

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133069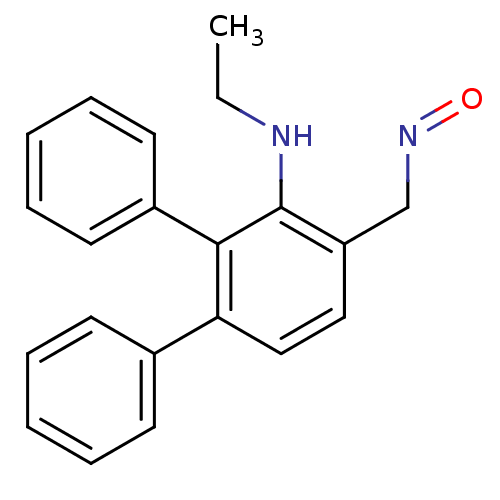 (3'-Ethylamino-[1,1';2',1'']terphenyl-4'-carbaldehy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029978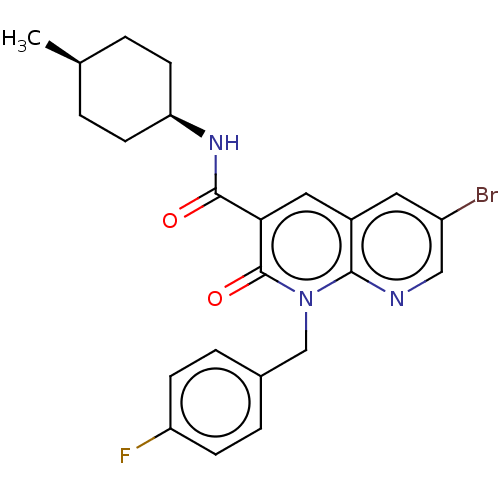 (CHEMBL3353441) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133070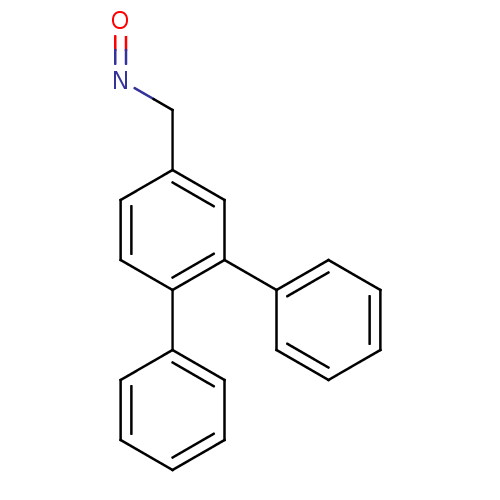 (CHEMBL335465 | [1,1';2',1'']Terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029963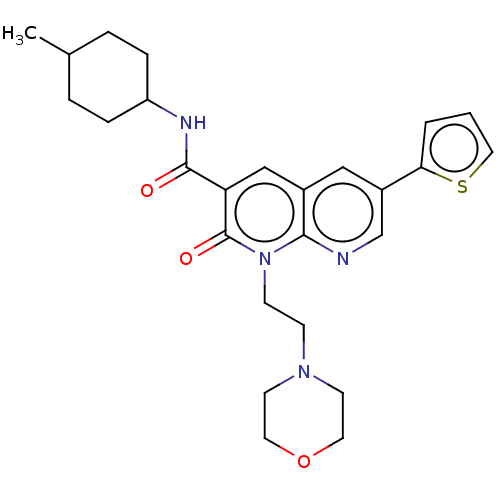 (CHEMBL3353452) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029958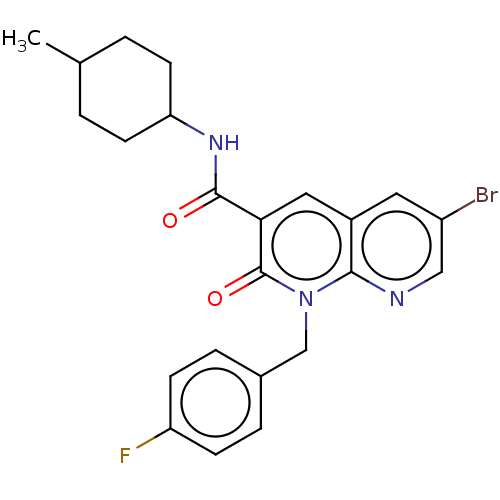 (CHEMBL3353439) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50381886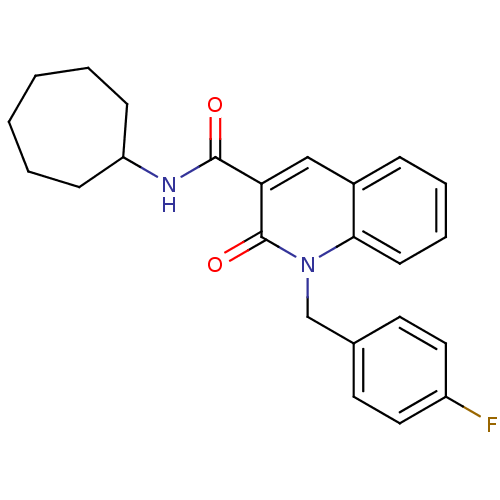 (CHEMBL2022363) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB2 receptor expressed in human HEK293 cell membrane after 90 mins | Eur J Med Chem 52: 284-94 (2012) Article DOI: 10.1016/j.ejmech.2012.03.031 BindingDB Entry DOI: 10.7270/Q2PK0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133070 (CHEMBL335465 | [1,1';2',1'']Terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029962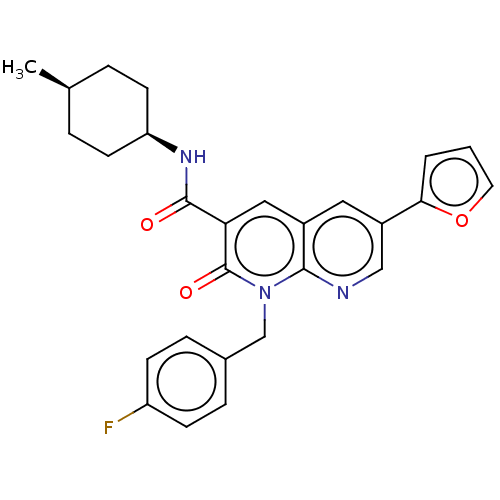 (CHEMBL3353450) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogenetic Corporation Curated by PDSP Ki Database | Mol Pharmacol 38: 681-8 (1990) BindingDB Entry DOI: 10.7270/Q29C6VWG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029965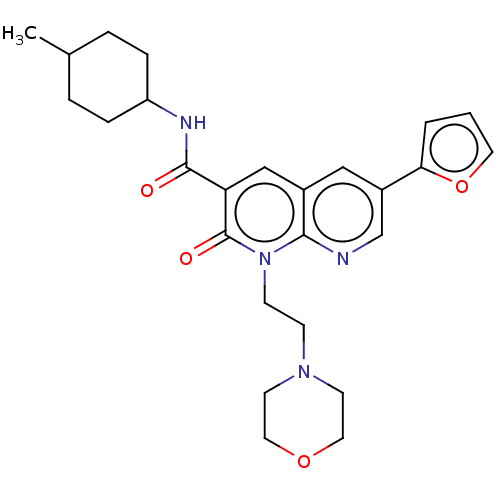 (CHEMBL3353454) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50346462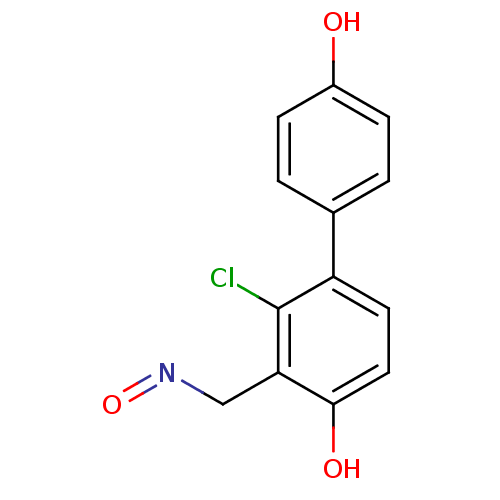 ((E)-2-chloro-4,4'-dihydroxybiphenyl-3-carbaldehyde...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human full-length ERbeta receptor by competitive radiometric binding assay | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133069 (3'-Ethylamino-[1,1';2',1'']terphenyl-4'-carbaldehy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029989 (CHEMBL3353437) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50346463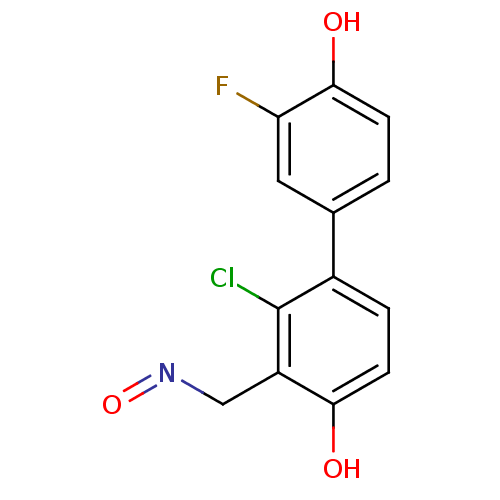 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human full-length ERbeta receptor by competitive radiometric binding assay | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180022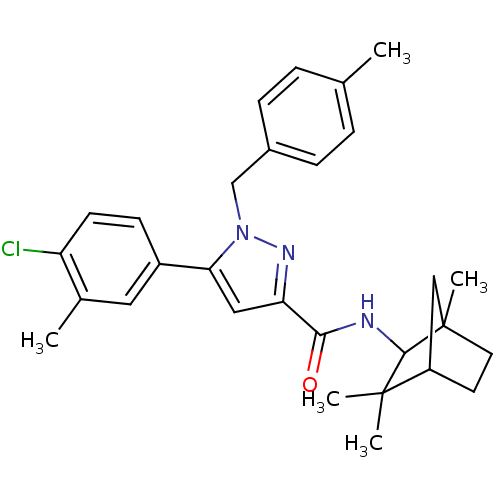 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human recombinant CB1 receptor expressed