 Found 44 hits with Last Name = 'maddry' and Initial = 'ja'
Found 44 hits with Last Name = 'maddry' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043393
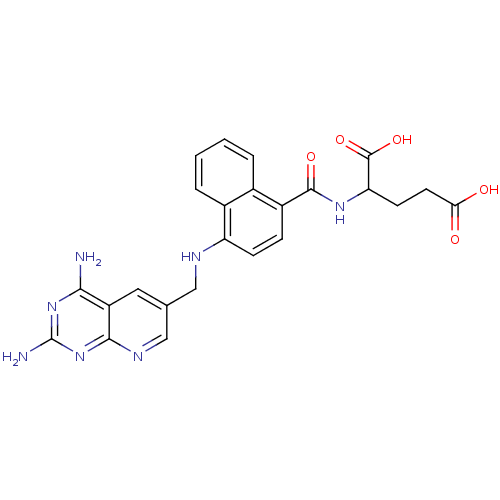
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C24H23N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h1-6,9,11,18,27H,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043396
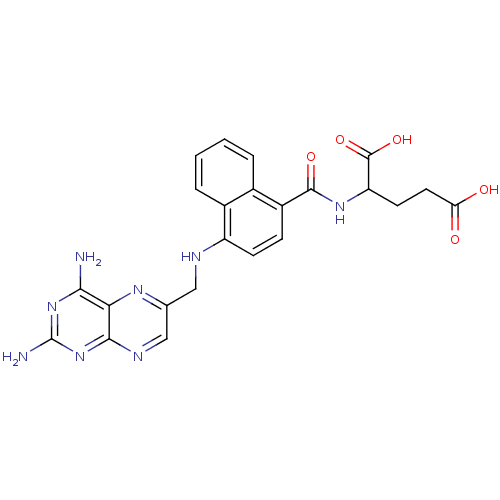
(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-na...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C23H22N8O5/c24-19-18-20(31-23(25)30-19)27-10-11(28-18)9-26-15-6-5-14(12-3-1-2-4-13(12)15)21(34)29-16(22(35)36)7-8-17(32)33/h1-6,10,16,26H,7-9H2,(H,29,34)(H,32,33)(H,35,36)(H4,24,25,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043399
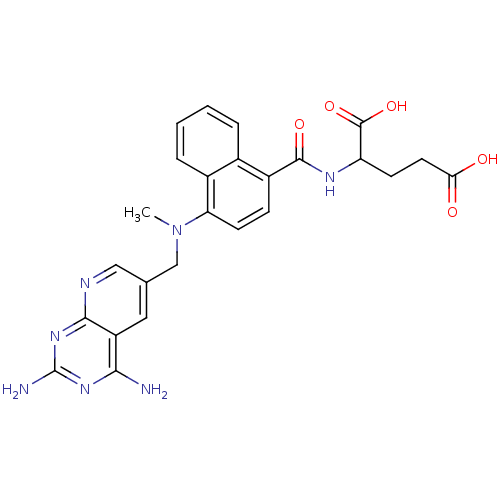
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2c1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C25H25N7O5/c1-32(12-13-10-17-21(26)30-25(27)31-22(17)28-11-13)19-8-6-16(14-4-2-3-5-15(14)19)23(35)29-18(24(36)37)7-9-20(33)34/h2-6,8,10-11,18H,7,9,12H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,26,27,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043395
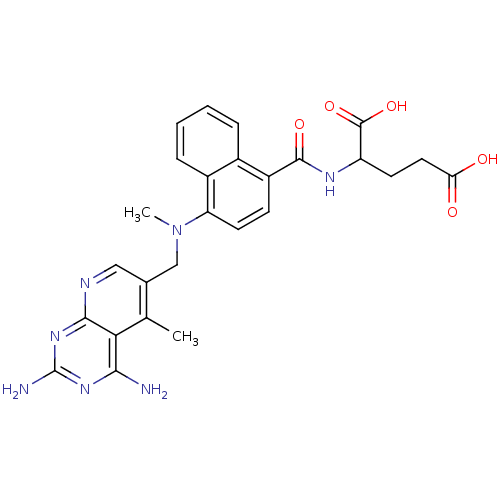
(2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2c1C)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C26H27N7O5/c1-13-14(11-29-23-21(13)22(27)31-26(28)32-23)12-33(2)19-9-7-17(15-5-3-4-6-16(15)19)24(36)30-18(25(37)38)8-10-20(34)35/h3-7,9,11,18H,8,10,12H2,1-2H3,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043400
