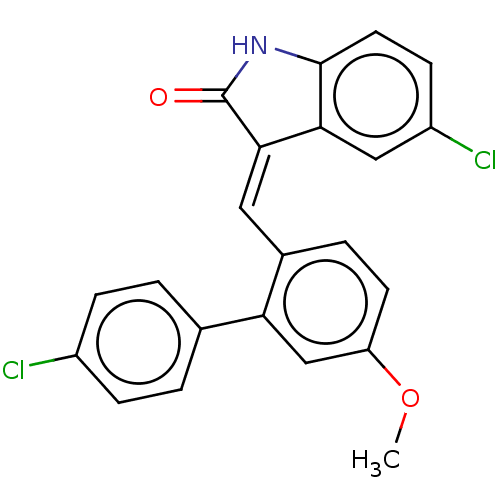 (CHEMBL3265103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016475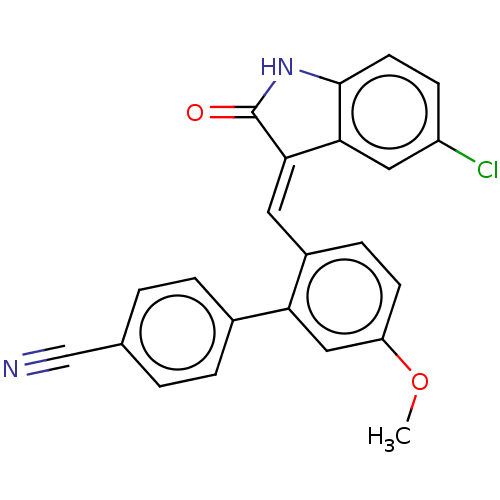 (CHEMBL3265100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016477 (CHEMBL3265102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016474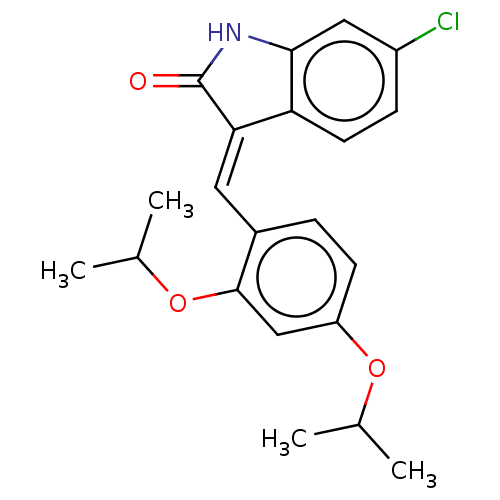 (CHEMBL3265099) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016481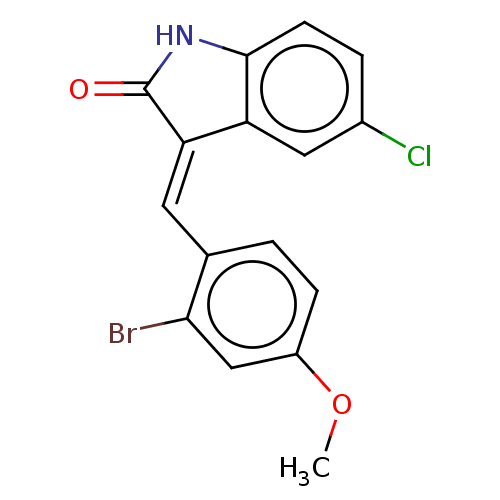 (CHEMBL3259872) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016468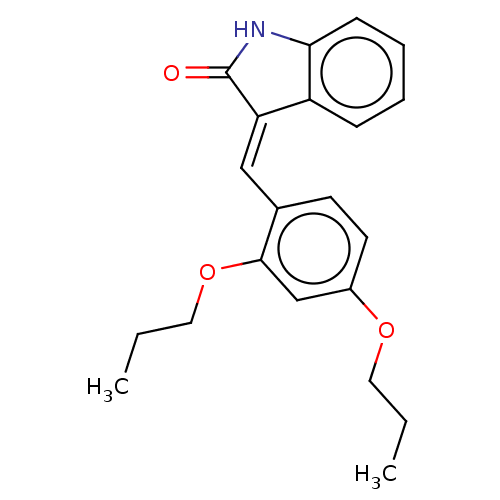 (CHEMBL3265096) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 in human MCF7 cells assessed as inhibition of MDM2-p53 interaction | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016468 (CHEMBL3265096) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016473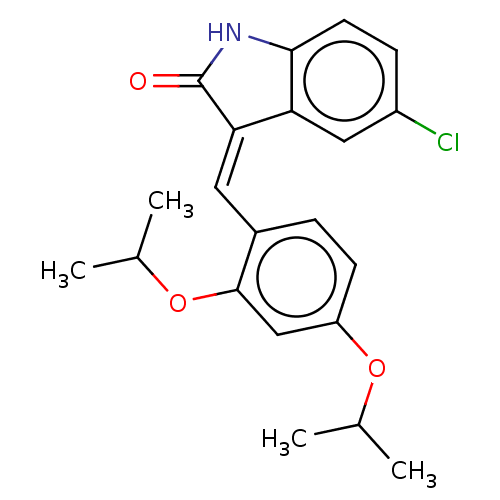 (CHEMBL3265098) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016472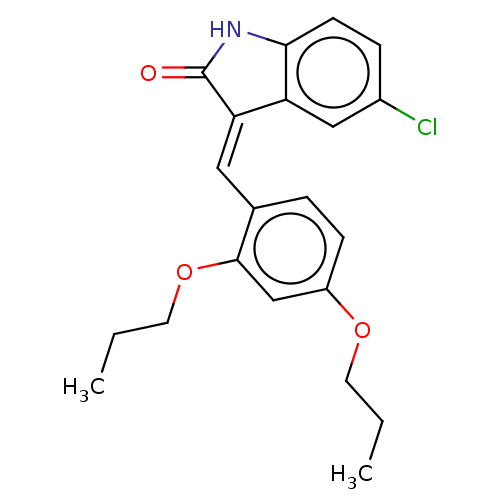 (CHEMBL3265097) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50016471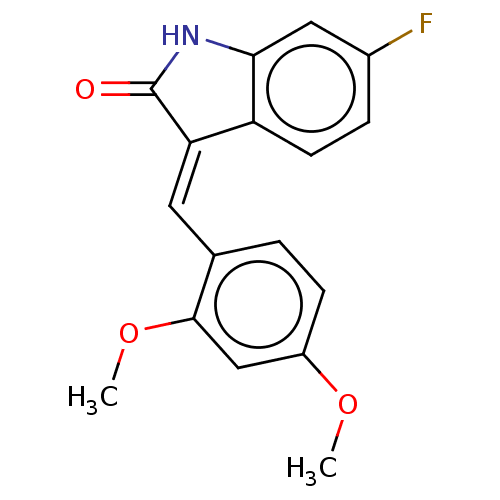 (CHEMBL3265095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College Curated by ChEMBL | Assay Description Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay | Eur J Med Chem 81: 277-88 (2014) Article DOI: 10.1016/j.ejmech.2014.05.027 BindingDB Entry DOI: 10.7270/Q2736SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50282506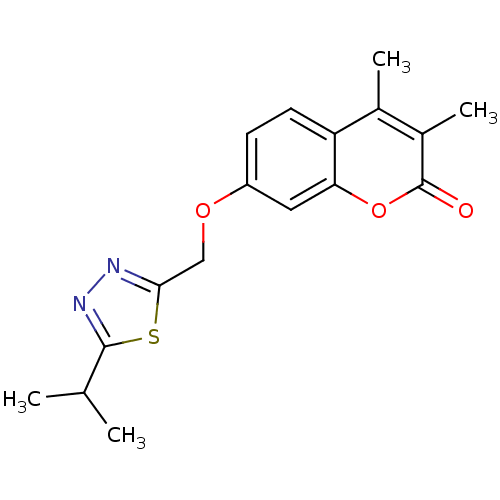 (7-(5-Isopropyl-[1,3,4]thiadiazol-2-ylmethoxy)-3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-B (unknown origin) | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073116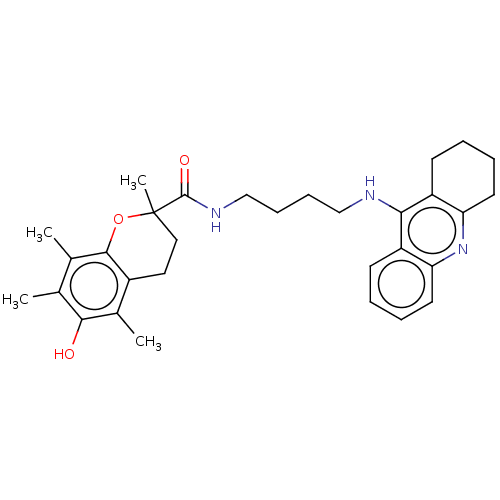 (CHEMBL3410952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073114 (CHEMBL3410954) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073115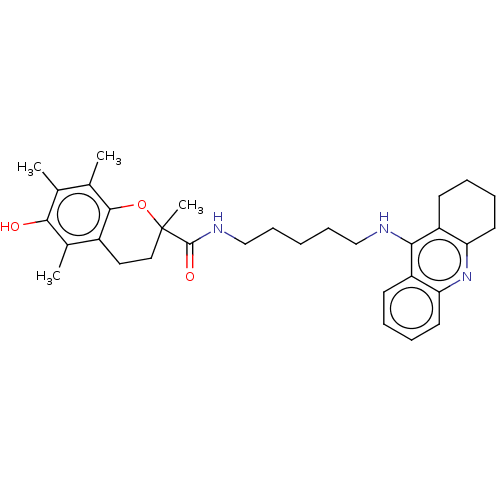 (CHEMBL3410953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 5 mins followed by NADPH addition and