

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143314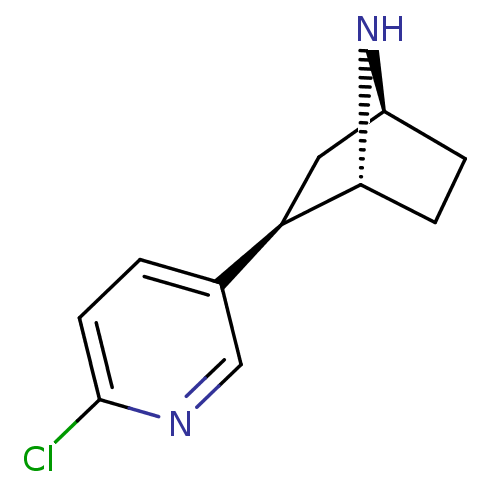 ((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100717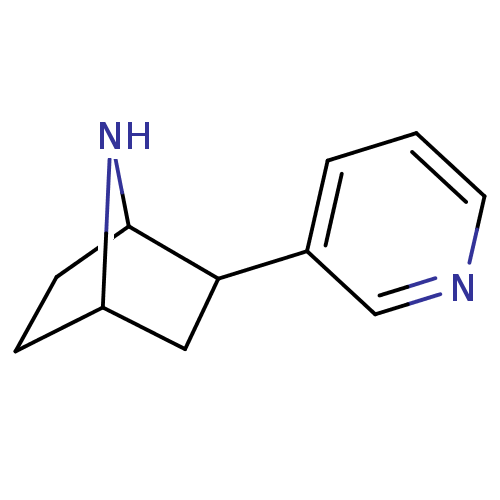 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100715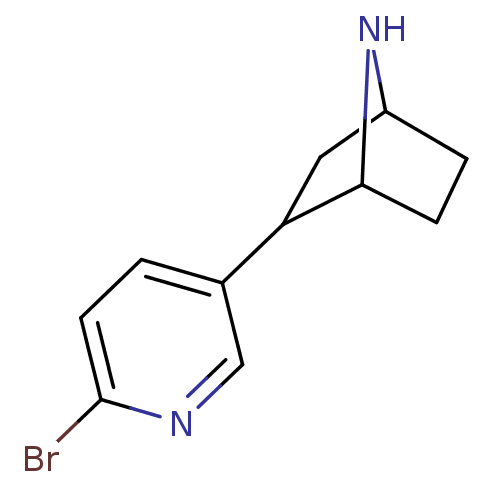 (2-(6-Bromo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hepta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143320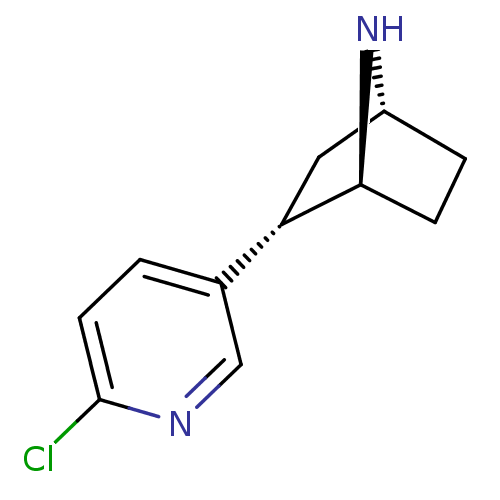 ((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100707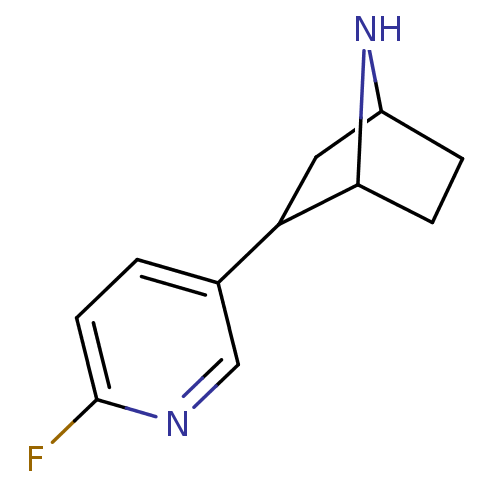 ((R)-2-(6-Fluoro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100717 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Non-specific binding in presence of 300 uM nicotine at nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex membranes | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143320 ((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Non-specific