in HEK293 cell membranes after 90 mins | Eur J Med Chem 90: 526-36 (2015) Article DOI: 10.1016/j.ejmech.2014.11.066 BindingDB Entry DOI: 10.7270/Q270833D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180022 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins | Eur J Med Chem 74: 524-32 (2014) Article DOI: 10.1016/j.ejmech.2013.10.070 BindingDB Entry DOI: 10.7270/Q21V5HXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180022 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50016479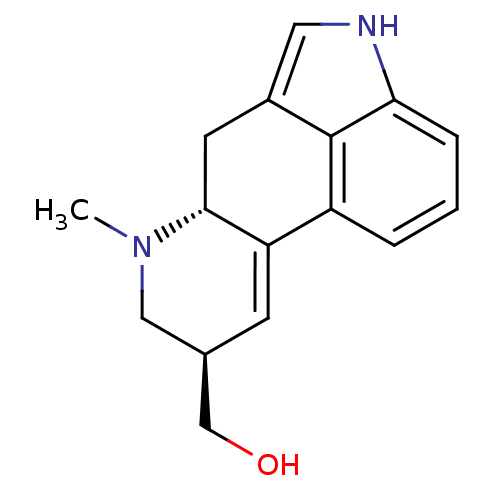 ((7-Methyl-4,6,6a,7,8,9-hexahydro-indolo[4,3-fg]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 89: 3630-4 (1992) Article DOI: 10.1073/pnas.89.8.3630 BindingDB Entry DOI: 10.7270/Q2VH5M9X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029972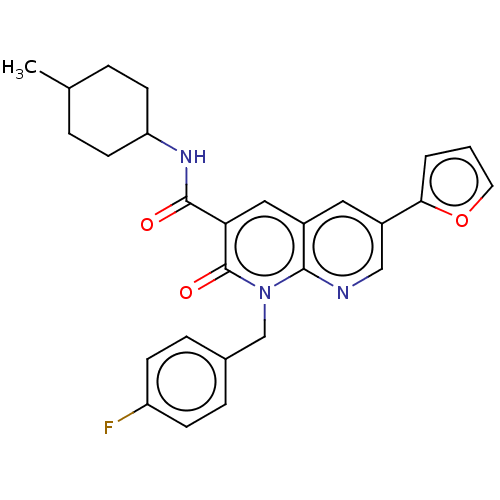 (CHEMBL3353448) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029964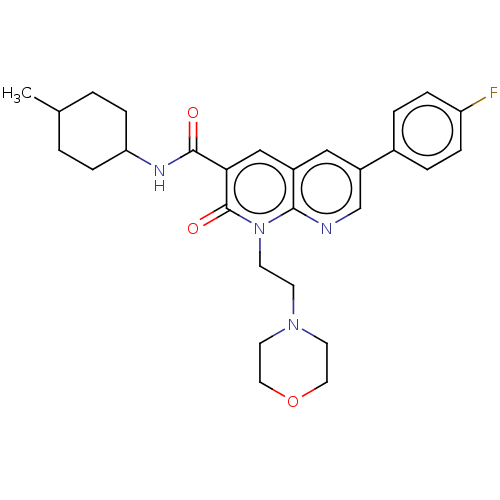 (CHEMBL3353453) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 89: 3630-4 (1992) Article DOI: 10.1073/pnas.89.8.3630 BindingDB Entry DOI: 10.7270/Q2VH5M9X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50082777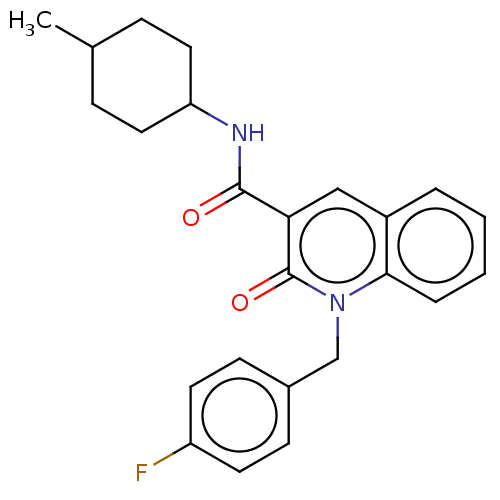 (CHEMBL3422784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins | Eur J Med Chem 97: 10-8 (2015) Article DOI: 10.1016/j.ejmech.2015.04.034 BindingDB Entry DOI: 10.7270/Q2VQ34DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50082769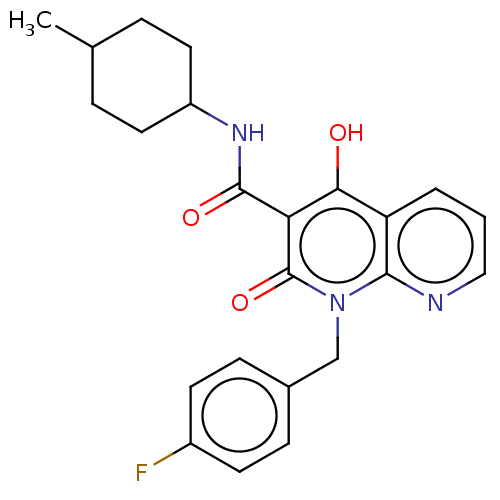 (CHEMBL3422790) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins | Eur J Med Chem 97: 10-8 (2015) Article DOI: 10.1016/j.ejmech.2015.04.034 BindingDB Entry DOI: 10.7270/Q2VQ34DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258652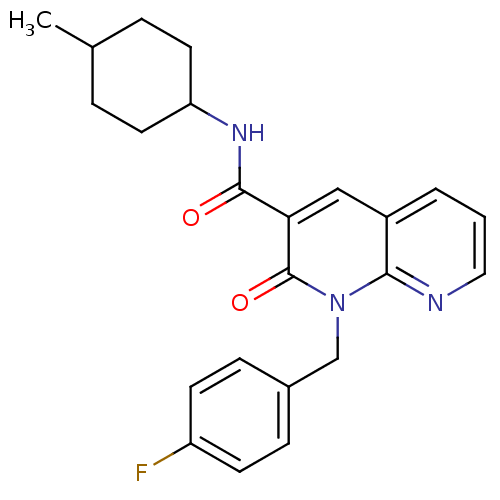 (CHEMBL466651 | N-(4-Methylcyclohexyl)-1-(p-fluorob...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029974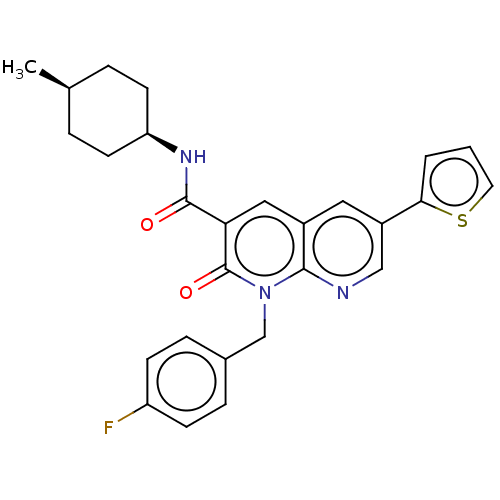 (CHEMBL3353446) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50495633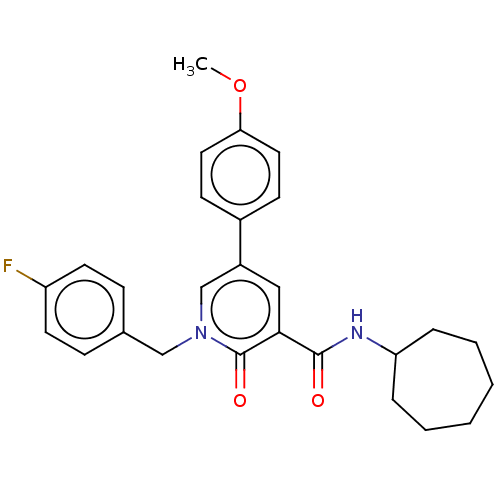 (CHEMBL3114181) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins | Eur J Med Chem 74: 524-32 (2014) Article DOI: 10.1016/j.ejmech.2013.10.070 BindingDB Entry DOI: 10.7270/Q21V5HXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50574656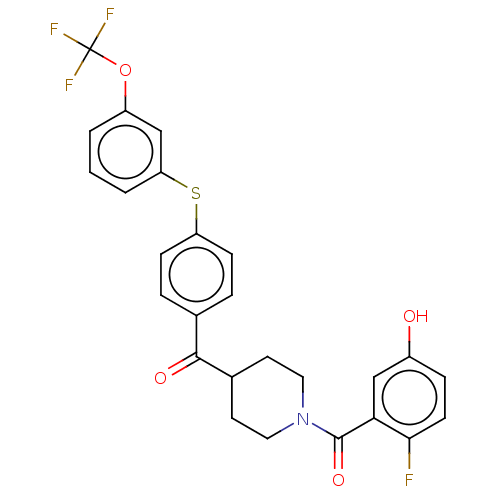 (CHEMBL4866490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MAGL assessed as dissociation constant using 4-NPA as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113679 BindingDB Entry DOI: 10.7270/Q2NV9P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50574654 (CHEMBL4870906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MAGL assessed as dissociation constant using 4-NPA as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113679 BindingDB Entry DOI: 10.7270/Q2NV9P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50574655 (CHEMBL4852896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MAGL assessed as dissociation constant using 4-NPA as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113679 BindingDB Entry DOI: 10.7270/Q2NV9P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133071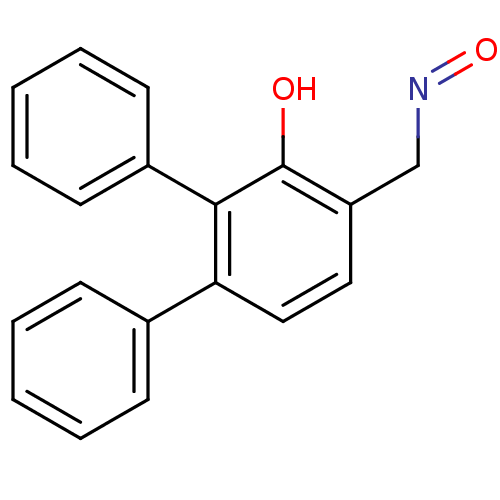 (3'-Hydroxy-[1,1';2',1'']terphenyl-4'-carbaldehyde ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029979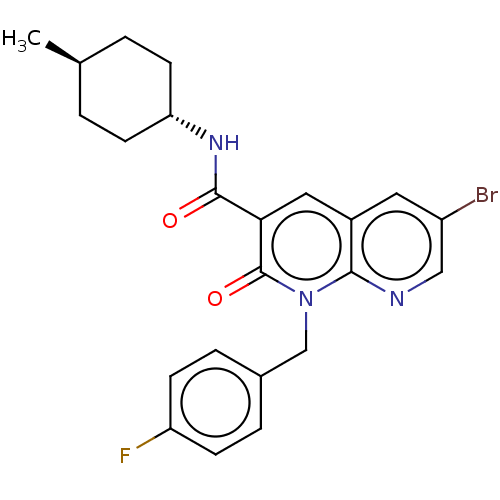 (CHEMBL3353440) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50016479 ((7-Methyl-4,6,6a,7,8,9-hexahydro-indolo[4,3-fg]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 89: 