

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.574 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Rattus norvegicus) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.772 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P4 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50324492 (CHEMBL1214871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Beta-1,4-galactosyltransferase 1 | J Med Chem 53: 5607-19 (2010) Article DOI: 10.1021/jm100612r BindingDB Entry DOI: 10.7270/Q2WS8TFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50447747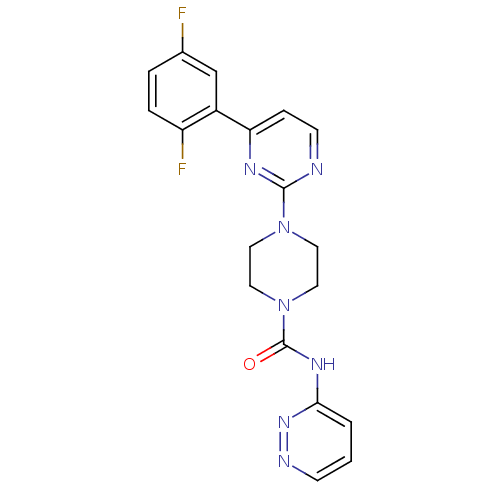 (CHEMBL3113272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHOK1 cells using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189733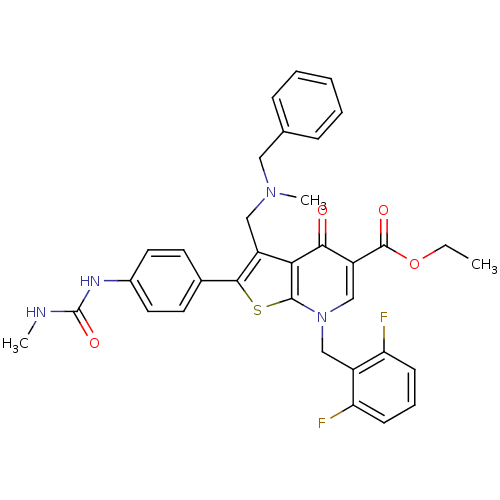 (CHEMBL379629 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189704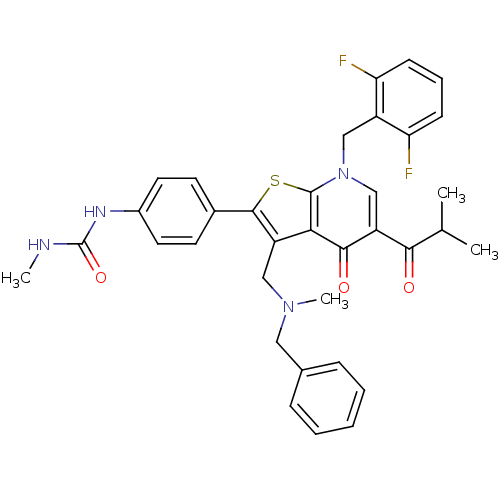 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of LHRH-stimulated arachidonic acid release in CHO cells expressing human LHRH receptor | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189716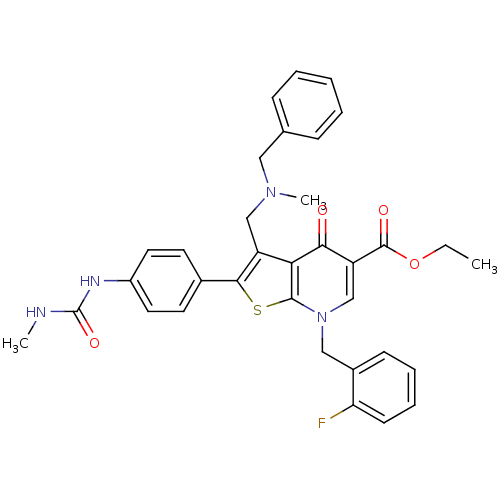 (CHEMBL210294 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50447748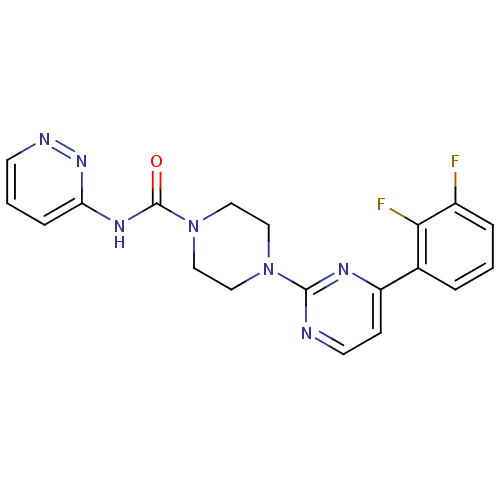 (CHEMBL3113271) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHOK1 cells using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50447745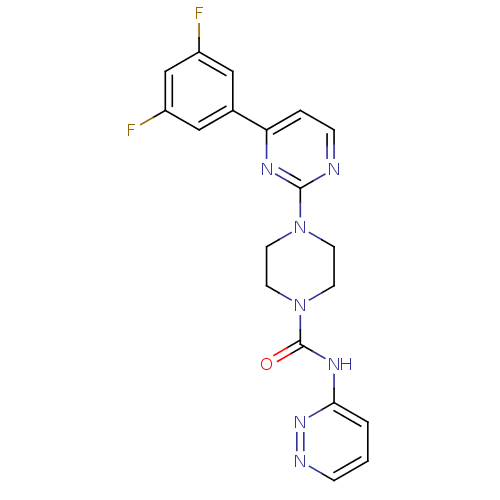 (CHEMBL3113274) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHOK1 cells using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189703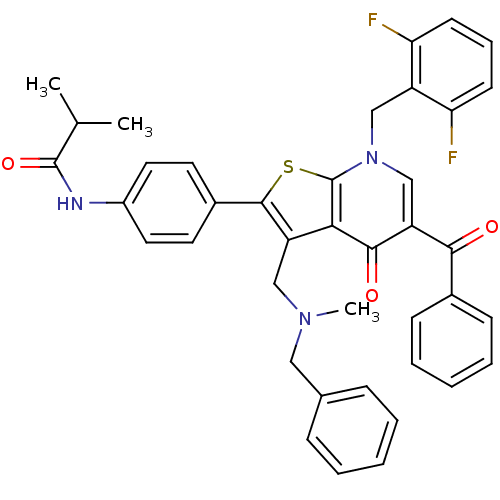 (CHEMBL208812 | N-(4-(7-(2,6-difluorobenzyl)-5-benz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189701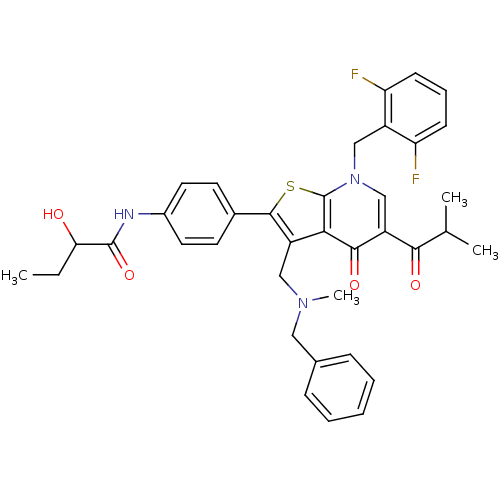 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189730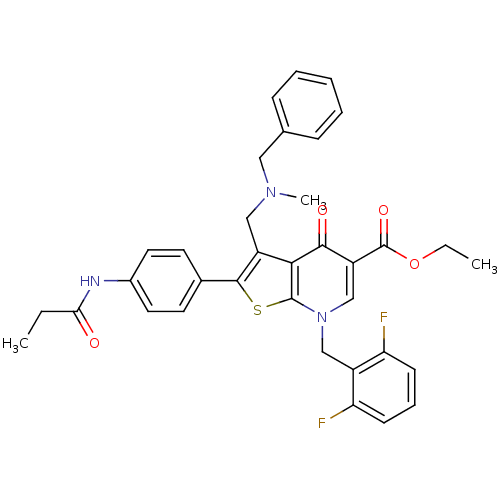 (CHEMBL210709 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189701 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of LHRH-stimulated arachidonic acid release in CHO cells expressing human LHRH receptor | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189704 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50447747 (CHEMBL3113272) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189720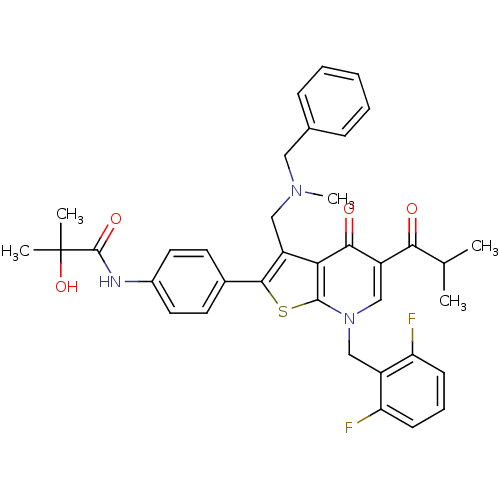 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50067485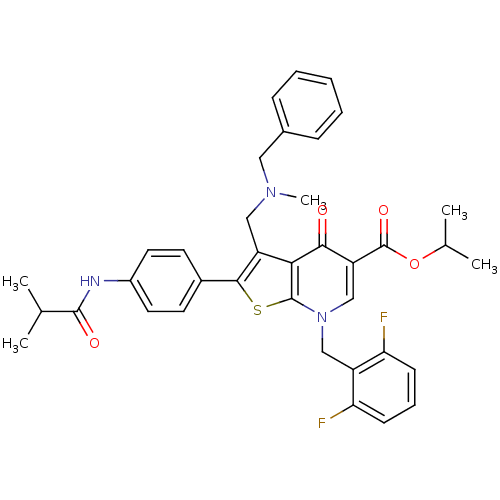 (3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description