 Found 9 hits with Last Name = 'littlefield' and Initial = 'es'
Found 9 hits with Last Name = 'littlefield' and Initial = 'es' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein
(Human rhinovirus B) | BDBM50065621

(CHEMBL94688 | [(S)-1-((S)-1-{(S)-3-Carbamoyl-1-[2-...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)\C=C1/CCCOC1=O Show InChI InChI=1S/C33H42N4O7/c1-22(2)18-27(37-33(42)44-21-24-12-7-4-8-13-24)31(40)36-28(19-23-10-5-3-6-11-23)30(39)35-26(15-16-29(34)38)20-25-14-9-17-43-32(25)41/h3-8,10-13,20,22,26-28H,9,14-19,21H2,1-2H3,(H2,34,38)(H,35,39)(H,36,40)(H,37,42)/b25-20+/t26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Catalytic rate constant (Kobs/[I]) of the compound was evaluated against human rhinovirus (HRV) serotype 14 3C Protease (3CP) |
J Med Chem 41: 2806-18 (1998)
Article DOI: 10.1021/jm980068d
BindingDB Entry DOI: 10.7270/Q29G5KZQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288762
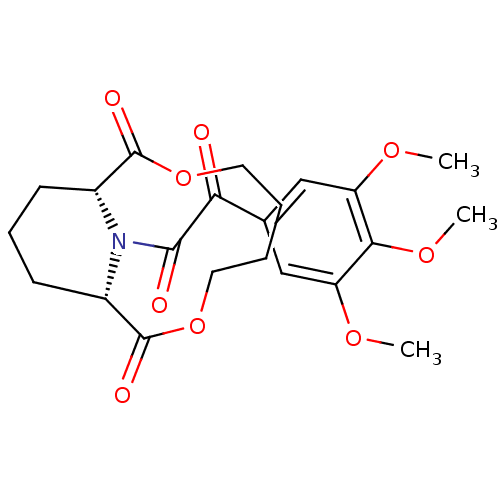
((1S,10R)-14-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-ace...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCCOC2=O Show InChI InChI=1S/C22H27NO9/c1-28-16-11-13(12-17(29-2)19(16)30-3)18(24)20(25)23-14-7-6-8-15(23)22(27)32-10-5-4-9-31-21(14)26/h11-12,14-15H,4-10H2,1-3H3/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288763

((1S,9R)-5-Benzyloxymethyl-13-[2-oxo-2-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(COCc1ccccc1)COC2=O Show InChI InChI=1S/C29H33NO10/c1-35-23-12-20(13-24(36-2)26(23)37-3)25(31)27(32)30-21-10-7-11-22(30)29(34)40-17-19(16-39-28(21)33)15-38-14-18-8-5-4-6-9-18/h4-6,8-9,12-13,19,21-22H,7,10-11,14-17H2,1-3H3/t19?,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288765