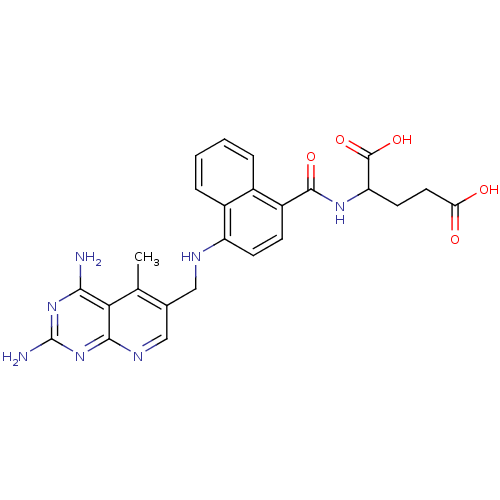
(2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...)Show SMILES Cc1c(CNc2ccc(C(=O)NC(CCC(O)=O)C(O)=O)c3ccccc23)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C25H25N7O5/c1-12-13(11-29-22-20(12)21(26)31-25(27)32-22)10-28-17-7-6-16(14-4-2-3-5-15(14)17)23(35)30-18(24(36)37)8-9-19(33)34/h2-7,11,18,28H,8-10H2,1H3,(H,30,35)(H,33,34)(H,36,37)(H4,26,27,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043398
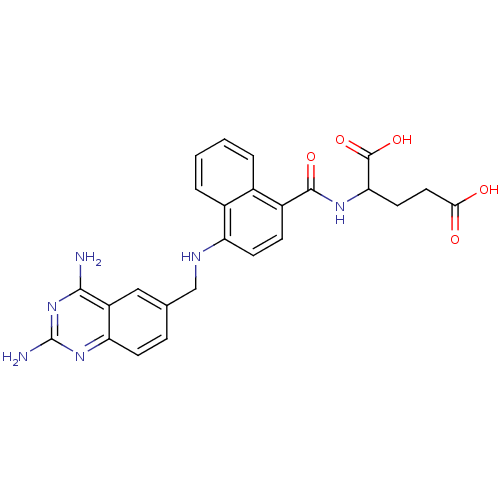
(2-({4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)ccc2n1 Show InChI InChI=1S/C25H24N6O5/c26-22-17-11-13(5-7-19(17)30-25(27)31-22)12-28-18-8-6-16(14-3-1-2-4-15(14)18)23(34)29-20(24(35)36)9-10-21(32)33/h1-8,11,20,28H,9-10,12H2,(H,29,34)(H,32,33)(H,35,36)(H4,26,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043394
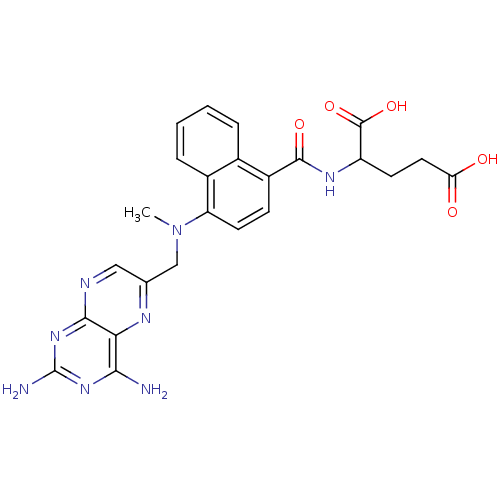
(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C24H24N8O5/c1-32(11-12-10-27-21-19(28-12)20(25)30-24(26)31-21)17-8-6-15(13-4-2-3-5-14(13)17)22(35)29-16(23(36)37)7-9-18(33)34/h2-6,8,10,16H,7,9,11H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,25,26,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043397
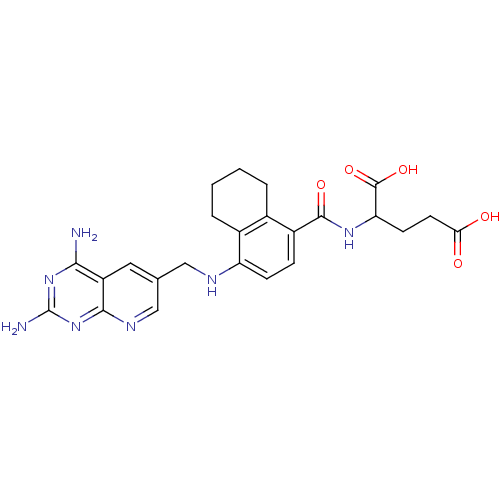
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4CCCCc34)cnc2n1 Show InChI InChI=1S/C24H27N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h5-6,9,11,18,27H,1-4,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50043394