The Compound was tested for the concentration to inhibit 50% of [125 I ]leuprorelin binding to the cloned human Leutinizing releasing hormone recepto... | J Med Chem 41: 4190-5 (1998) Article DOI: 10.1021/jm9803673 BindingDB Entry DOI: 10.7270/Q2CR5V19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50067485 (3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189704 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to monkey recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189723 (CHEMBL540109 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189727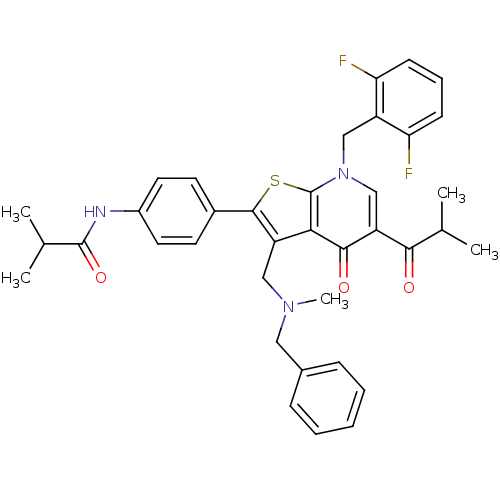 (CHEMBL211503 | N-(4-(7-(2,6-difluorobenzyl)-3-((be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189707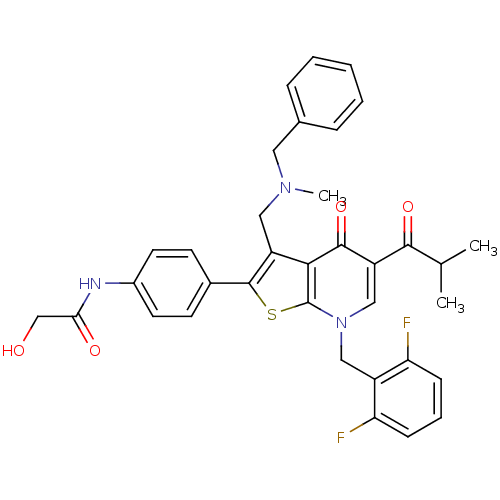 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50447746 (CHEMBL3113273) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50115988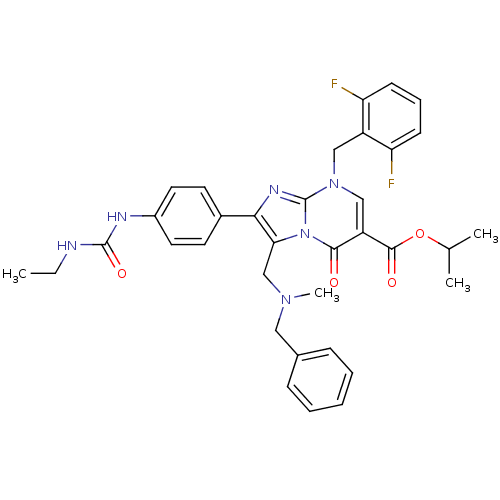 (3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 12: 2073-7 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50369395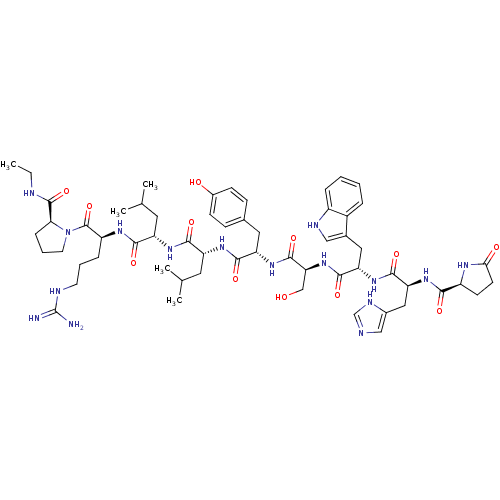 (ELIGARD | LEUPROLIDE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description The Compound was tested