

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085666 ((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085674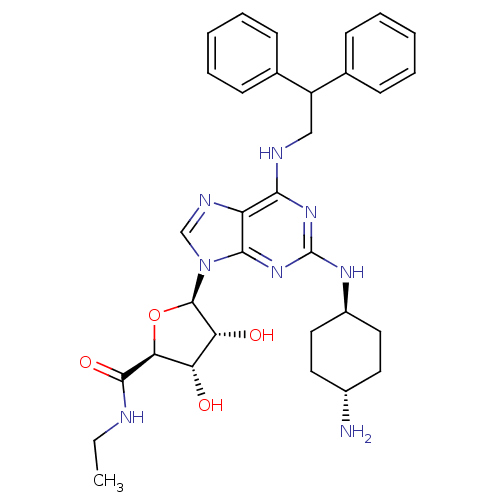 ((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199865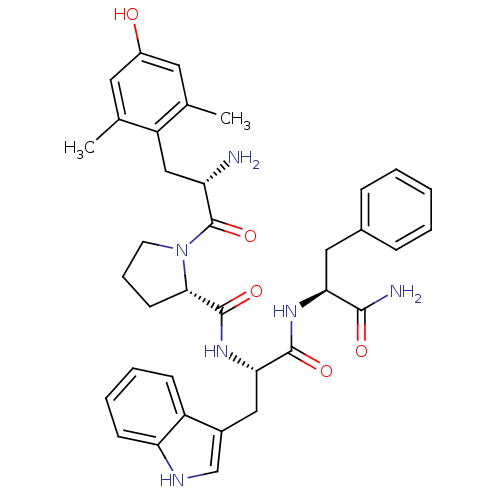 ((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells | Bioorg Med Chem 16: 6286-96 (2008) Article DOI: 10.1016/j.bmc.2008.04.020 BindingDB Entry DOI: 10.7270/Q2ZG6S2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50199865 ((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | Bioorg Med Chem 16: 6286-96 (2008) Article DOI: 10.1016/j.bmc.2008.04.020 BindingDB Entry DOI: 10.7270/Q2ZG6S2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085671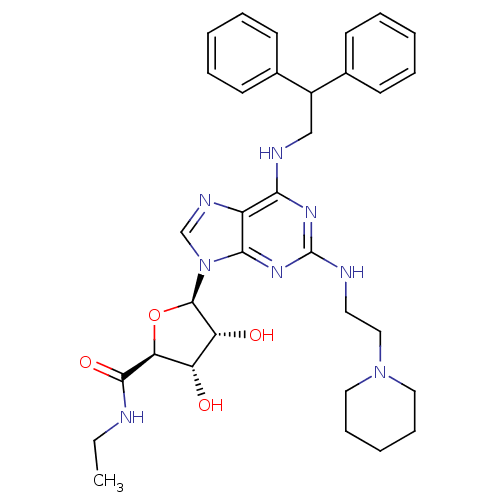 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50272031 ((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells | Bioorg Med Chem 16: 6286-96 (2008) Article DOI: 10.1016/j.bmc.2008.04.020 BindingDB Entry DOI: 10.7270/Q2ZG6S2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085668 ((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085662 