(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C24H24N8O5/c1-32(11-12-10-27-21-19(28-12)20(25)30-24(26)31-21)17-8-6-15(13-4-2-3-5-14(13)17)22(35)29-16(23(36)37)7-9-18(33)34/h2-6,8,10,16H,7,9,11H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,25,26,27,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.000190 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibition against P. carinii DHFR (dihydrofolate reductase) |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50016325
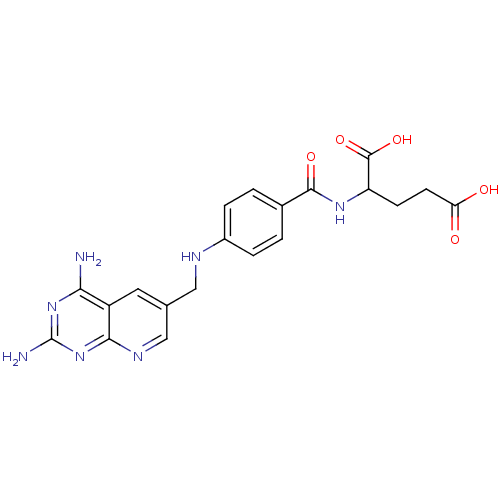
(2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H21N7O5/c21-16-13-7-10(9-24-17(13)27-20(22)26-16)8-23-12-3-1-11(2-4-12)18(30)25-14(19(31)32)5-6-15(28)29/h1-4,7,9,14,23H,5-6,8H2,(H,25,30)(H,28,29)(H,31,32)(H4,21,22,24,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.000530 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition against Pneumocystis carinii Dihydrofolate reductase |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50043393