for the concentration to inhibit 50% of [125 I ]leuprorelin binding to the cloned human Leutinizing releasing hormone recepto... | J Med Chem 41: 4190-5 (1998) Article DOI: 10.1021/jm9803673 BindingDB Entry DOI: 10.7270/Q2CR5V19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50369395 (ELIGARD | LEUPROLIDE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50447745 (CHEMBL3113274) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383116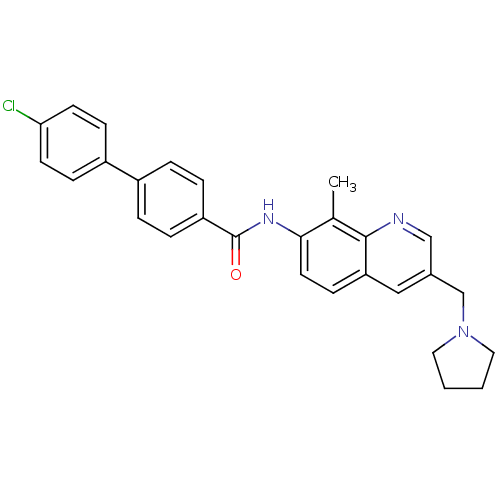 (CHEMBL2031736) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189712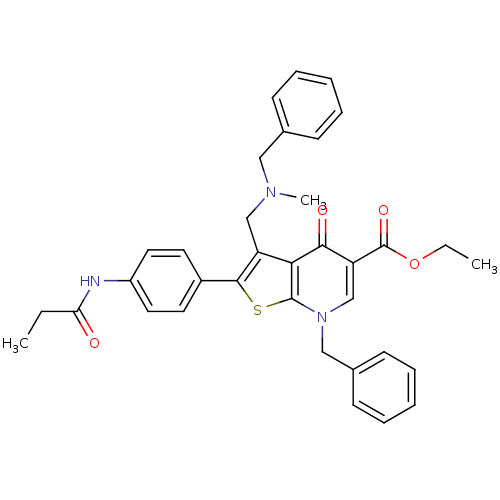 (CHEMBL379198 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50115989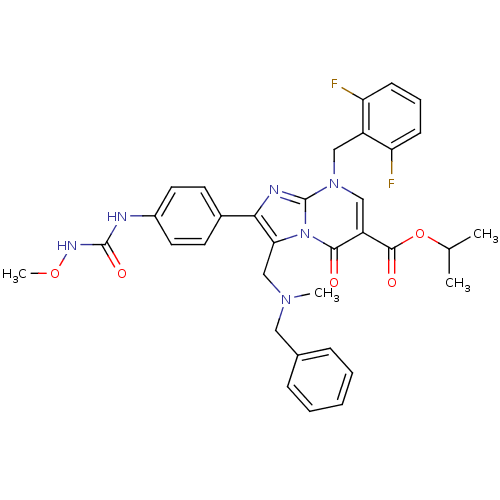 (3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 12: 2073-7 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189736 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189713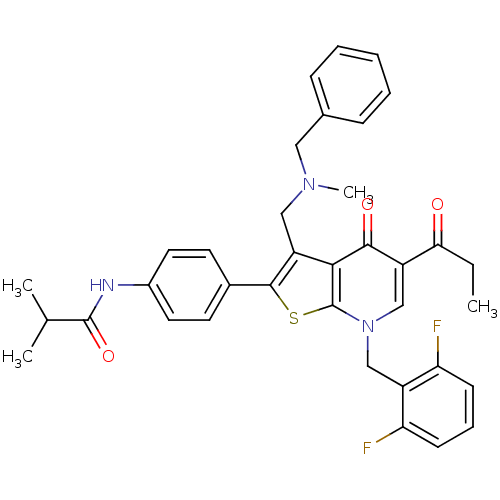 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50447749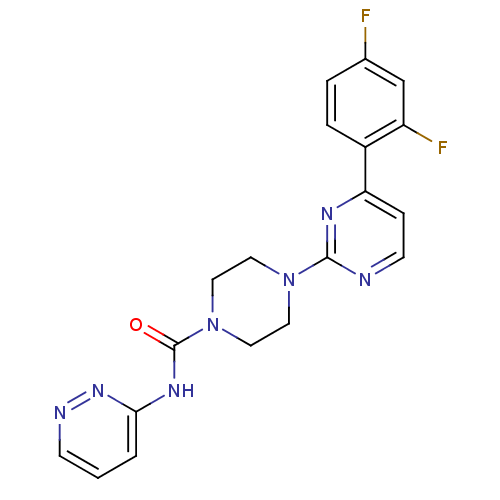 (CHEMBL3113270) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50369395 (ELIGARD | LEUPROLIDE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to rat anterior pituitary LHRH receptor | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50115998 (3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 12: 2073-7 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50369395 (ELIGARD | LEUPROLIDE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description The compound was tested for the concentration to inhibit 50% of [125 I]leuprorelin binding to Leutinizing releasing hormone receptor in the membrane ... | J Med Chem 41: 4190-5 (1998) Article DOI: 10.1021/jm9803673 BindingDB Entry DOI: 10.7270/Q2CR5V19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383114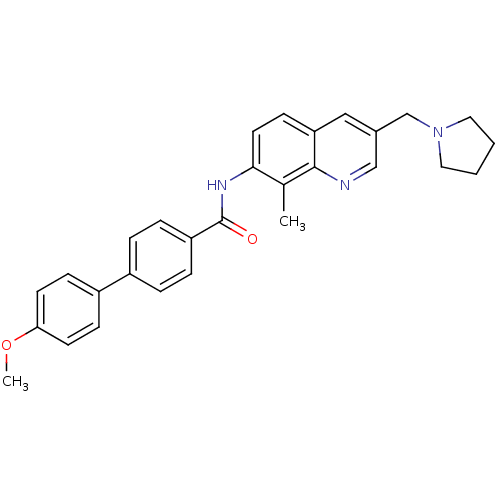 (CHEMBL2031734) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50351324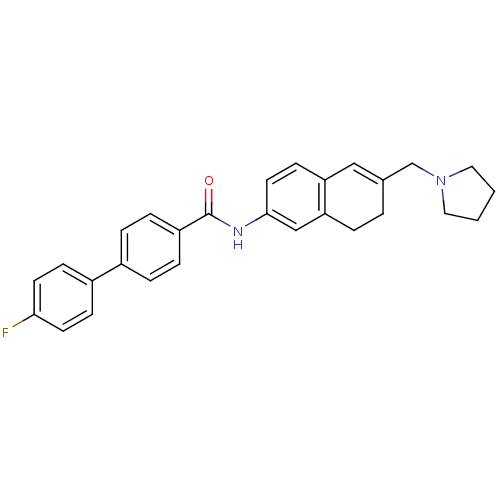 (CHEMBL1818901) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human 5HT2C receptor expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383112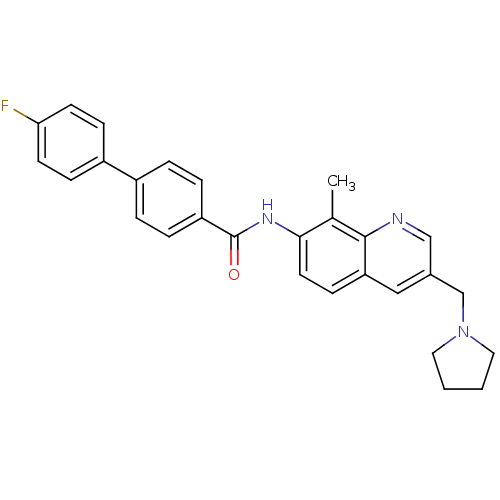 (CHEMBL2029372) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189740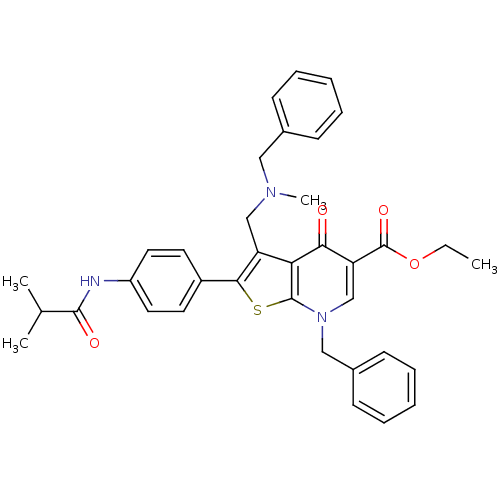 (CHEMBL210888 | ethyl 7-benzyl-3-((benzyl(methyl)am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50067485 (3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of LHRH-stimulated arachidonic