binding in presence of 300 uM nicotine at nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex membranes | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143314 ((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Non-specific binding in presence of 300 uM nicotine at nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex membranes | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100712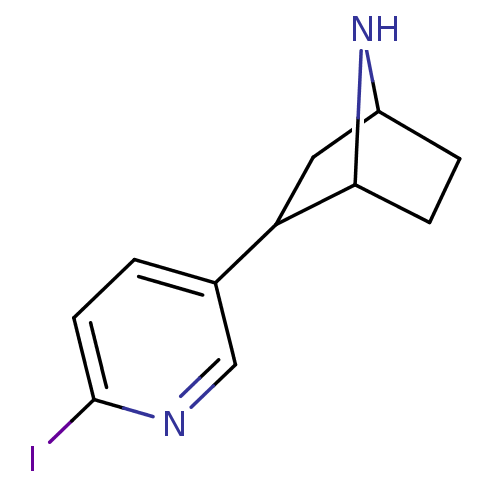 (2-(6-Iodo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100706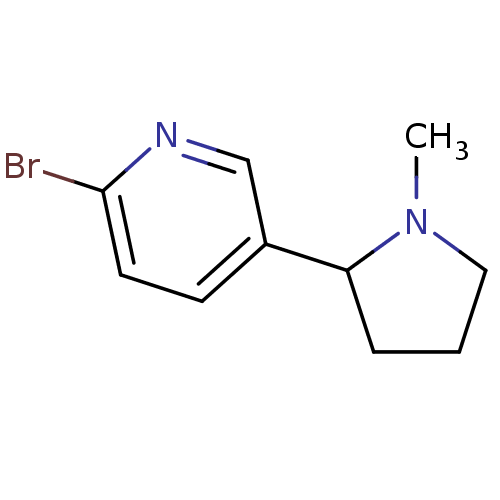 (2-Bromo-5-(1-methyl-pyrrolidin-2-yl)-pyridine | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-nicotine binding at the nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100712 (2-(6-Iodo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Non-specific binding in presence of 300 uM nicotine at nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex membranes | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50078056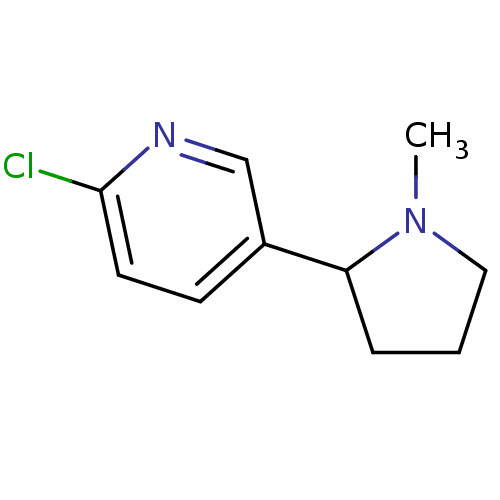 (2-Chloro-5-(1-methyl-pyrrolidin-2-yl)-pyridine | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-nicotine binding at the nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Non-specific binding in presence of 300 uM nicotine at nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex membranes | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100709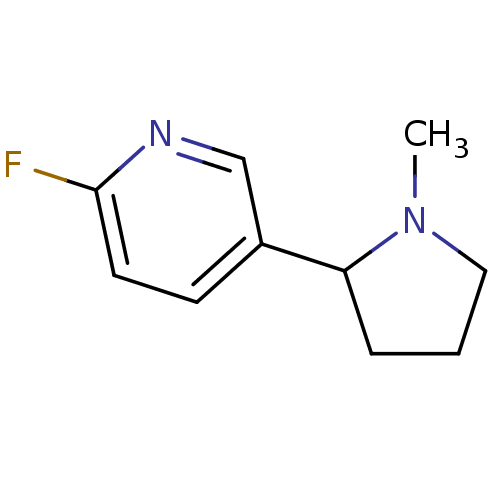 (2-Fluoro-5-(1-methyl-pyrrolidin-2-yl)-pyridine | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-nicotine binding at the nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50004108 ((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-nicotine binding at the nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100713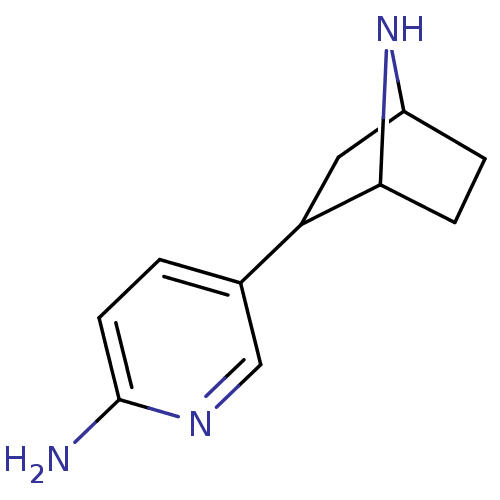 (5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridin-2-ylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100708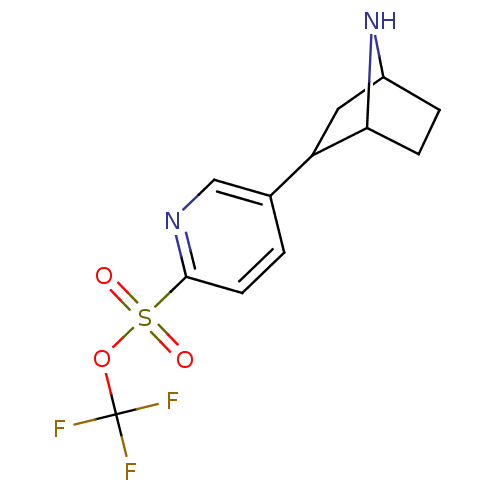 (5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridine-2-sulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100711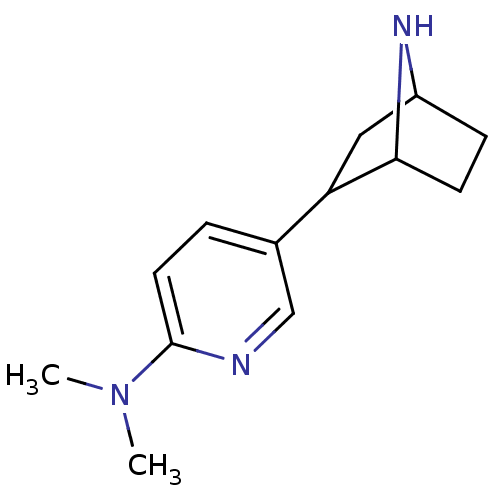 (CHEMBL56677 | [5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100716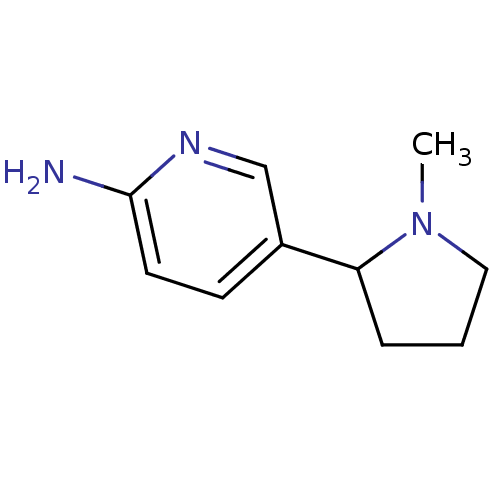 (5-(1-Methyl-pyrrolidin-2-yl)-pyridin-2-ylamine | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-nicotine binding at the nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100714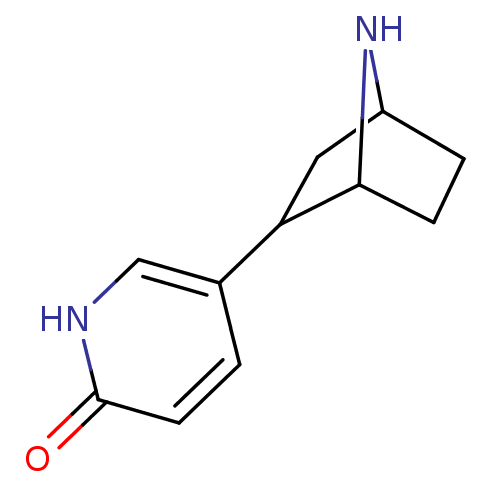 (5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridin-2-ol | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]pibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50253141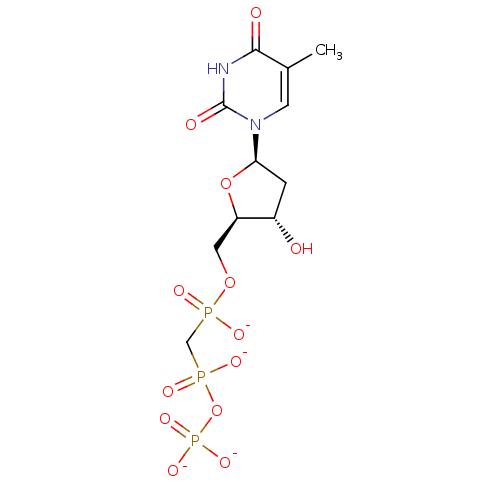 ([(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | J Med Chem 51: 