(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C24H23N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h1-6,9,11,18,27H,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.000530 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibition against Dihydrofolate reductase from P. carinii |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50043393

(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C24H23N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h1-6,9,11,18,27H,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.000530 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibition against Dihydrofolate reductase from P. carinii |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50043393

(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C24H23N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h1-6,9,11,18,27H,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibition against Dihydrofolate reductase from T. gondii |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50016325

(2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H21N7O5/c21-16-13-7-10(9-24-17(13)27-20(22)26-16)8-23-12-3-1-11(2-4-12)18(30)25-14(19(31)32)5-6-15(28)29/h1-4,7,9,14,23H,5-6,8H2,(H,25,30)(H,28,29)(H,31,32)(H4,21,22,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition against rat liver Dihydrofolate reductase |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50016325

(2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H21N7O5/c21-16-13-7-10(9-24-17(13)27-20(22)26-16)8-23-12-3-1-11(2-4-12)18(30)25-14(19(31)32)5-6-15(28)29/h1-4,7,9,14,23H,5-6,8H2,(H,25,30)(H,28,29)(H,31,32)(H4,21,22,24,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00210 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition against Toxoplasma gondii Dihydrofolate reductase |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50043394

(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C24H24N8O5/c1-32(11-12-10-27-21-19(28-12)20(25)30-24(26)31-21)17-8-6-15(13-4-2-3-5-14(13)17)22(35)29-16(23(36)37)7-9-18(33)34/h2-6,8,10,16H,7,9,11H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,25,26,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibition against rat liver DHFR (dihydrofolate reductase) |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM15339
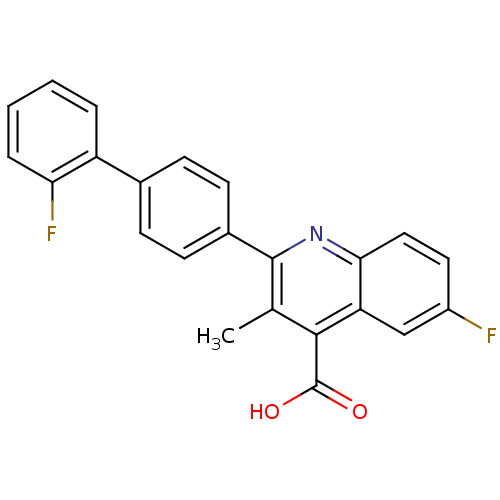
(6-fluoro-2-[4-(2-fluorophenyl)phenyl]-3-methyl-qui...)Show SMILES Cc1c(nc2ccc(F)cc2c1C(O)=O)-c1ccc(cc1)-c1ccccc1F Show InChI InChI=1S/C23H15F2NO2/c1-13-21(23(27)28)18-12-16(24)10-11-20(18)26-22(13)15-8-6-14(7-9-15)17-4-2-3-5-19(17)25/h2-12H,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289961
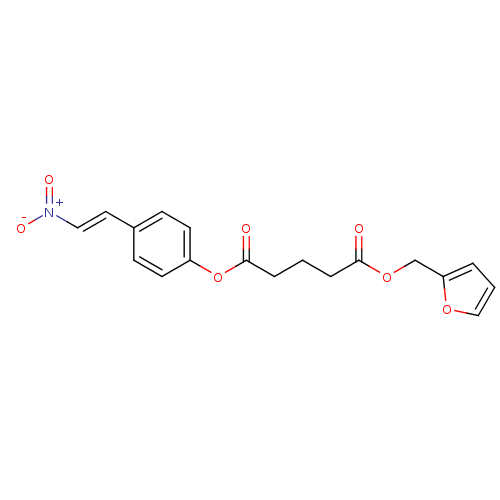
(CHEMBL65376 | Pentanedioic acid furan-2-ylmethyl e...)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)OCc2ccco2)cc1 Show InChI InChI=1S/C18H17NO7/c20-17(25-13-16-3-2-12-24-16)4-1-5-18(21)26-15-8-6-14(7-9-15)10-11-19(22)23/h2-3,6-12H,1,4-5,13H2/b11-10+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576030
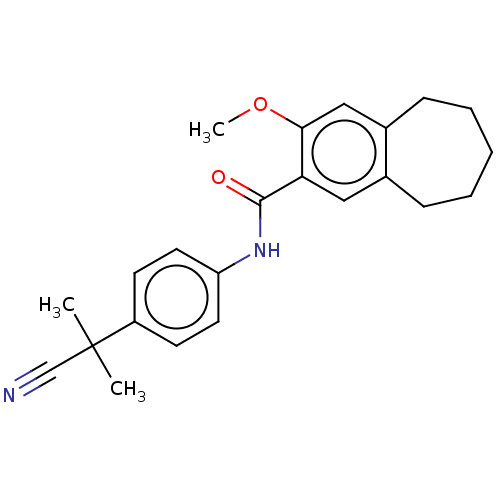
(CHEMBL4860581)Show SMILES COc1cc2CCCCCc2cc1C(=O)Nc1ccc(cc1)C(C)(C)C#N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289957