acid release in CHO cells expressing human LHRH receptor | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50115995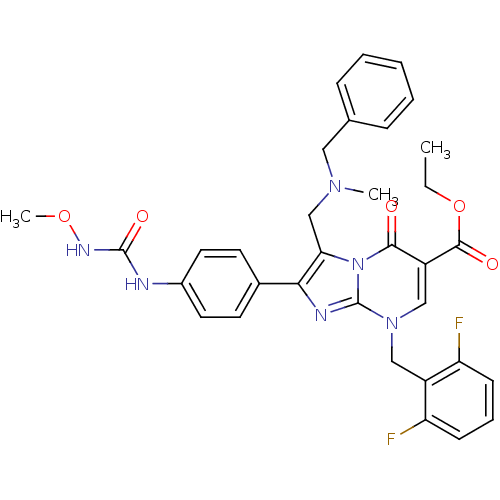 (3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 12: 2073-7 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50447746 (CHEMBL3113273) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHOK1 cells using AMCAA as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 22: 1468-78 (2014) Article DOI: 10.1016/j.bmc.2013.12.023 BindingDB Entry DOI: 10.7270/Q20C4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383096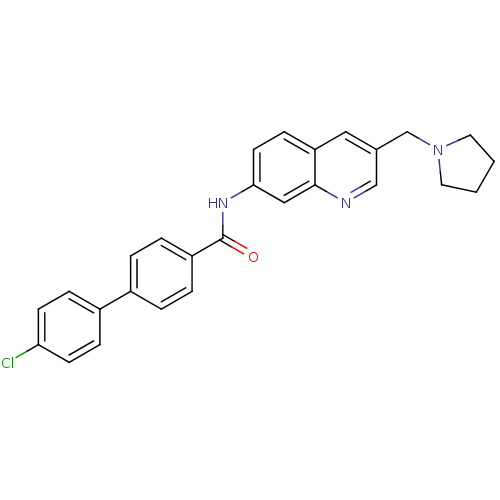 (CHEMBL2031716) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383091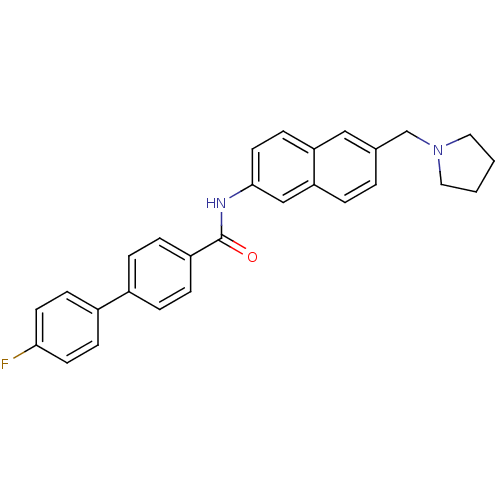 (CHEMBL2031573) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50369395 (ELIGARD | LEUPROLIDE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description The compound was tested for the concentration to inhibit 50% of [125 I]leuprorelin binding to Leutinizing releasing hormone receptor the membrane fra... | J Med Chem 41: 4190-5 (1998) Article DOI: 10.1021/jm9803673 BindingDB Entry DOI: 10.7270/Q2CR5V19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 785 total ) | Next | Last >> |