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-((1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50331552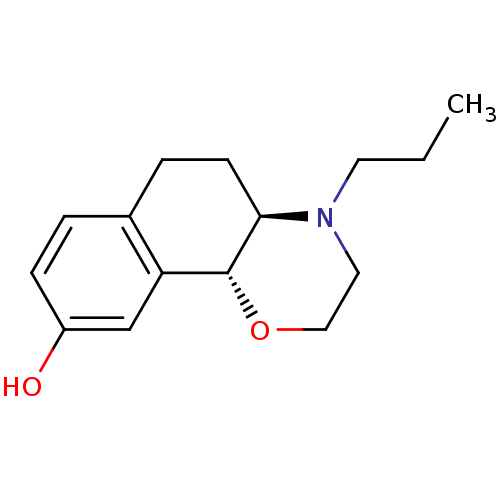 (CHEMBL1288585 | [11C]-(+)-(4aR,10bR)-4-propyl-3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Binding affinity to human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50341447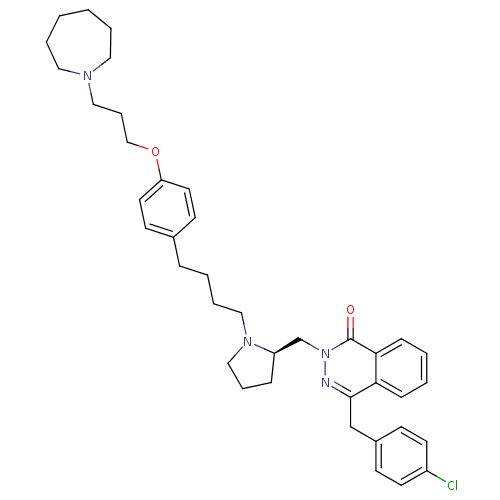 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205359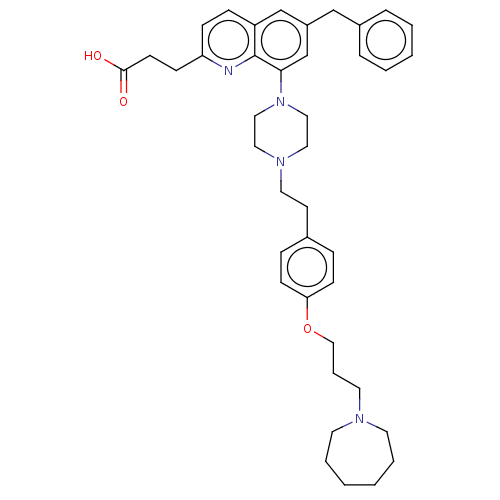 (CHEMBL3917428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132057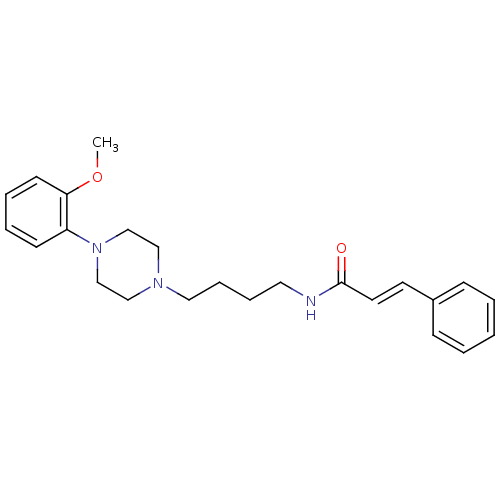 (CHEMBL1180430 | CHEMBL126128 | N-{4-[4-(2-Methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132069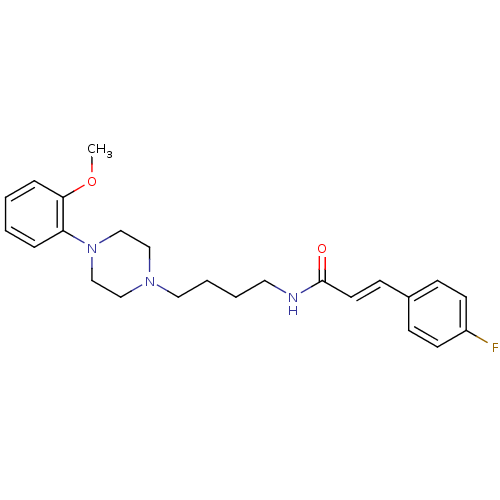 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50294115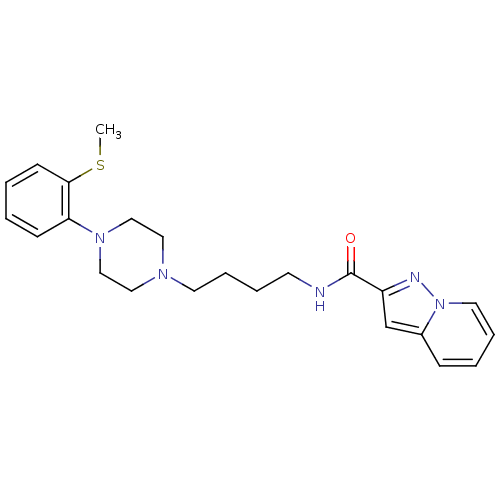 (CHEMBL550222 | N-(4-(4-(2-(methylthio)phenyl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in CHO cells | J Med Chem 52: 4923-35 (2009) Article DOI: 10.1021/jm900690y BindingDB Entry DOI: 10.7270/Q2PK0G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205360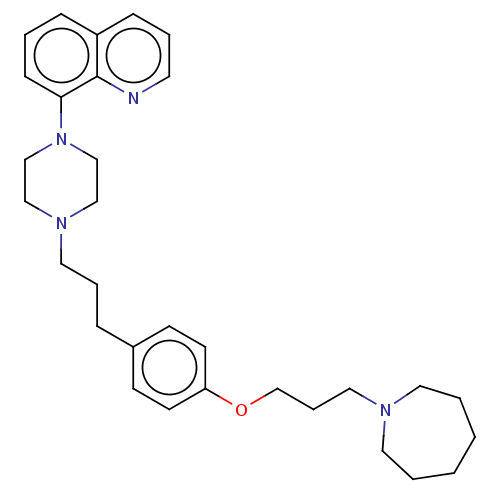 (CHEMBL3921827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474799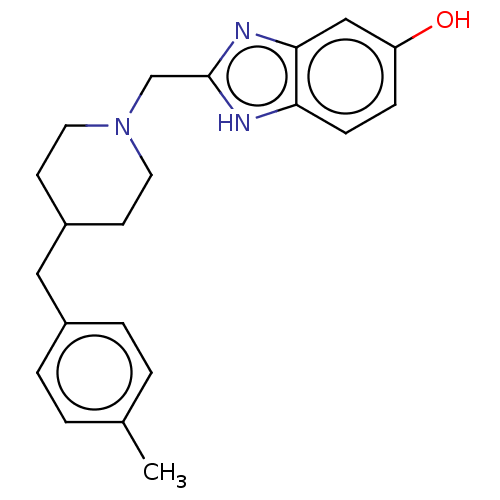 (CHEMBL416690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132047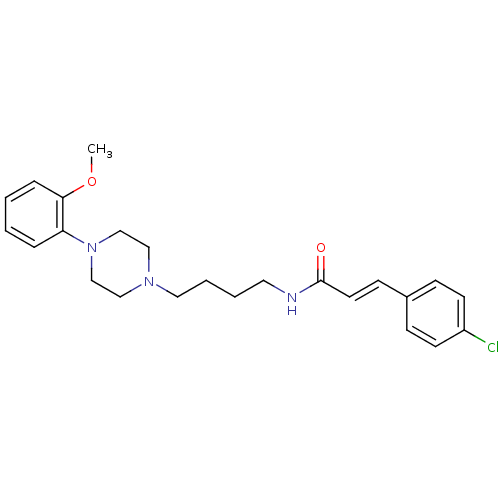 (3-(4-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205353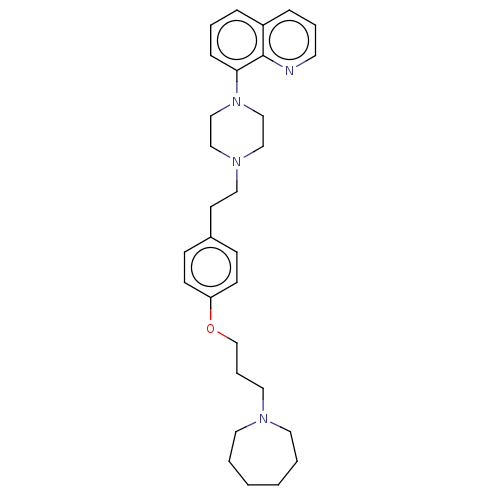 (CHEMBL3947980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205367 (CHEMBL3917794) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50331549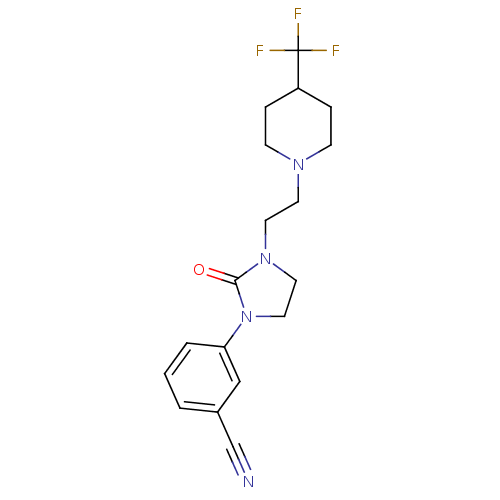 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50294115 (CHEMBL550222 | N-(4-(4-(2-(methylthio)phenyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from wild type human cloned dopamine D4 receptor expressed in HEK293 cells | J Med Chem 52: 4923-35 (2009) Article DOI: 10.1021/jm900690y BindingDB Entry DOI: 10.7270/Q2PK0G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205361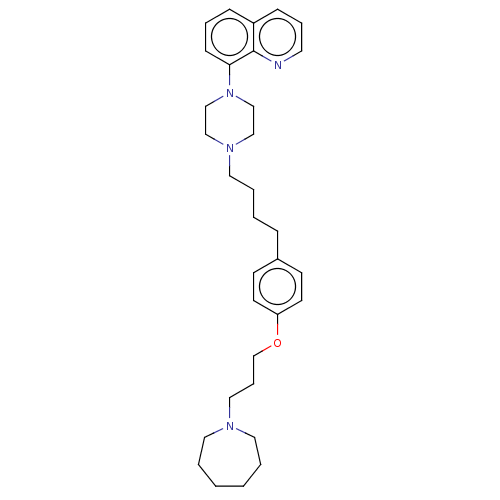 (CHEMBL3893197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474791 (CHEMBL65693) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205358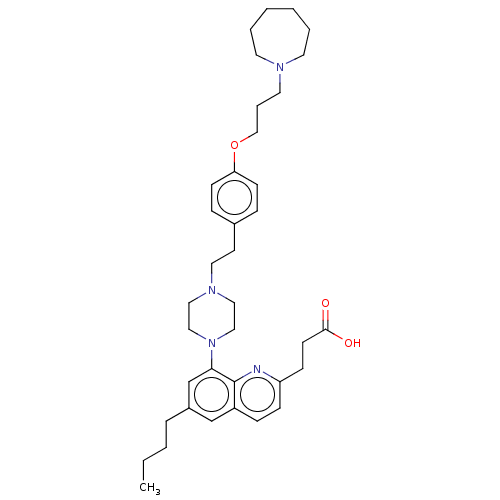 (CHEMBL3898341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085661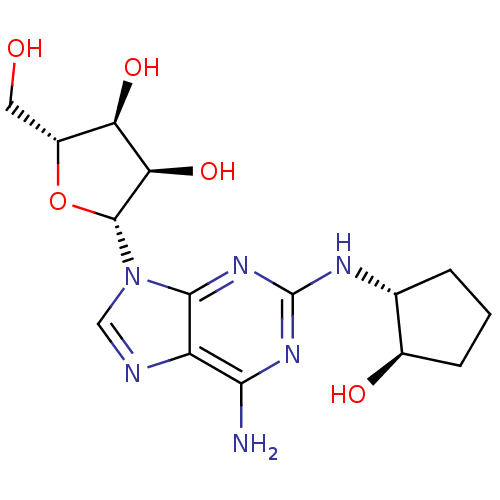 ((2R,3R,4S,5R)-2-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474809 (CHEMBL65454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474789 (CHEMBL68134) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50204862 (CHEMBL436521 | N-(2-((4-benzylpiperidin-1-yl)methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474796 (CHEMBL64941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells | Bioorg Med Chem 16: 6286-96 (2008) Article DOI: 10.1016/j.bmc.2008.04.020 BindingDB Entry DOI: 10.7270/Q2ZG6S2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | Bioorg Med Chem 16: 6286-96 (2008) Article DOI: 10.1016/j.bmc.2008.04.020 BindingDB Entry DOI: 10.7270/Q2ZG6S2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50294117 (2-((4-(2-methoxyphenyl)piperazin-1-yl)methyl)H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D4 receptor expressed in CHO cells | J Med Chem 52: 4923-35 (2009) Article DOI: 10.1021/jm900690y BindingDB Entry DOI: 10.7270/Q2PK0G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50341448 