6460-70 (2008) Article DOI: 10.1021/jm800692a BindingDB Entry DOI: 10.7270/Q29P32JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50253100 ([(2R,3S,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | J Med Chem 51: 6460-70 (2008) Article DOI: 10.1021/jm800692a BindingDB Entry DOI: 10.7270/Q29P32JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50253142 ([(2R,3S,5R)-5-(2-amino-6-oxo-6,9-dihydro-1H-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | J Med Chem 51: 6460-70 (2008) Article DOI: 10.1021/jm800692a BindingDB Entry DOI: 10.7270/Q29P32JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100710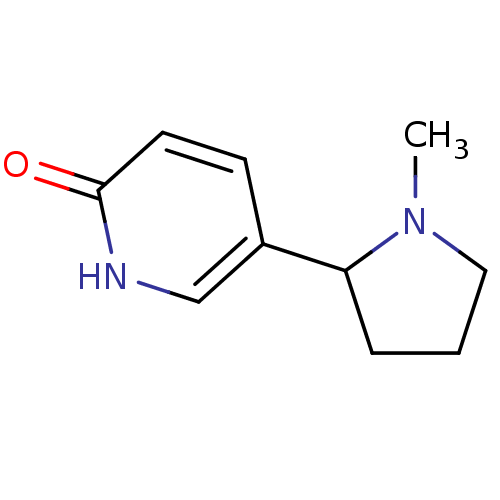 (5-(1-methylpyrrolidin-2-yl)pyridin-2-ol | 6-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-nicotine binding at the nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50377969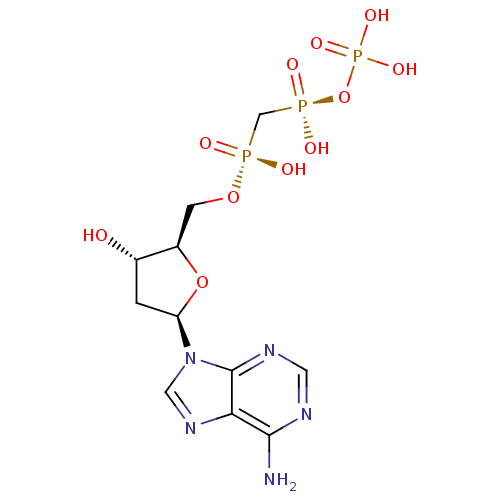 (CHEMBL1162297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | J Med Chem 51: 6460-70 (2008) Article DOI: 10.1021/jm800692a BindingDB Entry DOI: 10.7270/Q29P32JF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100728 (US8507676, 78) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100726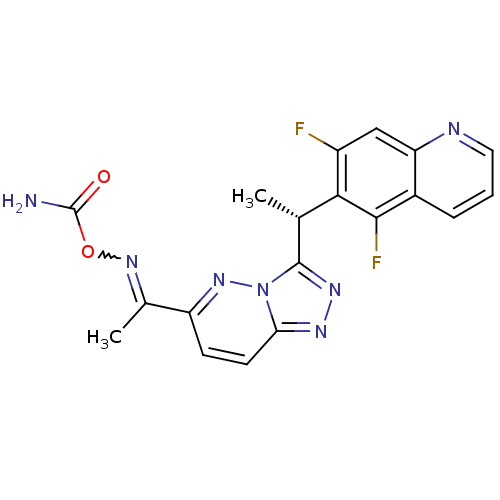 (US8507676, 77-S) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163201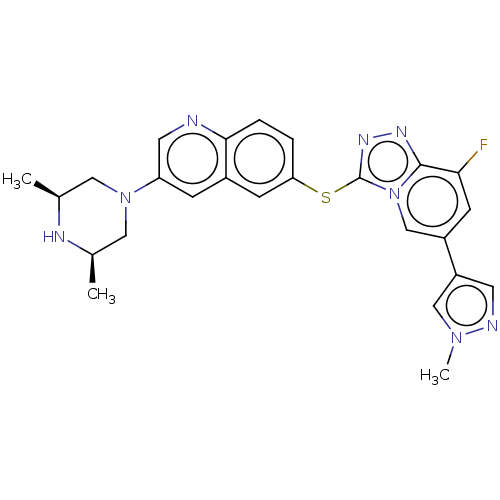 (US9062045, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163213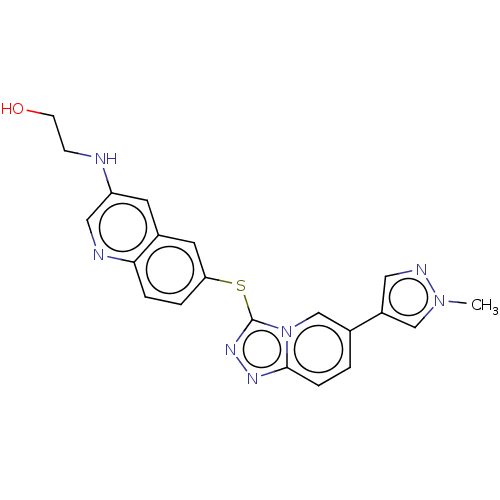 (US9062045, 53) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163224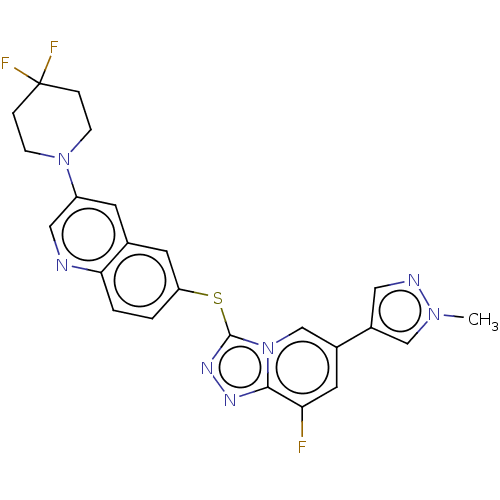 (US9062045, 67) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163174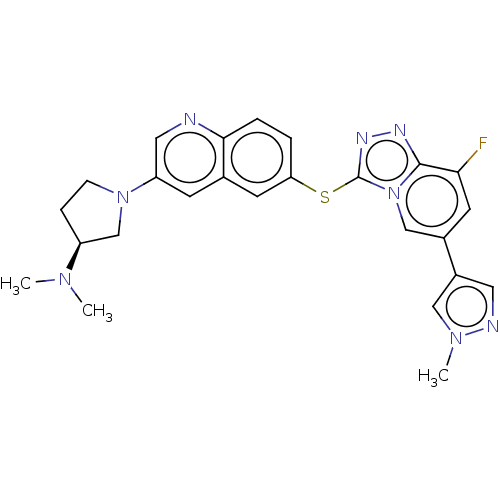 (US9062045, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163205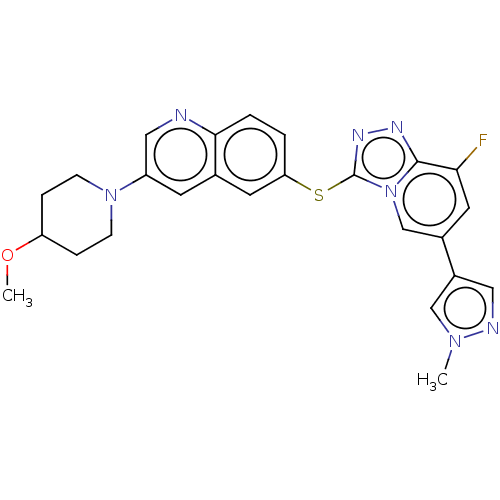 (US9062045, 44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163169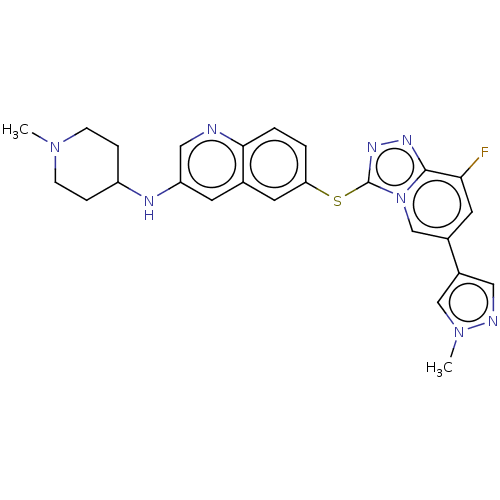 (US9062045, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100724 (US8507676, 75-S) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163215 (US9062045, 55) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100659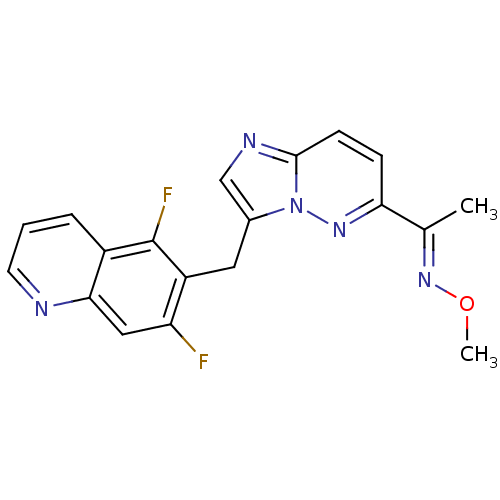 (US8507676, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163183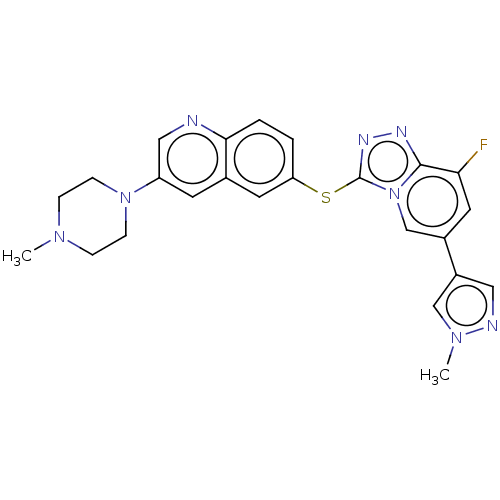 (US9062045, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163189 (US9062045, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163180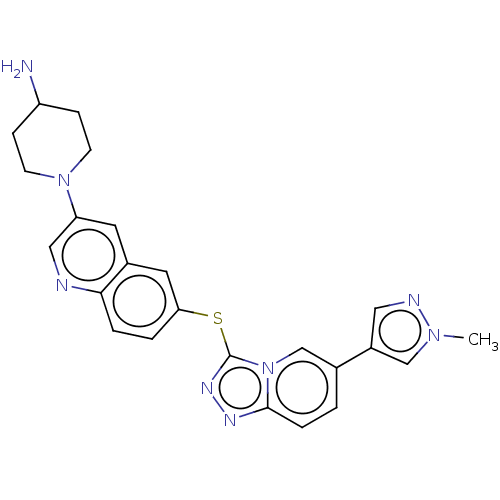 (US9062045, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163212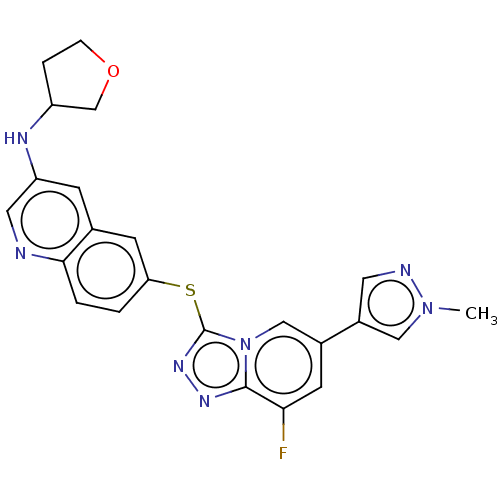 (US9062045, 52) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163164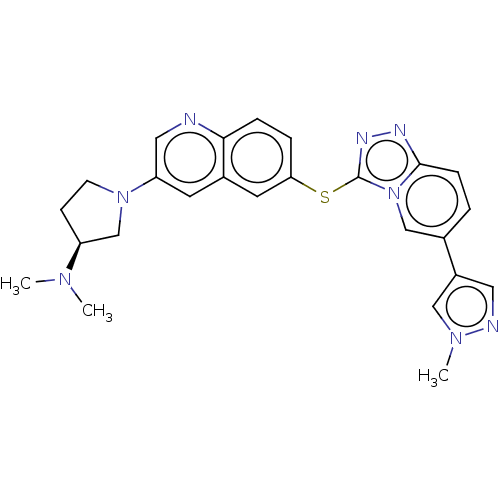 (US9062045, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163217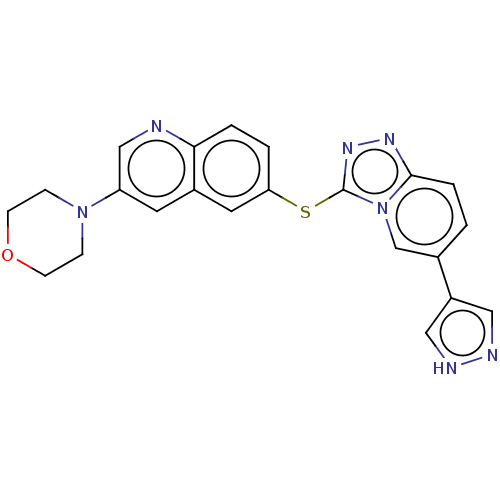 (US9062045, 57) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163175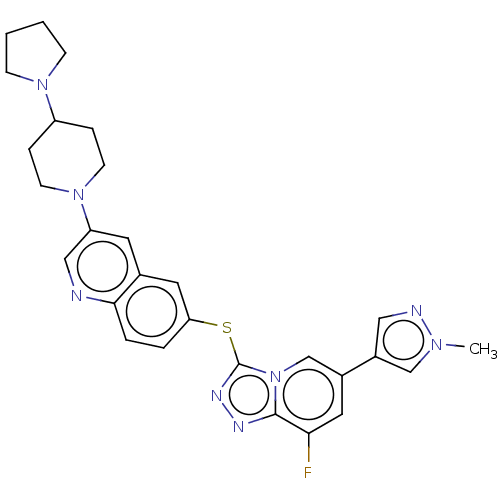 (US9062045, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163204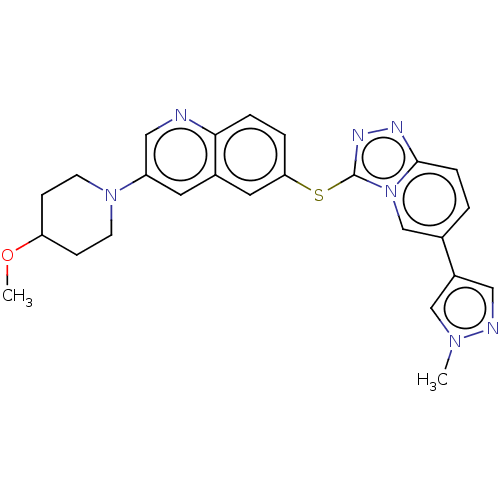 (US9062045, 43) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163234 (US9062045, 78) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163199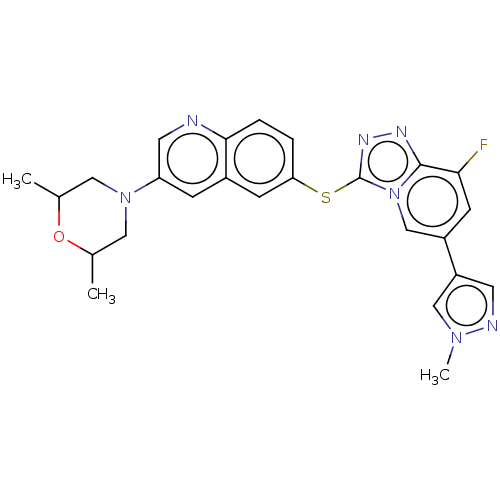 (US9062045, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163198 (US9062045, 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-F... | US Patent US9062045 (2015) BindingDB Entry DOI: 10.7270/Q23F4NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100674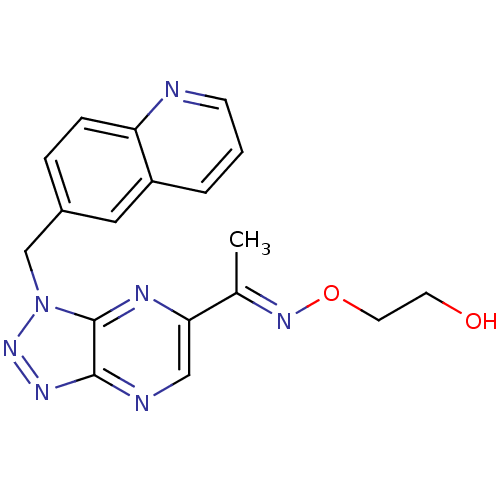 (US8507676, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100673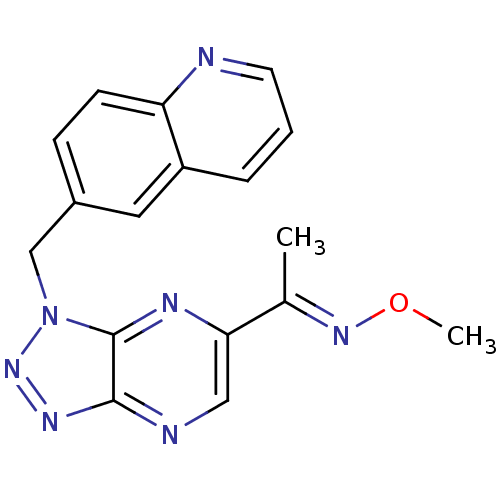 (US8507676, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 364 total ) | Next | Last >> |