3630-4 (1992) Article DOI: 10.1073/pnas.89.8.3630 BindingDB Entry DOI: 10.7270/Q2VH5M9X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50495626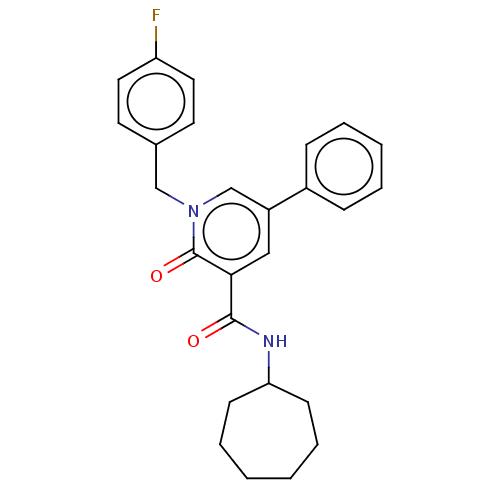 (CHEMBL3114182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins | Eur J Med Chem 74: 524-32 (2014) Article DOI: 10.1016/j.ejmech.2013.10.070 BindingDB Entry DOI: 10.7270/Q21V5HXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogenetic Corporation Curated by PDSP Ki Database | Mol Pharmacol 38: 681-8 (1990) BindingDB Entry DOI: 10.7270/Q29C6VWG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029959 (CHEMBL3353442 | US11564928, Compound 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50605682 (CHEMBL5176915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01806 BindingDB Entry DOI: 10.7270/Q2Q81J4P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029928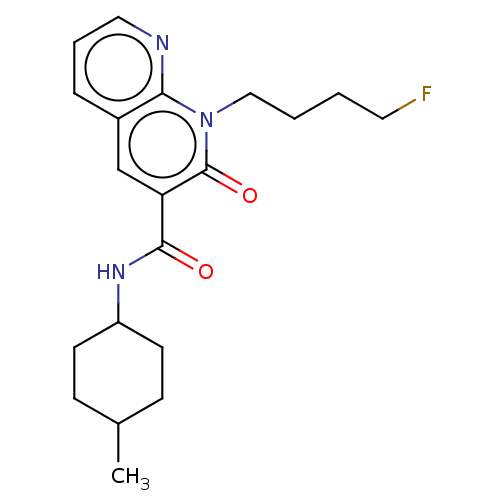 (CHEMBL3353436) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029980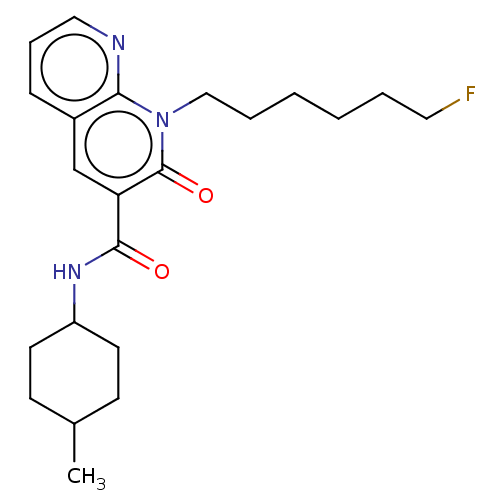 (CHEMBL3353438) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50082768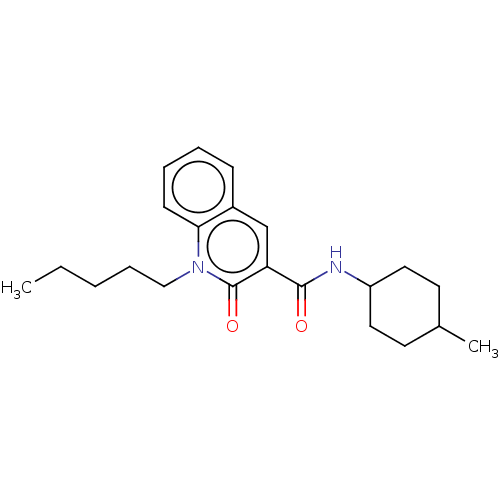 (CHEMBL3422788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins | Eur J Med Chem 97: 10-8 (2015) Article DOI: 10.1016/j.ejmech.2015.04.034 BindingDB Entry DOI: 10.7270/Q2VQ34DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50556542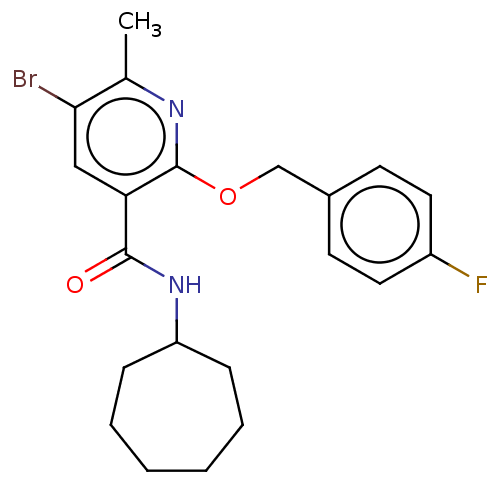 (CHEMBL4780771) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112858 BindingDB Entry DOI: 10.7270/Q2SQ943D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50556532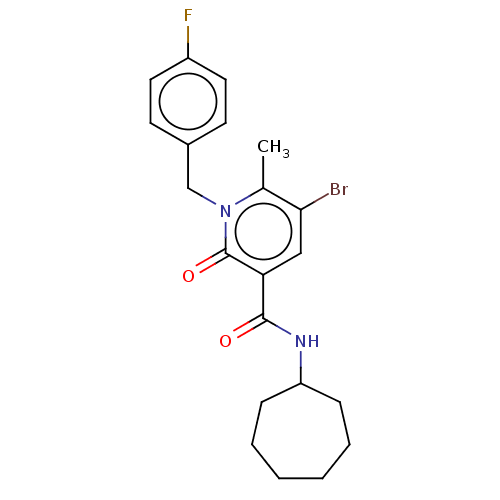 (CHEMBL4753891) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112858 BindingDB Entry DOI: 10.7270/Q2SQ943D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029960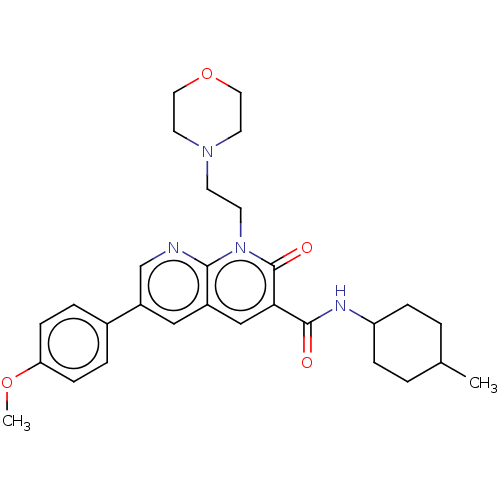 (CHEMBL3353451) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 89: 3630-4 (1992) Article DOI: 10.1073/pnas.89.8.3630 BindingDB Entry DOI: 10.7270/Q2VH5M9X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from recombinant human CB2R expressed in CHO cell membranes incubated for 90 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112858 BindingDB Entry DOI: 10.7270/Q2SQ943D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cell membranes after 90 mins | Eur J Med Chem 154: 155-171 (2018) Article DOI: 10.1016/j.ejmech.2018.05.019 BindingDB Entry DOI: 10.7270/Q24F1T87 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133071 (3'-Hydroxy-[1,1';2',1'']terphenyl-4'-carbaldehyde ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50381882 (CHEMBL2022694) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB2 receptor expressed in human HEK293 cell membrane after 90 mins | Eur J Med Chem 52: 284-94 (2012) Article DOI: 10.1016/j.ejmech.2012.03.031 BindingDB Entry DOI: 10.7270/Q2PK0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180036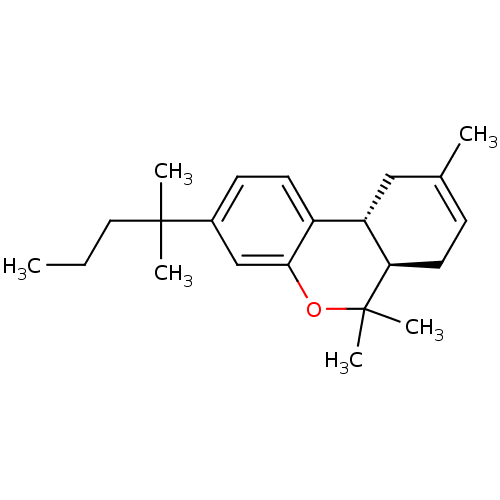 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB2 receptor expressed in human HEK293 cell membrane after 90 mins | Eur J Med Chem 52: 284-94 (2012) Article DOI: 10.1016/j.ejmech.2012.03.031 BindingDB Entry DOI: 10.7270/Q2PK0H6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258570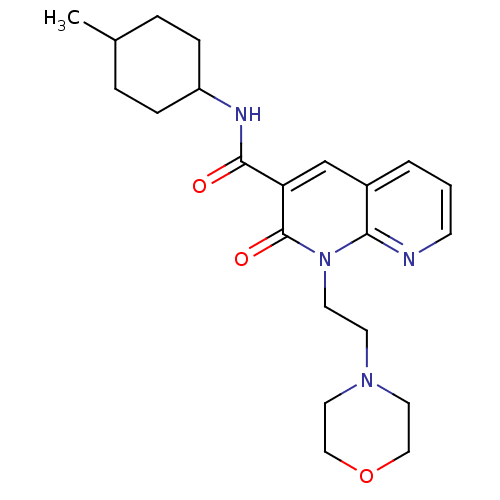 (CHEMBL466223 | N-(4-Methylcyclohexyl)-1-(2-morphol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029976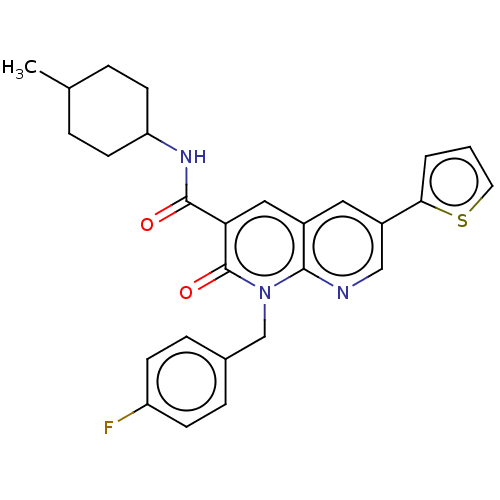 (CHEMBL3353444) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1406 total ) | Next | Last >> |