measured after 20 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112465 BindingDB Entry DOI: 10.7270/Q23X8BCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079508 (CHEMBL3417300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180 secs b... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079522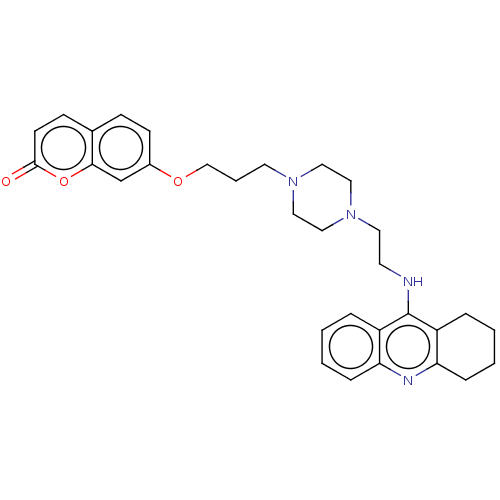 (CHEMBL3417311) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073116 (CHEMBL3410952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079515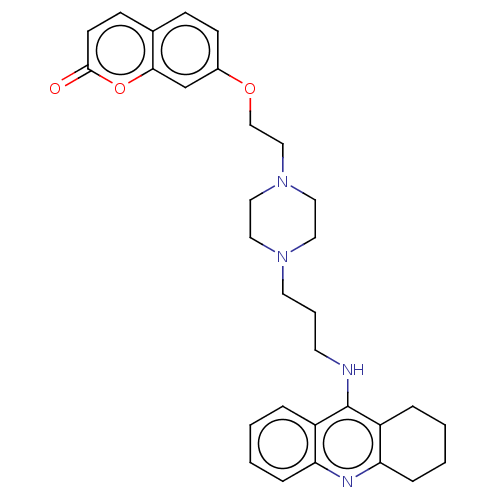 (CHEMBL3417307) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073113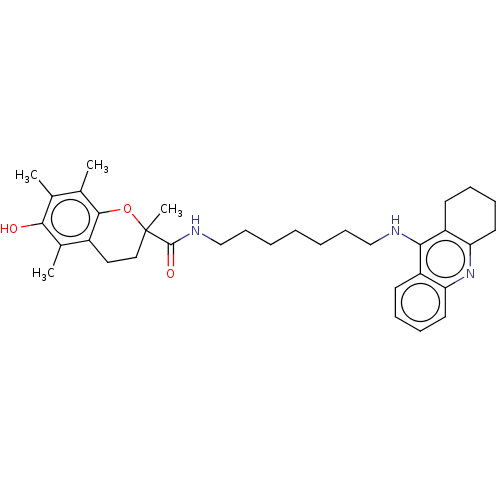 (CHEMBL3410955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079518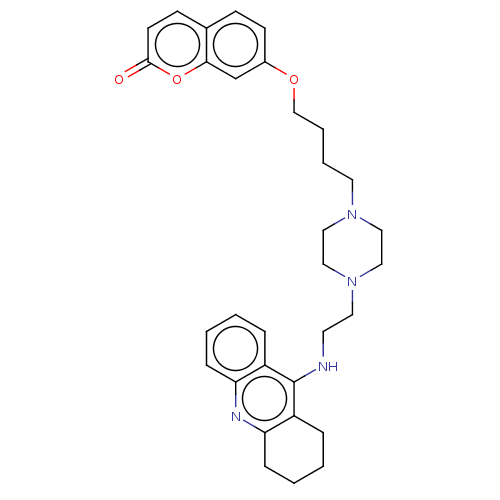 (CHEMBL3417310) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073114 (CHEMBL3410954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 5 mins followed by NADPH addition and measured after 20 min... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112465 BindingDB Entry DOI: 10.7270/Q23X8BCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079513 (CHEMBL3417305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079514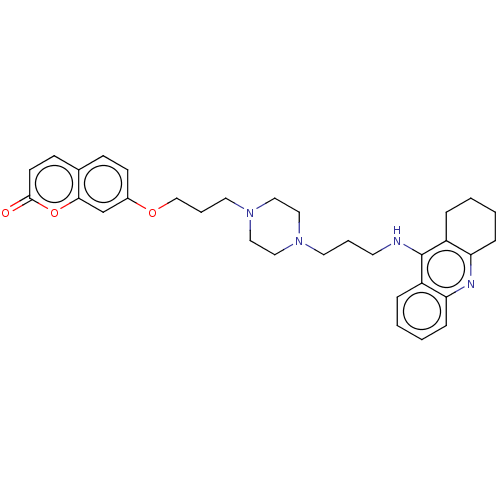 (CHEMBL3417306) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445346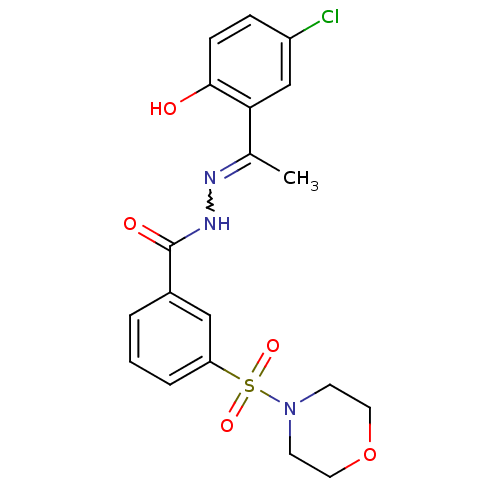 (CHEMBL3104250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) using H3K4me2 as substrate by fluorescence assay | Eur J Med Chem 166: 432-444 (2019) Article DOI: 10.1016/j.ejmech.2019.01.075 BindingDB Entry DOI: 10.7270/Q29S1VGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079506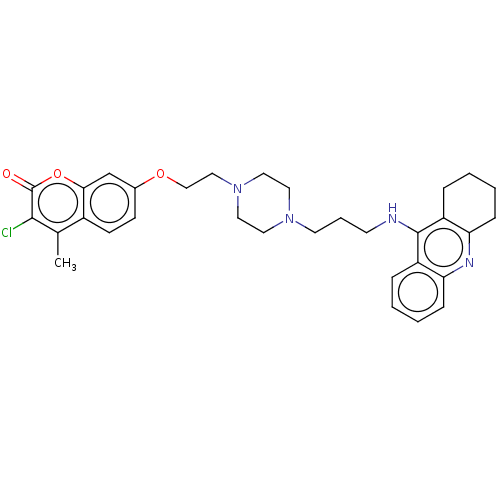 (CHEMBL3417299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180 secs b... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180 se... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079514 (CHEMBL3417306) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079506 (CHEMBL3417299) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079517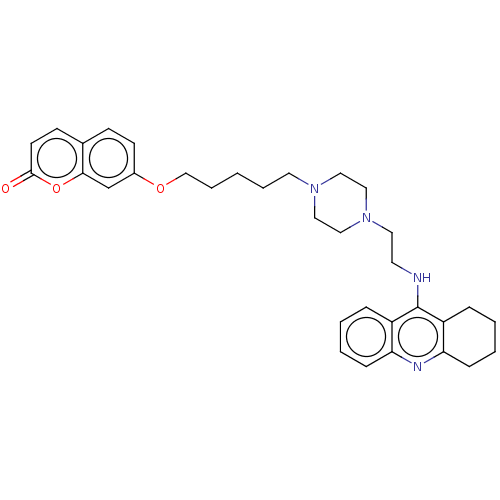 (CHEMBL3417309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079508 (CHEMBL3417300) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073112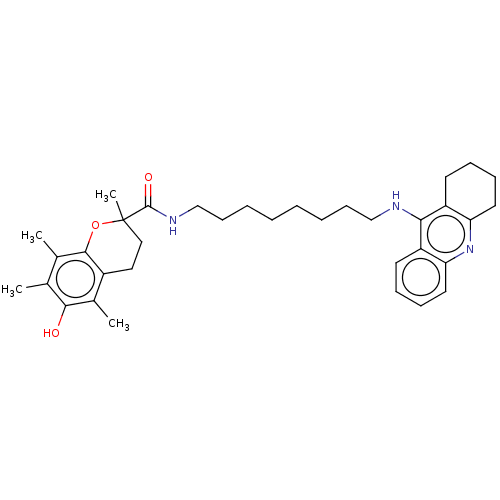 (CHEMBL3410956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073115 (CHEMBL3410953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 5 mins followed by NADPH addition and measured after... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112465 BindingDB Entry DOI: 10.7270/Q23X8BCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079513 (CHEMBL3417305) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079511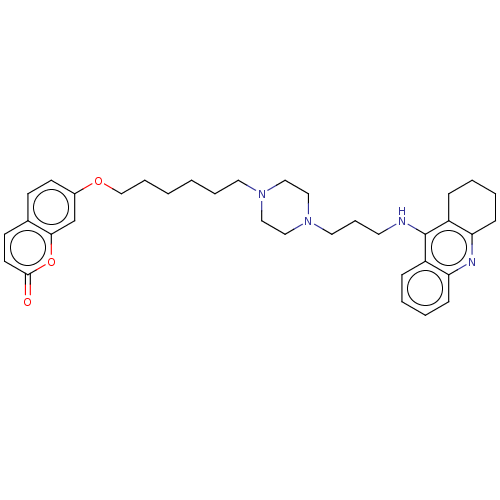 (CHEMBL3417303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079515 (CHEMBL3417307) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079510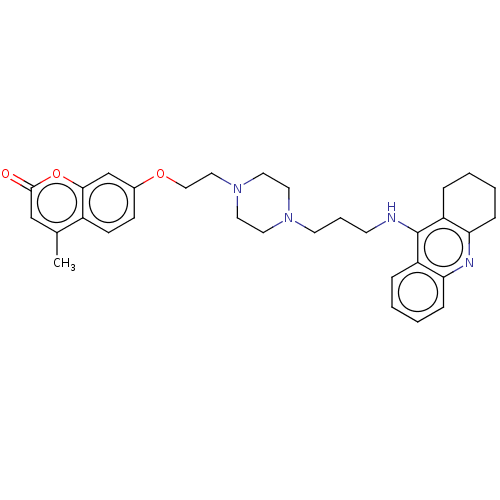 (CHEMBL3417302) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 203 total ) | Next | Last >> |