

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478430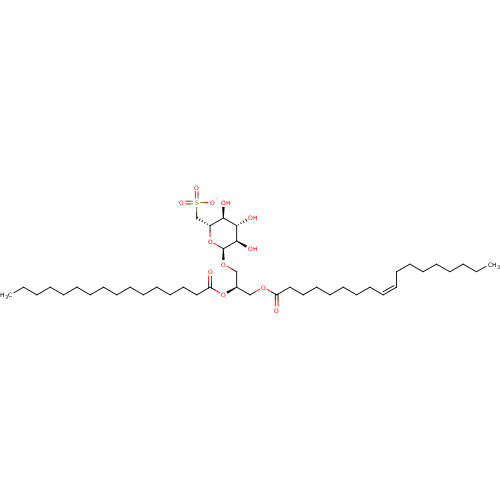 (CHEMBL500695) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030220 (4-[(10R,13R)-17-(1,5-Dimethyl-hexyl)-10,13-dimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of RNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478436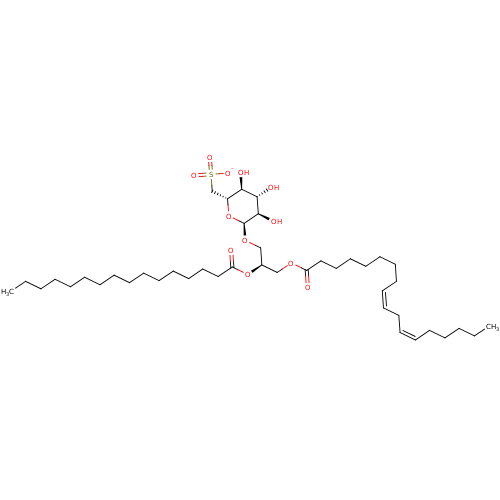 (CHEMBL448380) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478431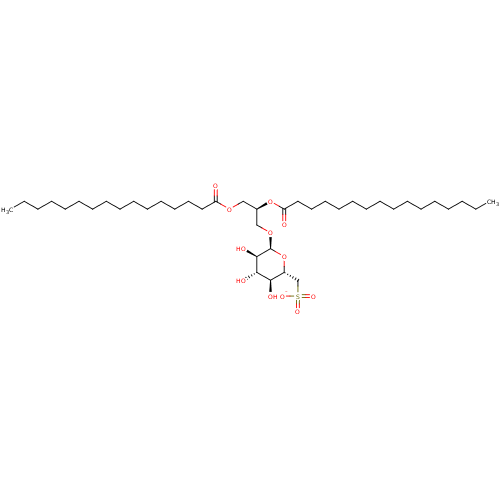 (CHEMBL455390) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030217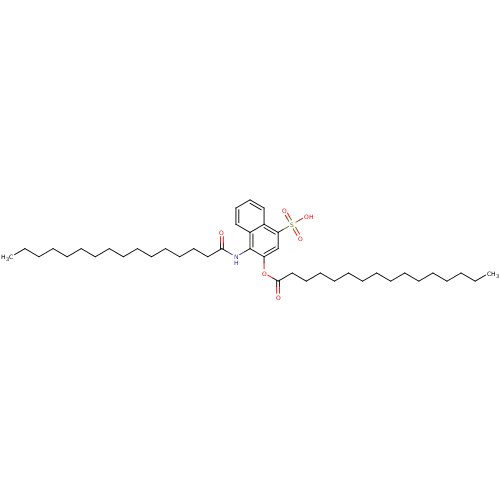 (CHEMBL313213 | Hexadecanoic acid 1-hexadecanoylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of RNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478497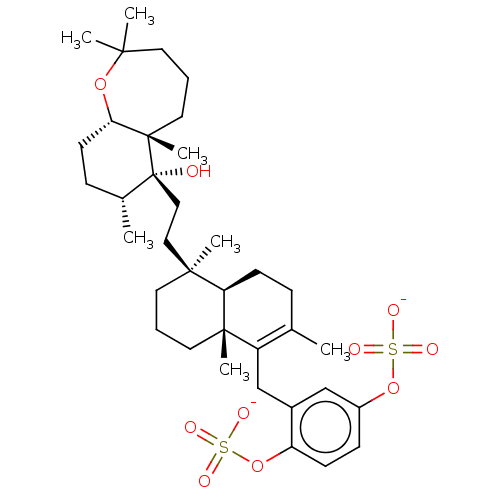 (Shaagrockol C) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478495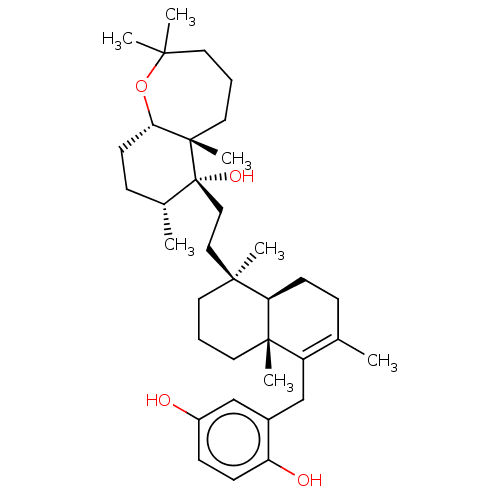 (CHEMBL450355) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478496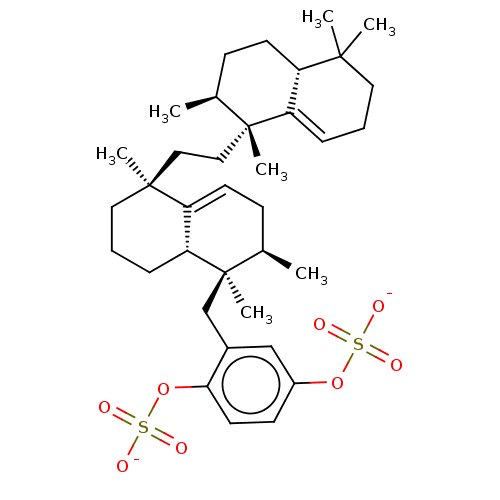 (TOXIUSOL SODIUM | Toxiusol) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030215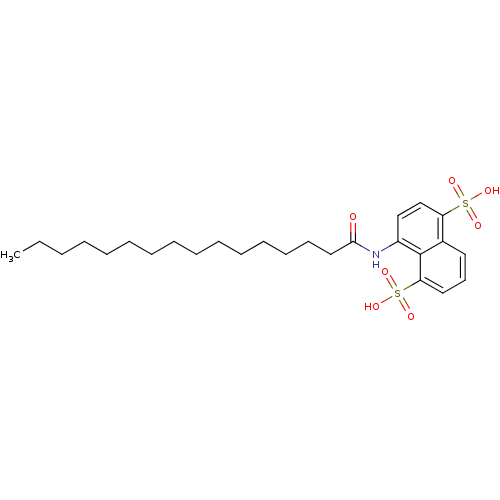 (4-Hexadecanoylamino-naphthalene-1,5-disulfonic aci...