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205365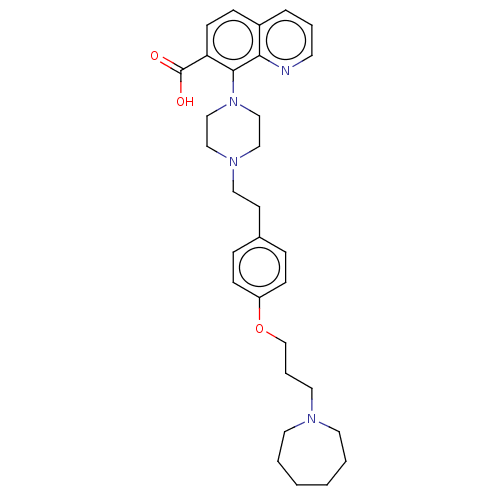 (CHEMBL3925977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205369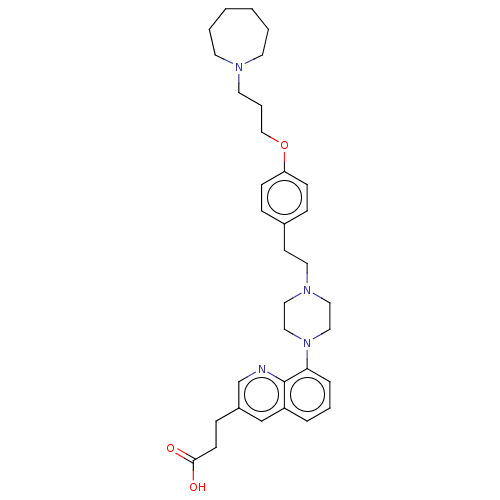 (CHEMBL3907368) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50174298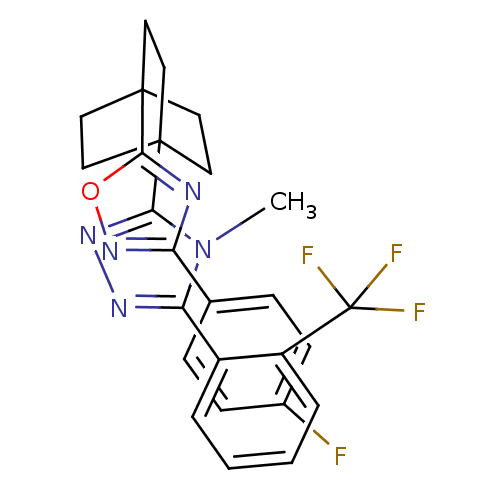 (3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50294116 (CHEMBL561763 | N-[3-[4-(2-Methylsulfanylphenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D2L receptor expressed in CHO cells | J Med Chem 52: 4923-35 (2009) Article DOI: 10.1021/jm900690y BindingDB Entry DOI: 10.7270/Q2PK0G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50143890 (2-(4-Benzyl-piperidin-1-ylmethyl)-3H-benzoimidazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205356 (CHEMBL3967709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205364 (CHEMBL3935303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205355 (CHEMBL3952802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205362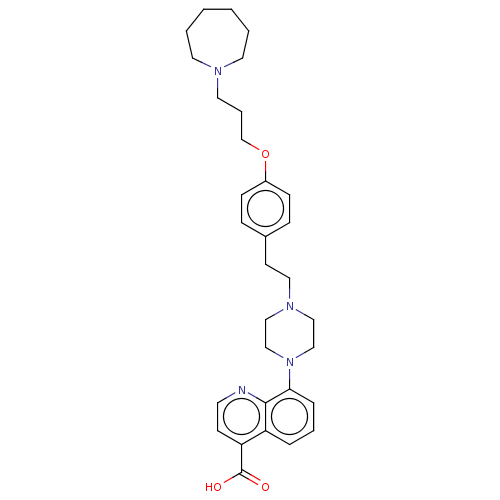 (CHEMBL3896541) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085664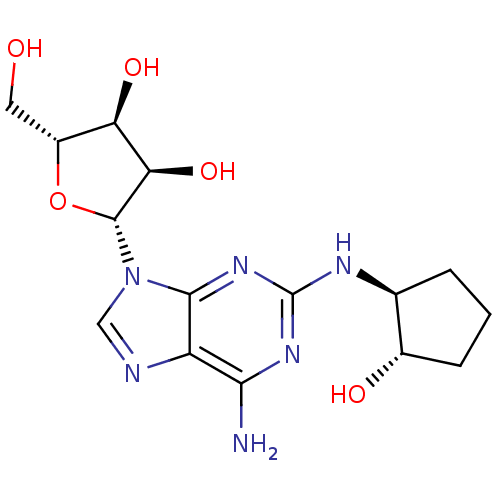 ((2R,3R,4S,5R)-2-[6-Amino-2-((1S,2S)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50119391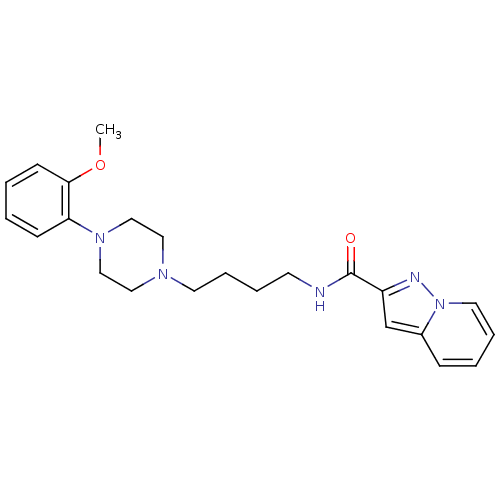 (CHEMBL142020 | N-(4-(4-(2-methoxyphenyl)piperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from wild type human cloned dopamine D4 receptor expressed in HEK293 cells | J Med Chem 52: 4923-35 (2009) Article DOI: 10.1021/jm900690y BindingDB Entry DOI: 10.7270/Q2PK0G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205354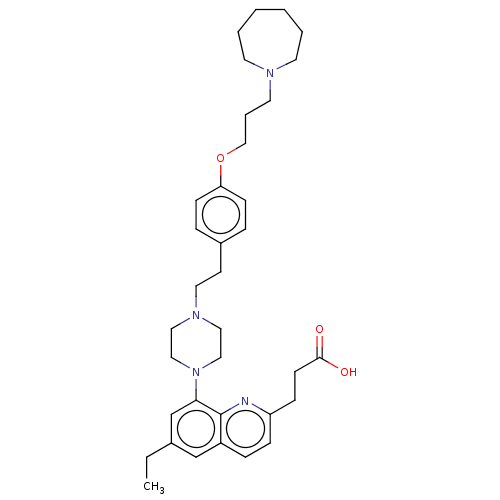 (CHEMBL3926412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AMP deaminase 3 (Homo sapiens (Human)) | BDBM50096172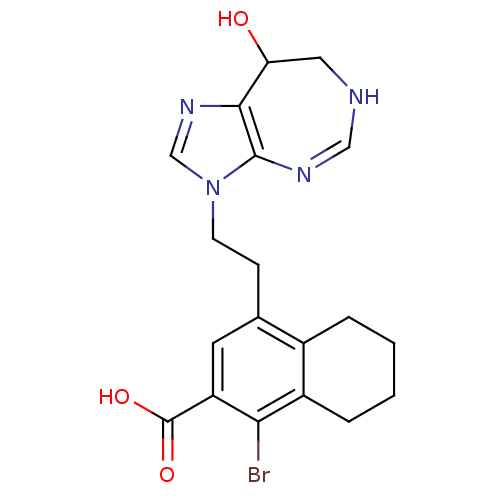 (1-Bromo-4-[2-(8-hydroxy-7,8-dihydro-6H-imidazo[4,5...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant AMPD3 | ACS Med Chem Lett 1: 286-289 (2010) Article DOI: 10.1021/ml100092a BindingDB Entry DOI: 10.7270/Q2X0683X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085663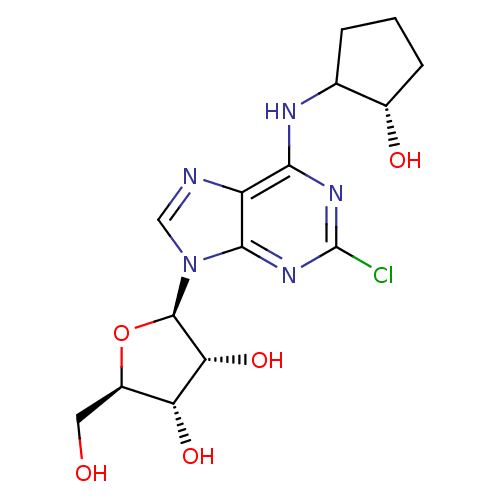 ((2R,3R,4S,5R)-2-[2-Chloro-6-((R)-(S)-2-hydroxy-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7839 total ) | Next | Last >> |