(CHEMBL304442 | Pentanedioic acid 4-((E)-2-nitro-vi...)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C19H17NO6/c21-18(25-16-5-2-1-3-6-16)7-4-8-19(22)26-17-11-9-15(10-12-17)13-14-20(23)24/h1-3,5-6,9-14H,4,7-8H2/b14-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289964
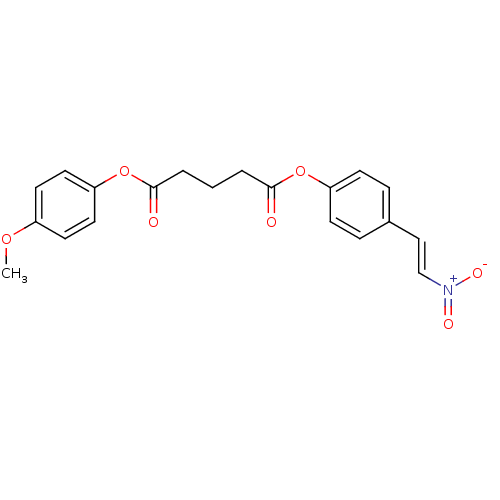
(CHEMBL63559 | Pentanedioic acid 4-methoxy-phenyl e...)Show SMILES COc1ccc(OC(=O)CCCC(=O)Oc2ccc(\C=C\[N+]([O-])=O)cc2)cc1 Show InChI InChI=1S/C20H19NO7/c1-26-16-9-11-18(12-10-16)28-20(23)4-2-3-19(22)27-17-7-5-15(6-8-17)13-14-21(24)25/h5-14H,2-4H2,1H3/b14-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289963

(CHEMBL3142195 | CHEMBL92506 | Pentanedioic acid (2...)Show SMILES CC(C)O[C@@H]1[C@@H](COC(=O)CCCC(=O)Oc2ccc(\C=C\[N+]([O-])=O)cc2)O[C@H]([C@@H]1OC(C)C)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C29H36N6O9/c1-17(2)41-25-21(44-29(26(25)42-18(3)4)34-16-33-24-27(30)31-15-32-28(24)34)14-40-22(36)6-5-7-23(37)43-20-10-8-19(9-11-20)12-13-35(38)39/h8-13,15-18,21,25-26,29H,5-7,14H2,1-4H3,(H2,30,31,32)/b13-12+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576027

(CHEMBL4852930) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576023
(CHEMBL4851250) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289961

(CHEMBL65376 | Pentanedioic acid furan-2-ylmethyl e...)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)OCc2ccco2)cc1 Show InChI InChI=1S/C18H17NO7/c20-17(25-13-16-3-2-12-24-16)4-1-5-18(21)26-15-8-6-14(7-9-15)10-11-19(22)23/h2-3,6-12H,1,4-5,13H2/b11-10+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of HER-2 expressed in NIH3T3 cell lines |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289957

(CHEMBL304442 | Pentanedioic acid 4-((E)-2-nitro-vi...)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C19H17NO6/c21-18(25-16-5-2-1-3-6-16)7-4-8-19(22)26-17-11-9-15(10-12-17)13-14-20(23)24/h1-3,5-6,9-14H,4,7-8H2/b14-13+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of HER-2 expressed in NIH3T3 cell lines |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289963

(CHEMBL3142195 | CHEMBL92506 | Pentanedioic acid (2...)Show SMILES CC(C)O[C@@H]1[C@@H](COC(=O)CCCC(=O)Oc2ccc(\C=C\[N+]([O-])=O)cc2)O[C@H]([C@@H]1OC(C)C)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C29H36N6O9/c1-17(2)41-25-21(44-29(26(25)42-18(3)4)34-16-33-24-27(30)31-15-32-28(24)34)14-40-22(36)6-5-7-23(37)43-20-10-8-19(9-11-20)12-13-35(38)39/h8-13,15-18,21,25-26,29H,5-7,14H2,1-4H3,(H2,30,31,32)/b13-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of HER-2 expressed in NIH3T3 cell lines |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50214741

(CHEMBL65096)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C20H20N2O5/c23-19(21-15-17-5-2-1-3-6-17)7-4-8-20(24)27-18-11-9-16(10-12-18)13-14-22(25)26/h1-3,5-6,9-14H,4,7-8,15H2,(H,21,23)/b14-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576022