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of RNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478495 (CHEMBL450355) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478500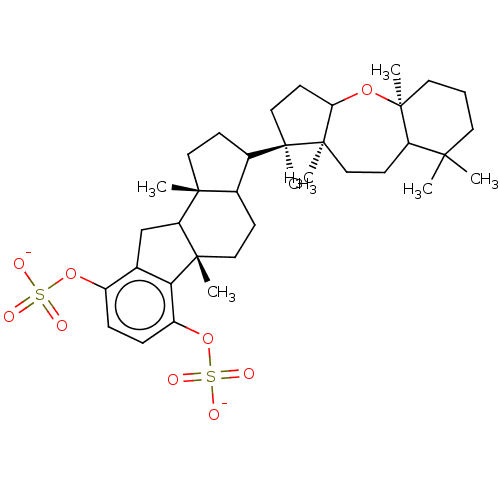 (TOXICOL A) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478493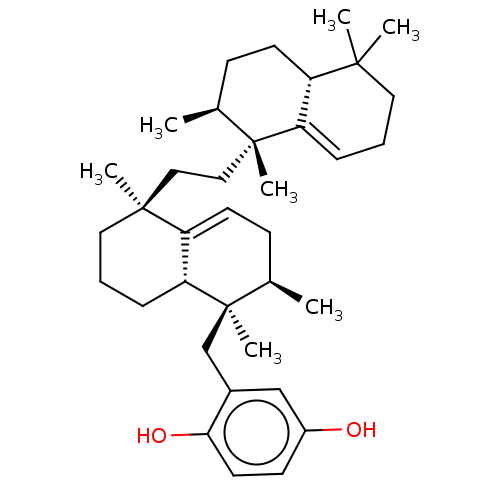 (CHEMBL480885) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478437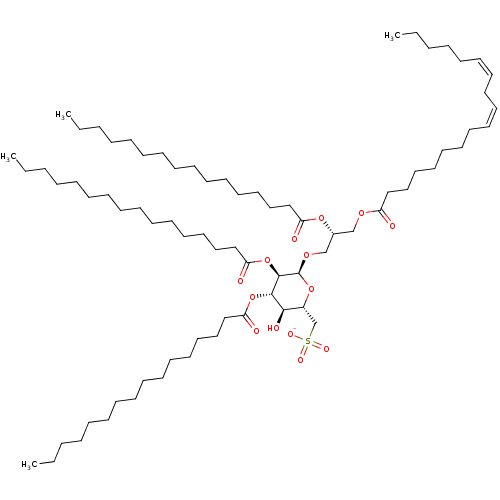 (CHEMBL506716) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478500 (TOXICOL A) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030218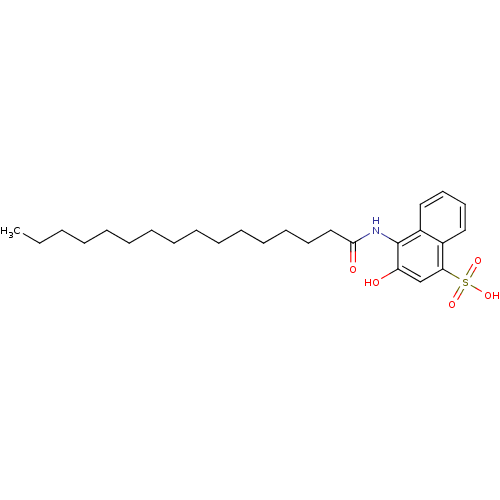 (4-Hexadecanoylamino-3-hydroxy-naphthalene-1-sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of RNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030218 (4-Hexadecanoylamino-3-hydroxy-naphthalene-1-sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of RNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478497 (Shaagrockol C) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030219 (Biphenyl-4,4'-dicarboxylic acid bis-(3,6-disulfo-n...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of RNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478499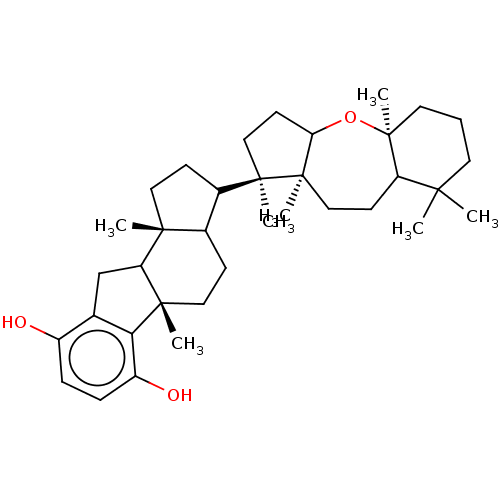 (TOXICOL B) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478493 (CHEMBL480885) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030216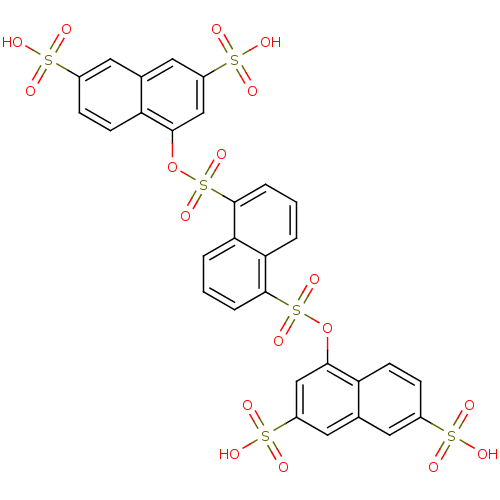 (4-[5-(3,6-disulfo-1-naphthyloxysulfonyl)-1-naphthy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of RNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478498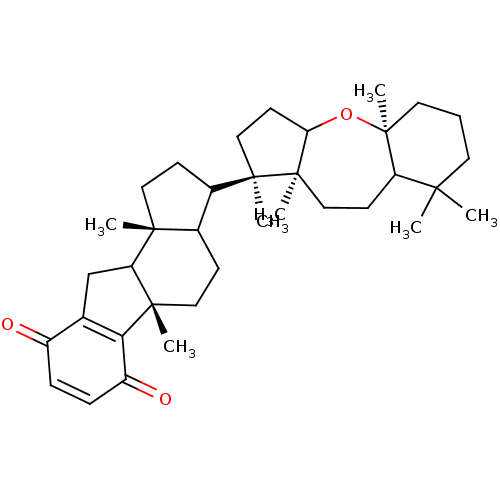 (CHEMBL519929) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030217 (CHEMBL313213 | Hexadecanoic acid 1-hexadecanoylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of DNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478496 (TOXIUSOL SODIUM | Toxiusol) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030215 (4-Hexadecanoylamino-naphthalene-1,5-disulfonic aci...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of DNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478494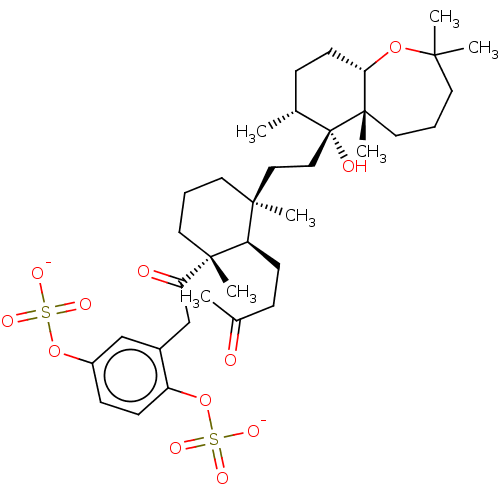 (SHAAGROCKOL B) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030219 (Biphenyl-4,4'-dicarboxylic acid bis-(3,6-disulfo-n...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of DNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030220 (4-[(10R,13R)-17-(1,5-Dimethyl-hexyl)-10,13-dimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of DNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478499 (TOXICOL B) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of DNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478494 (SHAAGROCKOL B) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478498 (CHEMBL519929) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNA dependent DNA polymerase activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by po... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50030219 (Biphenyl-4,4'-dicarboxylic acid bis-(3,6-disulfo-n...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of Ribonuclease H activity of reverse transcriptase | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50030217 (CHEMBL313213 | Hexadecanoic acid 1-hexadecanoylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of Ribonuclease H activity of reverse transcriptase | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50030220 (4-[(10R,13R)-17-(1,5-Dimethyl-hexyl)-10,13-dimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of Ribonuclease H activity of reverse transcriptase | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50478743 (LKTVRLIKFLY) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by quantitative ELISA | J Biol Chem 282: 15743-53 (2007) Article DOI: 10.1074/jbc.m609864200 BindingDB Entry DOI: 10.7270/Q2JQ13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50478742 (CHEMBL526172) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by quantitative ELISA | J Biol Chem 282: 15743-53 (2007) Article DOI: 10.1074/jbc.m609864200 BindingDB Entry DOI: 10.7270/Q2JQ13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50030215 (4-Hexadecanoylamino-naphthalene-1,5-disulfonic aci...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of Ribonuclease H activity of reverse transcriptase | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478432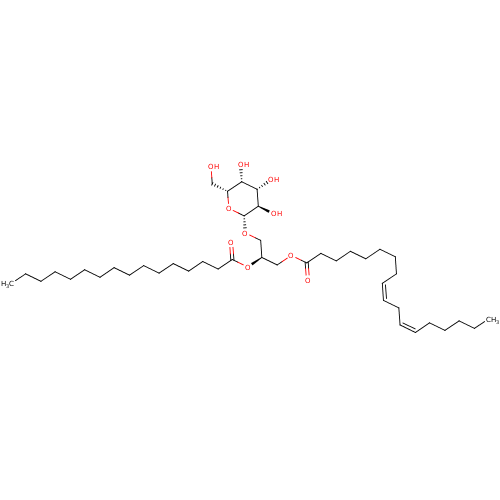 (CHEMBL448268) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50030218 (4-Hexadecanoylamino-3-hydroxy-naphthalene-1-sulfon...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of Ribonuclease H activity of reverse transcriptase | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478438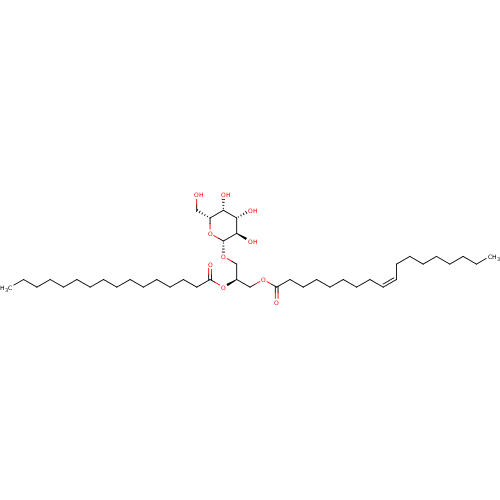 (CHEBI:90507 | CHEMBL499250) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478434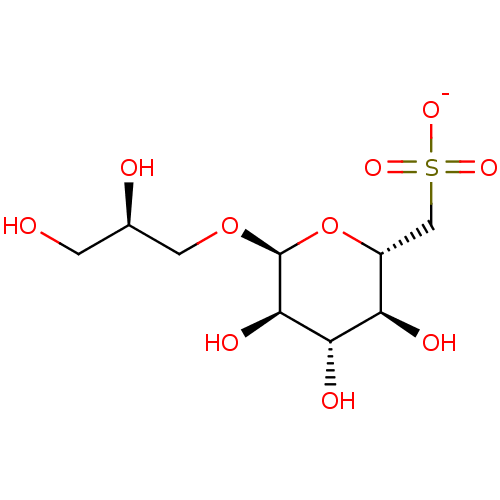 (CHEMBL470759) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030216 (4-[5-(3,6-disulfo-1-naphthyloxysulfonyl)-1-naphthy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of DNA-dependent DNA polymerase activity | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478433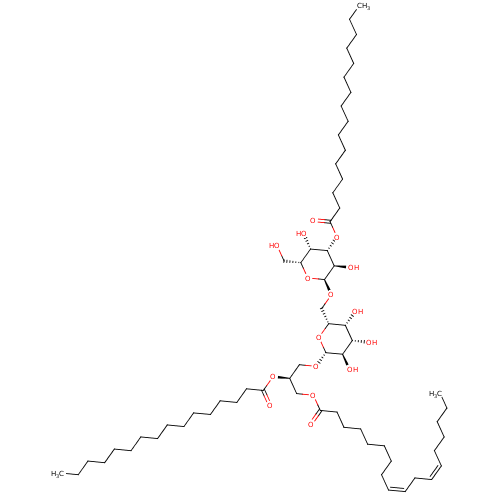 (CHEMBL447743) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478435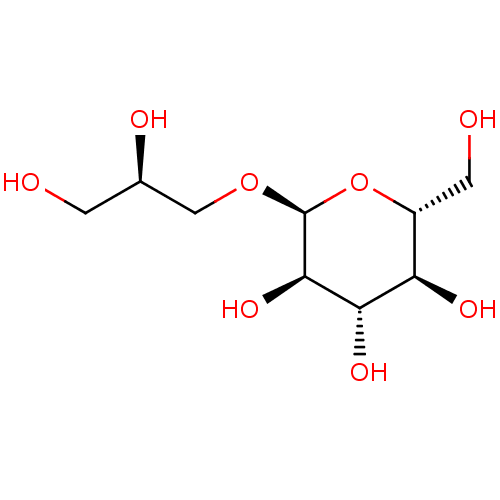 (CHEMBL450754) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478439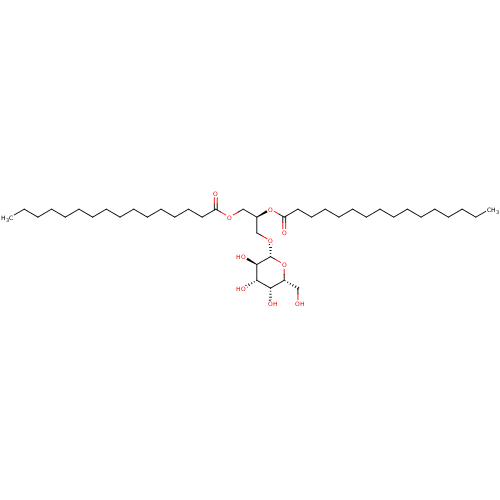 (CHEBI:63774 | CHEMBL443117) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478440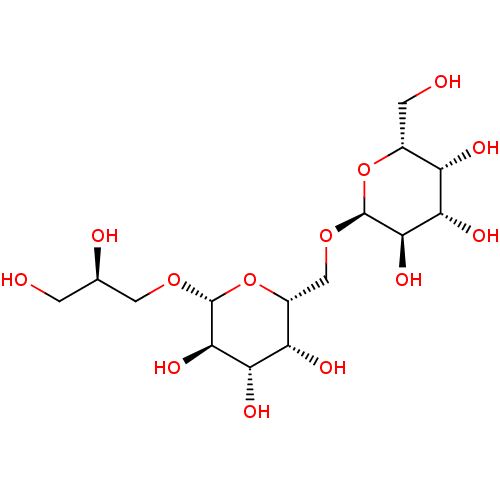 (CHEMBL472021) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478441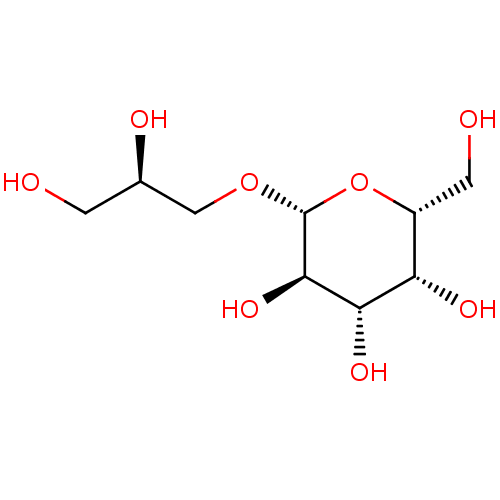 (CHEBI:15754 | CHEMBL446492) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase activity assessed as residual enzyme activity by poly(rA)n.oligo(dT)12-18-directed incorporation of [3H]dTTP... | J Nat Prod 61: 891-5 (1998) Article DOI: 10.1021/np970585j BindingDB Entry DOI: 10.7270/Q2RX9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50030216 (4-[5-(3,6-disulfo-1-naphthyloxysulfonyl)-1-naphthy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description The 50% inhibitory concentration was measured as inhibition of Ribonuclease H activity of reverse transcriptase | J Med Chem 37: 2513-9 (1994) BindingDB Entry DOI: 10.7270/Q2HQ3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478493 (CHEMBL480885) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNase H activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by poly(rA)n.oligo(dT)n-di... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478497 (Shaagrockol C) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sackler School of Medicine Curated by ChEMBL | Assay Description Inhibition of RNase H activity of HIV1 BH10 recombinant reverse transcriptase p66/p51 assessed as residual enzyme activity by poly(rA)n.oligo(dT)n-di... | J Nat Prod 56: 2120-5 (1993) Article DOI: 10.1021/np50102a014 BindingDB Entry DOI: 10.7270/Q2PZ5CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |