

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230326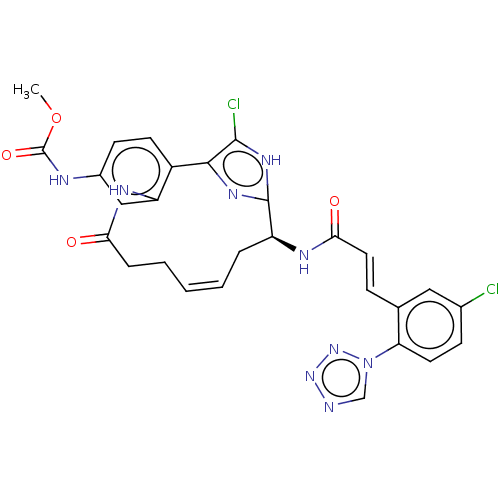 (CHEMBL4060950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230326 (CHEMBL4060950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269207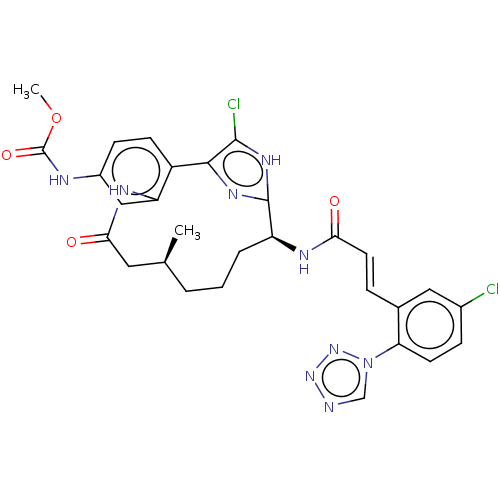 (CHEMBL4097522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269199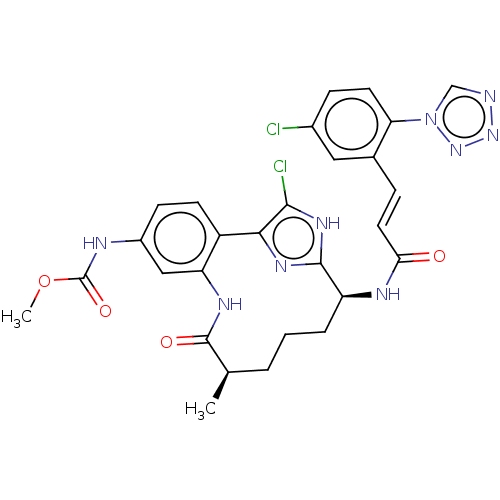 (CHEMBL4083769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250493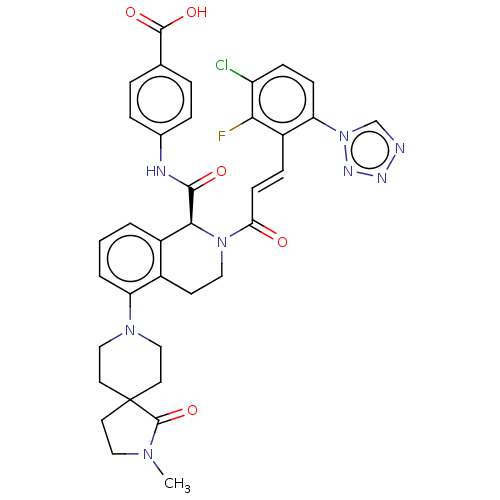 (CHEMBL4068445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250492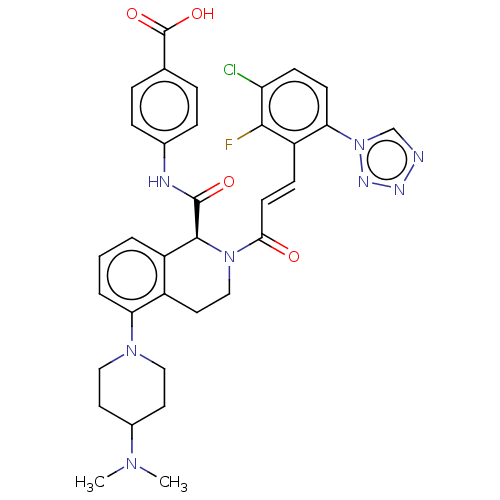 (CHEMBL4097304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269209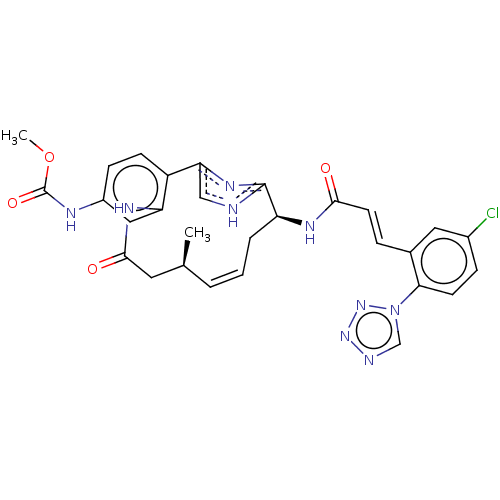 (CHEMBL4063677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269190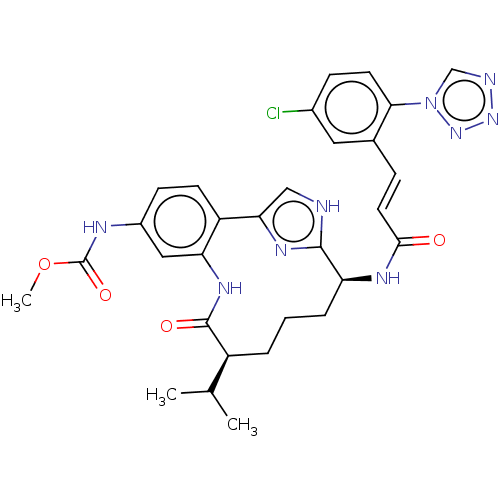 (CHEMBL4103982) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322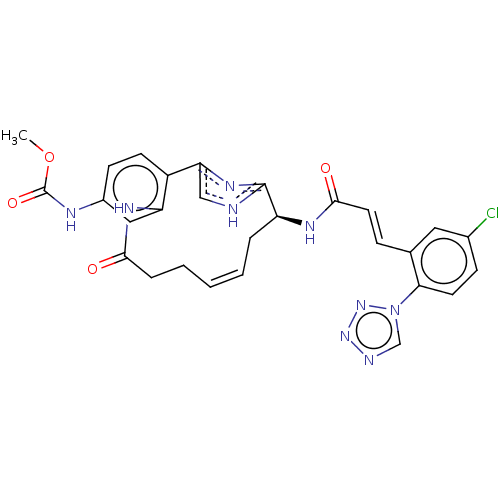 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514438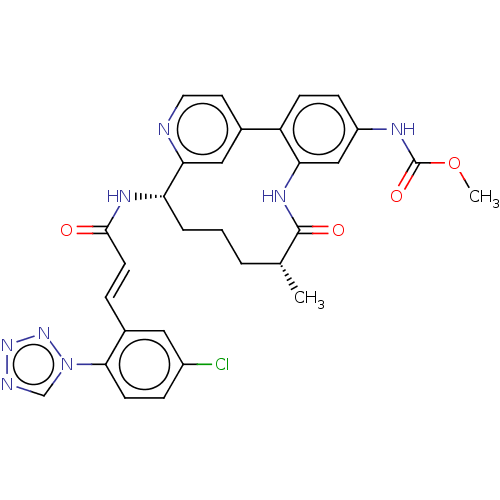 (CHEMBL4439729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269186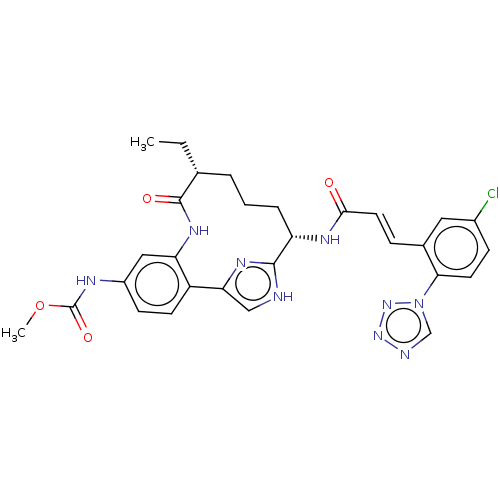 (CHEMBL4089185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269186 (CHEMBL4089185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514437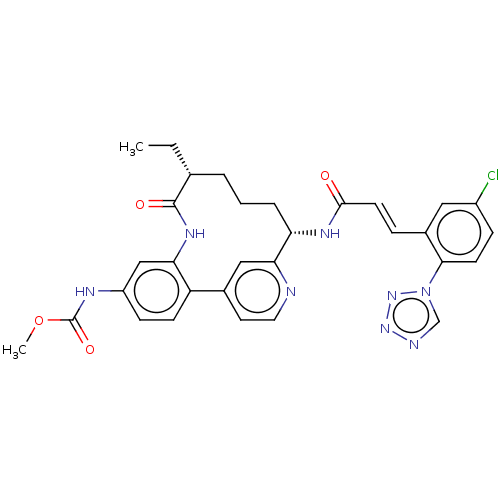 (CHEMBL4444690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM142872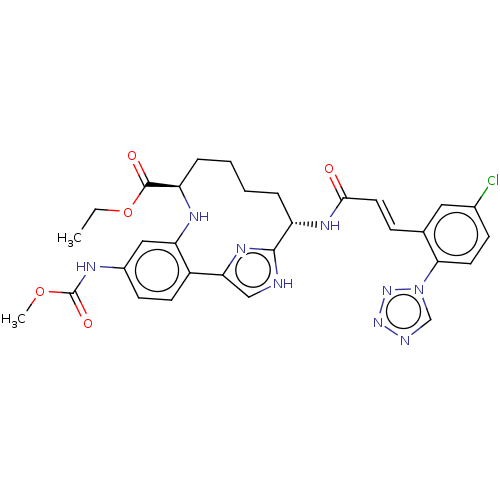 (US8940720, I-67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586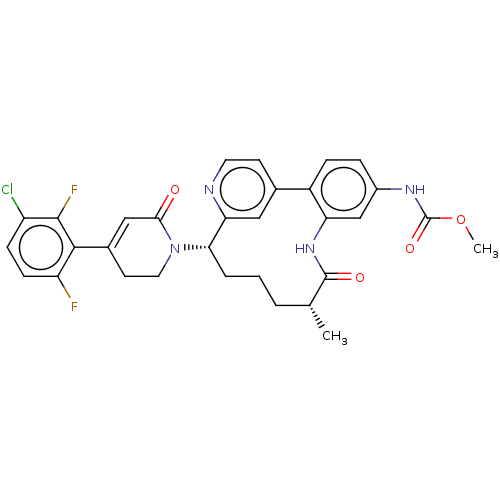 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762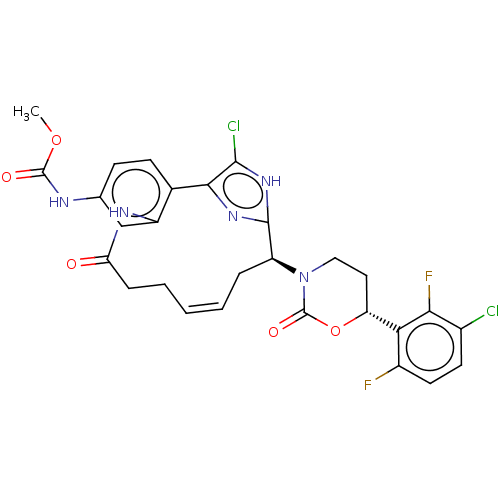 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50539655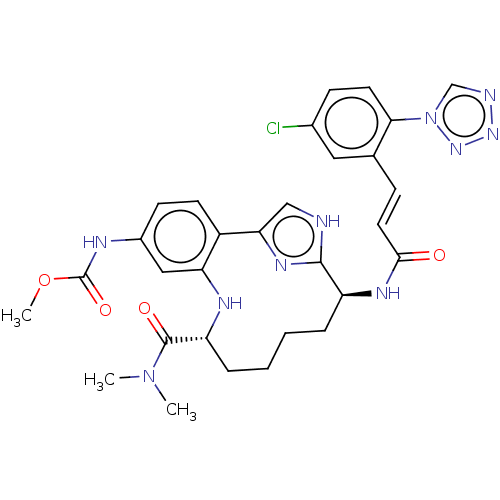 (CHEMBL4632633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269191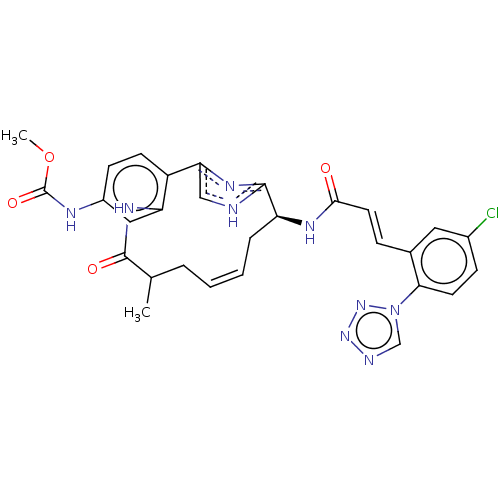 (CHEMBL4063746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269191 (CHEMBL4063746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250501 (CHEMBL4078562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269195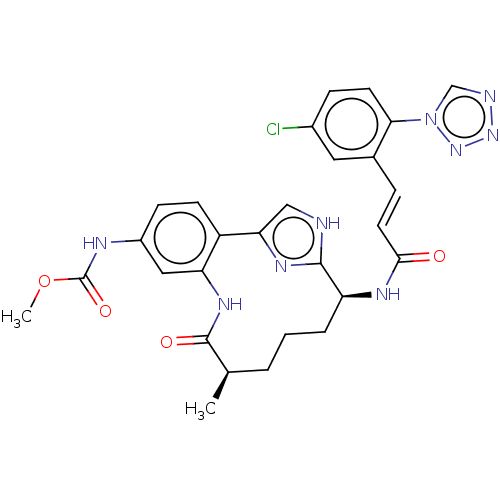 (CHEMBL4101766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269195 (CHEMBL4101766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50539654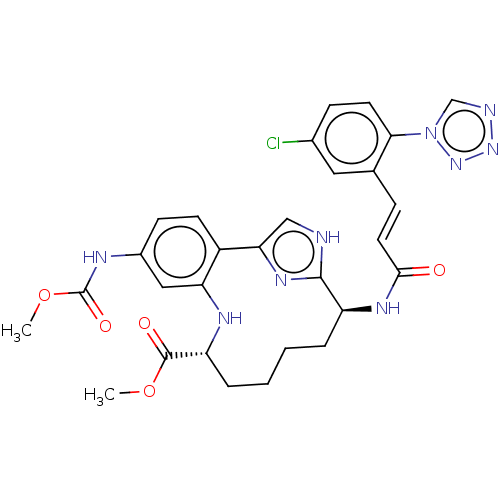 (CHEMBL4640111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230325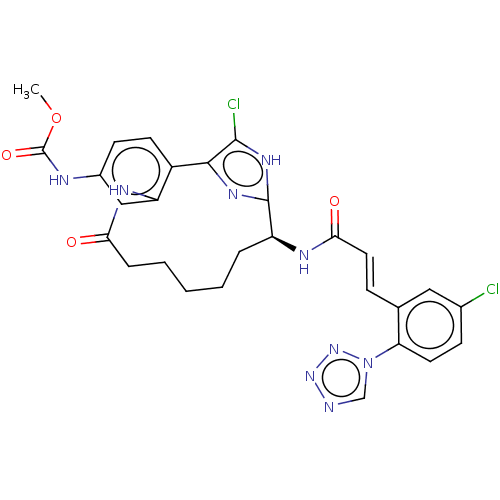 (CHEMBL4062923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230325 (CHEMBL4062923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250490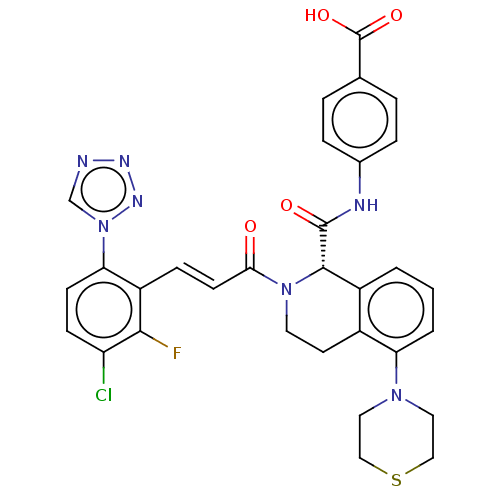 (CHEMBL4087166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541581 (CHEMBL4646341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541577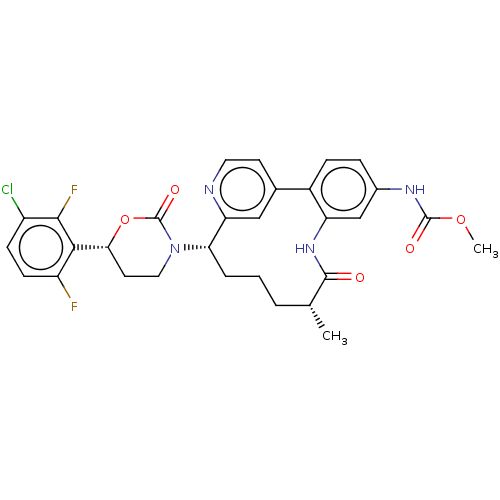 (CHEMBL4646441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM142873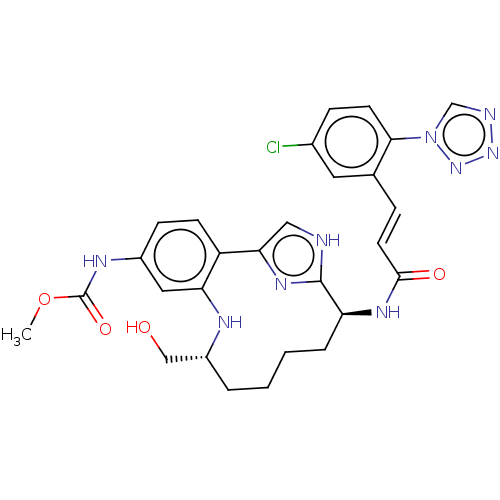 (US10487086, Example I-77 | US11136327, Example I-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM420081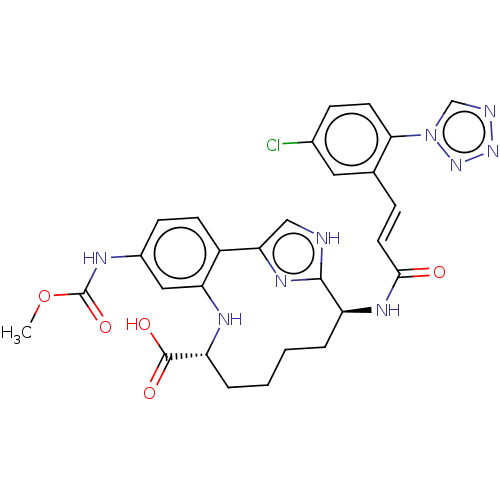 (US10487086, Example I-84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269206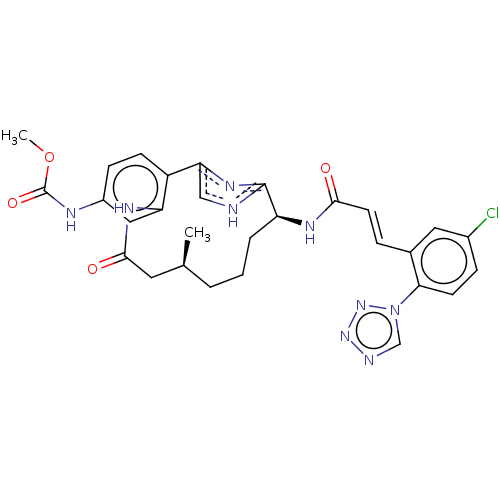 (CHEMBL4070961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541576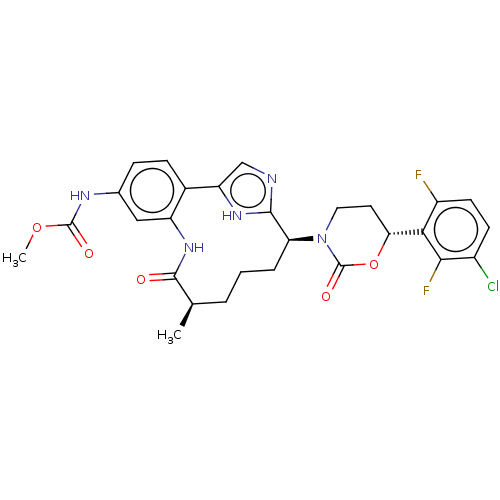 (CHEMBL4644510) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM349975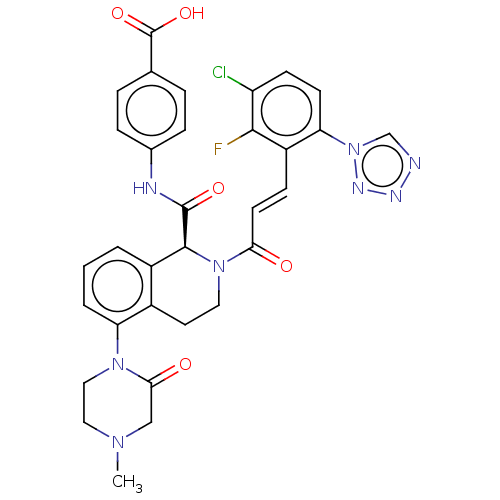 ((S,E)-4-(2-(3-(3-chloro-2-fluoro-6-(1H-tetrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230340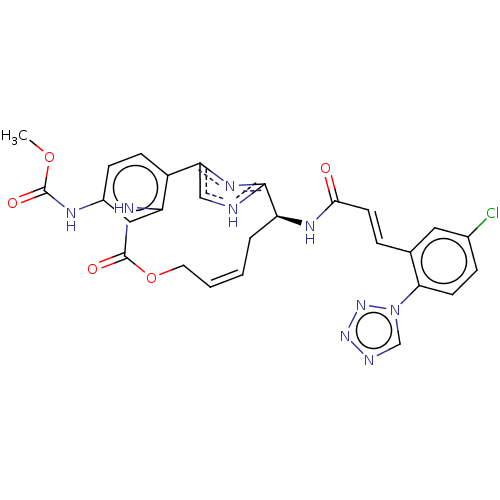 (CHEMBL4087980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541582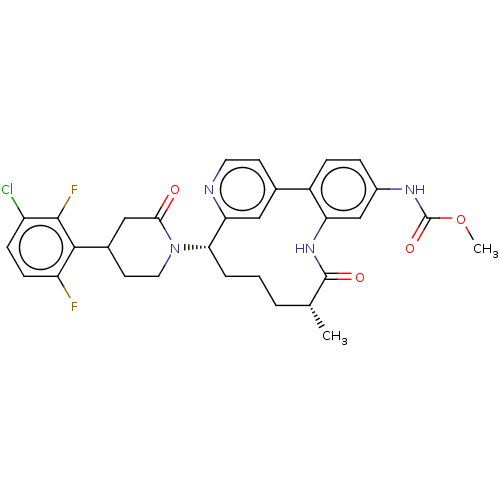 (CHEMBL4636247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269200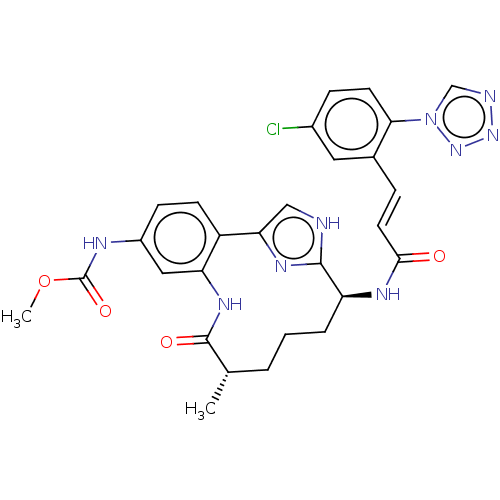 (CHEMBL4076558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50539651 (CHEMBL4647704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514439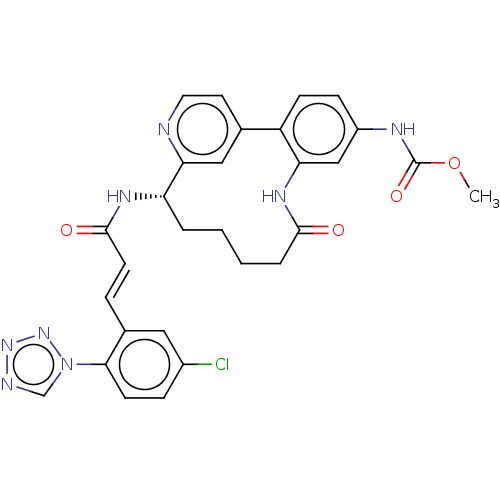 (CHEMBL4456818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM420078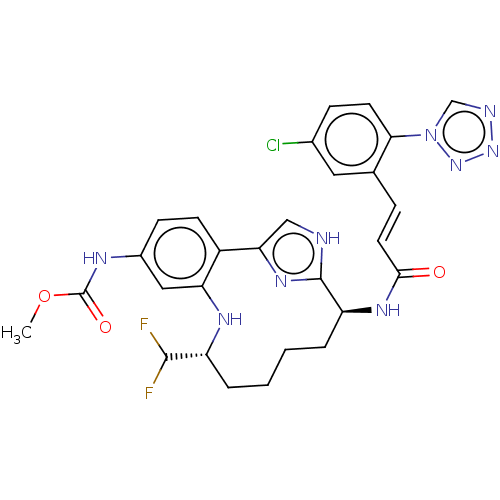 (US10487086, Example I-72 | US11136327, Example I-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250494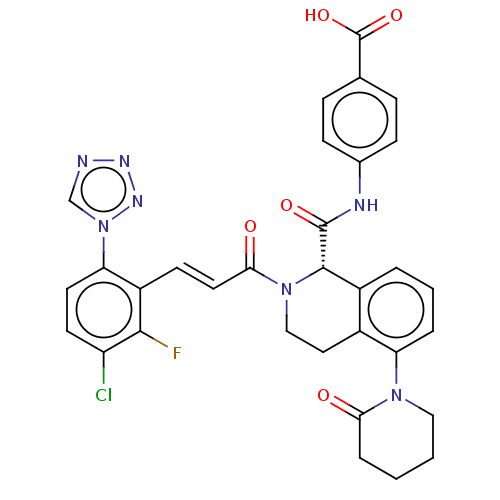 (CHEMBL4089581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250495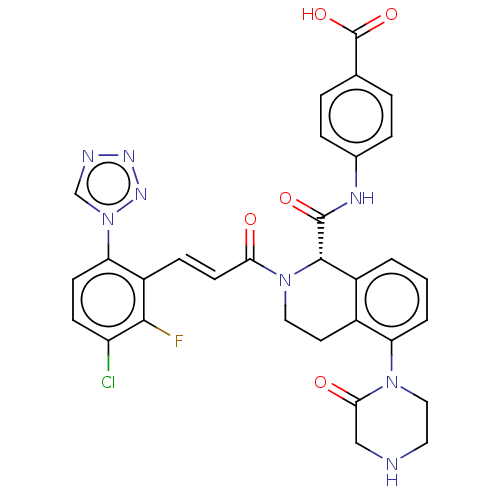 (CHEMBL4081710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541579 (CHEMBL4637480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525779 (CHEMBL4550408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 303 total ) | Next | Last >> |