(CHEMBL1498496) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50289964

(CHEMBL63559 | Pentanedioic acid 4-methoxy-phenyl e...)Show SMILES COc1ccc(OC(=O)CCCC(=O)Oc2ccc(\C=C\[N+]([O-])=O)cc2)cc1 Show InChI InChI=1S/C20H19NO7/c1-26-16-9-11-18(12-10-16)28-20(23)4-2-3-19(22)27-17-7-5-15(6-8-17)13-14-21(24)25/h5-14H,2-4H2,1H3/b14-13+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of HER-2 expressed in NIH3T3 cell lines |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50214742
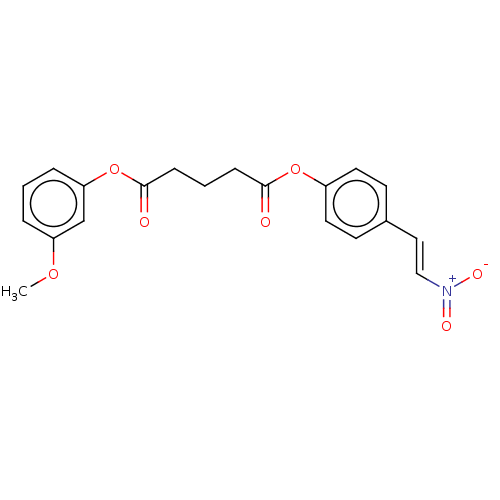
(CHEMBL302905)Show SMILES COc1cccc(OC(=O)CCCC(=O)Oc2ccc(\C=C\[N+]([O-])=O)cc2)c1 Show InChI InChI=1S/C20H19NO7/c1-26-17-4-2-5-18(14-17)28-20(23)7-3-6-19(22)27-16-10-8-15(9-11-16)12-13-21(24)25/h2,4-5,8-14H,3,6-7H2,1H3/b13-12+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50214743
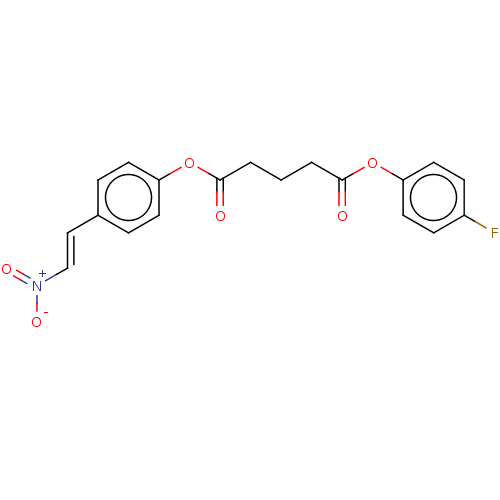
(CHEMBL63650)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)Oc2ccc(F)cc2)cc1 Show InChI InChI=1S/C19H16FNO6/c20-15-6-10-17(11-7-15)27-19(23)3-1-2-18(22)26-16-8-4-14(5-9-16)12-13-21(24)25/h4-13H,1-3H2/b13-12+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50214739

(CHEMBL68413)Show SMILES COc1ccccc1OC(=O)CCCC(=O)Oc1ccc(\C=C\[N+]([O-])=O)cc1 Show InChI InChI=1S/C20H19NO7/c1-26-17-5-2-3-6-18(17)28-20(23)8-4-7-19(22)27-16-11-9-15(10-12-16)13-14-21(24)25/h2-3,5-6,9-14H,4,7-8H2,1H3/b14-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50214738