((1S,9R)-5-(tert-Butyl-dimethyl-silanyloxymethyl)-1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO[Si](C)(C)C(C)(C)C)COC2=O Show InChI InChI=1S/C28H41NO10Si/c1-28(2,3)40(7,8)39-16-17-14-37-26(32)19-10-9-11-20(27(33)38-15-17)29(19)25(31)23(30)18-12-21(34-4)24(36-6)22(13-18)35-5/h12-13,17,19-20H,9-11,14-16H2,1-8H3/t17?,19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288764
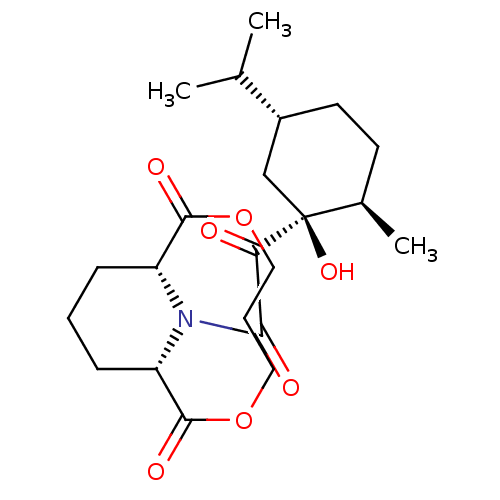
((1S,9R)-13-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C22H33NO7/c1-13(2)15-9-8-14(3)22(28,12-15)18(24)19(25)23-16-6-4-7-17(23)21(27)30-11-5-10-29-20(16)26/h13-17,28H,4-12H2,1-3H3/t14-,15-,16-,17+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288768
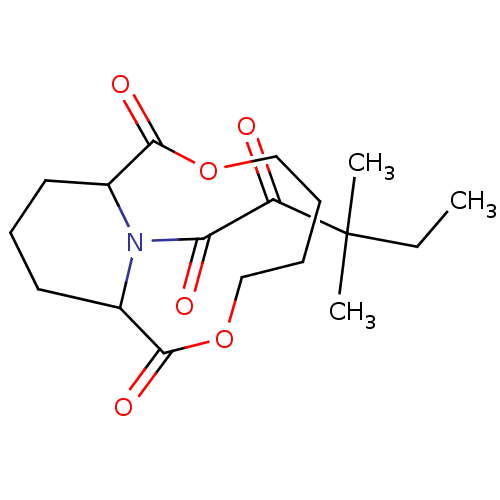
((1R,10S)-14-(3,3-Dimethyl-2-oxo-pentanoyl)-3,8-dio...)Show SMILES CCC(C)(C)C(=O)C(=O)N1C2CCCC1C(=O)OCCCCOC2=O Show InChI InChI=1S/C18H27NO6/c1-4-18(2,3)14(20)15(21)19-12-8-7-9-13(19)17(23)25-11-6-5-10-24-16(12)22/h12-13H,4-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288767
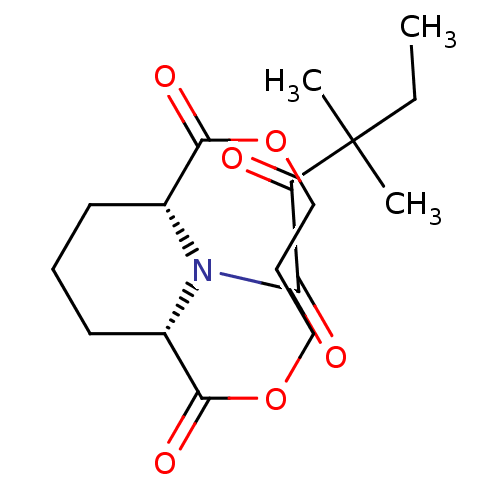
((1R,9S)-13-(3,3-Dimethyl-2-oxo-pentanoyl)-3,7-diox...)Show SMILES CCC(C)(C)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C17H25NO6/c1-4-17(2,3)13(19)14(20)18-11-7-5-8-12(18)16(22)24-10-6-9-23-15(11)21/h11-12H,4-10H2,1-3H3/t11-,12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288766
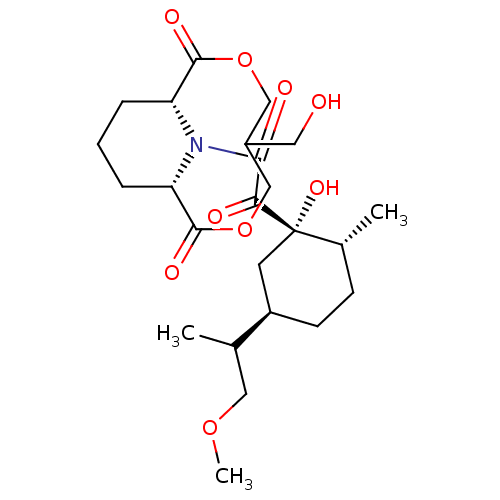
((1S,9R)-13-{2-[(1S,2R,5R)-1-Hydroxy-5-(2-methoxy-1...)Show SMILES COCC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO)COC2=O Show InChI InChI=1S/C24H37NO9/c1-14(11-32-3)17-8-7-15(2)24(31,9-17)20(27)21(28)25-18-5-4-6-19(25)23(30)34-13-16(10-26)12-33-22(18)29/h14-19,26,31H,4-13H2,1-3H3/t14?,15-,16?,17-,18-,19+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288769
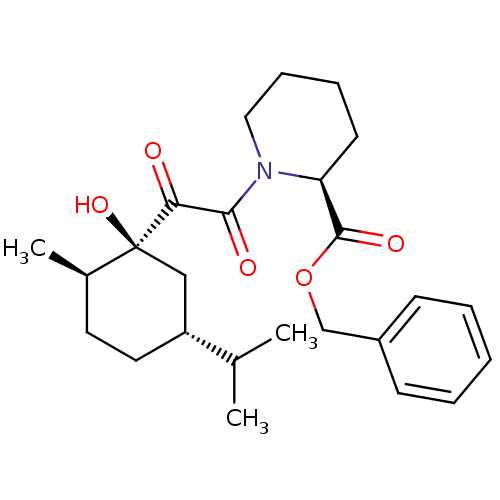
((S)-1-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-methy...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C25H35NO5/c1-17(2)20-13-12-18(3)25(30,15-20)22(27)23(28)26-14-8-7-11-21(26)24(29)31-16-19-9-5-4-6-10-19/h4-6,9-10,17-18,20-21,30H,7-8,11-16H2,1-3H3/t18-,20-,21+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data