(CHEMBL65594)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C19H18N2O5/c22-18(20-16-5-2-1-3-6-16)7-4-8-19(23)26-17-11-9-15(10-12-17)13-14-21(24)25/h1-3,5-6,9-14H,4,7-8H2,(H,20,22)/b14-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50214737
(CHEMBL63772)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)OCCc2ccccc2)cc1 Show InChI InChI=1S/C21H21NO6/c23-20(27-16-14-17-5-2-1-3-6-17)7-4-8-21(24)28-19-11-9-18(10-12-19)13-15-22(25)26/h1-3,5-6,9-13,15H,4,7-8,14,16H2/b15-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50214740
(CHEMBL67009)Show SMILES [O-][N+](=O)\C=C\c1ccc(OC(=O)CCCC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C20H19NO6/c22-19(26-15-17-5-2-1-3-6-17)7-4-8-20(23)27-18-11-9-16(10-12-18)13-14-21(24)25/h1-3,5-6,9-14H,4,7-8,15H2/b14-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ligand-stimulated autophosphorylation of EGFR/HER2 chimeric receptor expressed in NIH3T3 cells |
Bioorg Med Chem Lett 7: 2109-2114 (1997)
Article DOI: 10.1016/S0960-894X(97)00369-7
BindingDB Entry DOI: 10.7270/Q2N016H3 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576029
(CHEMBL4858433) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576025
(CHEMBL4866286) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576028
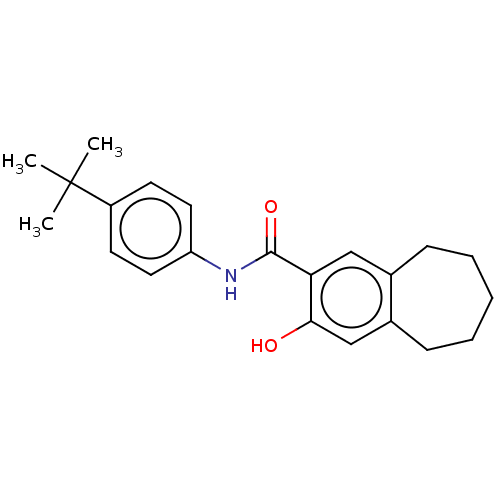
(CHEMBL4849065) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576024
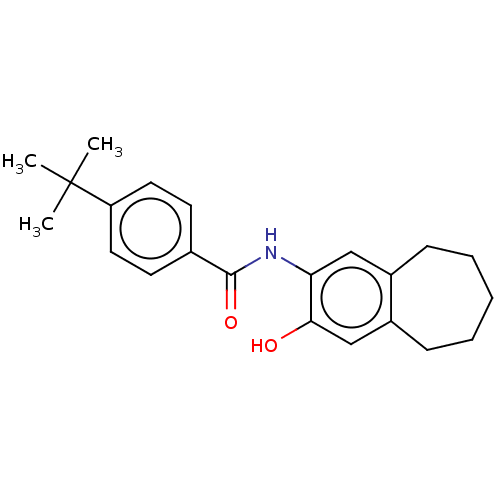
(CHEMBL4857838) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576032

(CHEMBL4869858) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576031
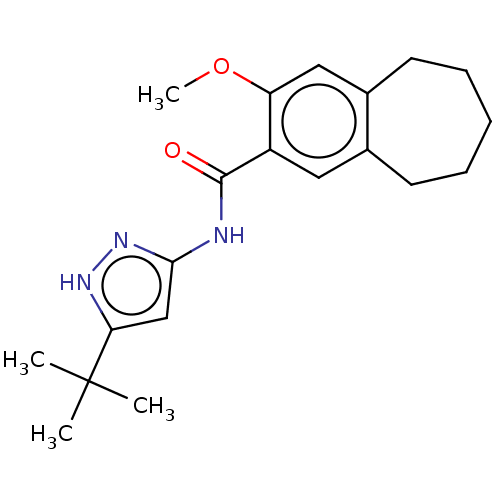
(CHEMBL4874245)Show SMILES COc1cc2CCCCCc2cc1C(=O)Nc1cc([nH]n1)C(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50576026
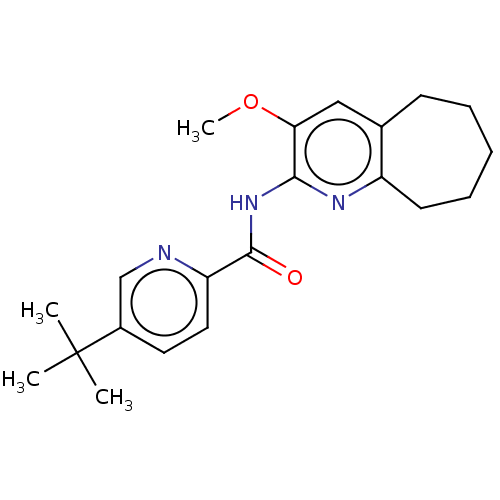
(CHEMBL4876364) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length C-terminal MYC/DDk-tagged DHODH using decylubiquinone as substrate measured for 1 hr by DCIP absorbance a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02183
BindingDB Entry DOI: 10.7270/Q2W3814Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data