

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546794 (CHEMBL4777335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546794 (CHEMBL4777335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546795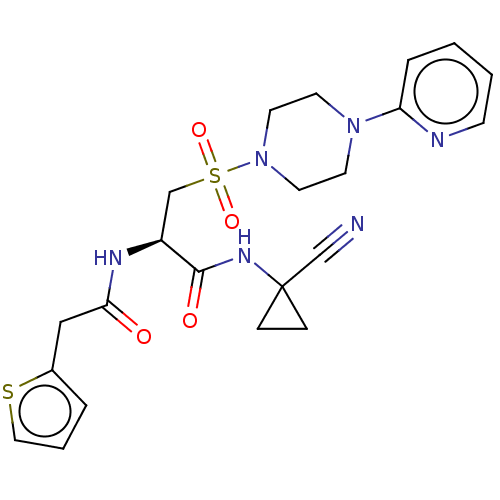 (CHEMBL4777740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546795 (CHEMBL4777740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546796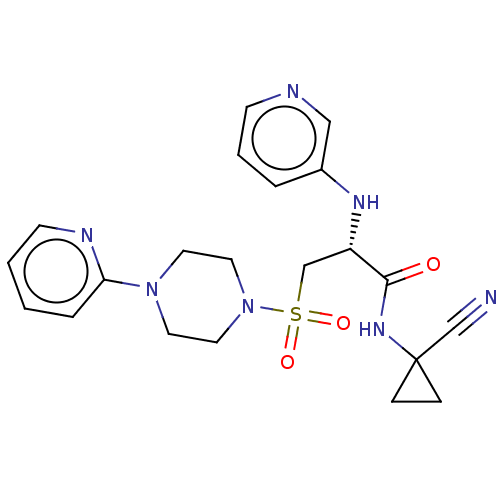 (CHEMBL4761229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546796 (CHEMBL4761229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546797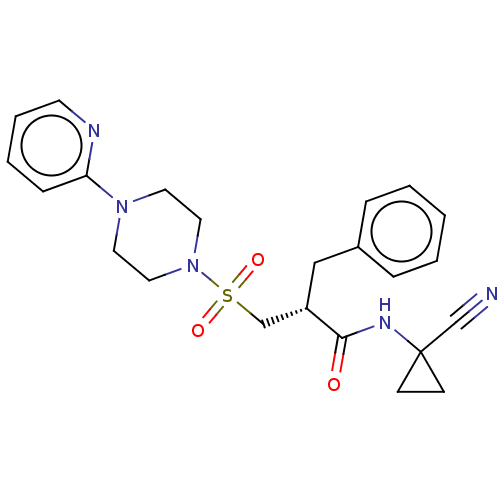 (CHEMBL4757049) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546797 (CHEMBL4757049) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546800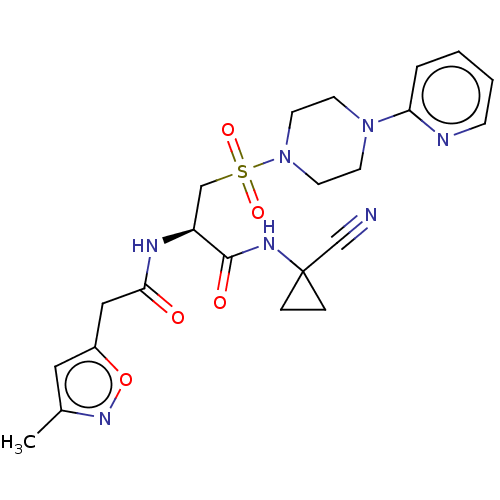 (CHEMBL4758443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546800 (CHEMBL4758443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546798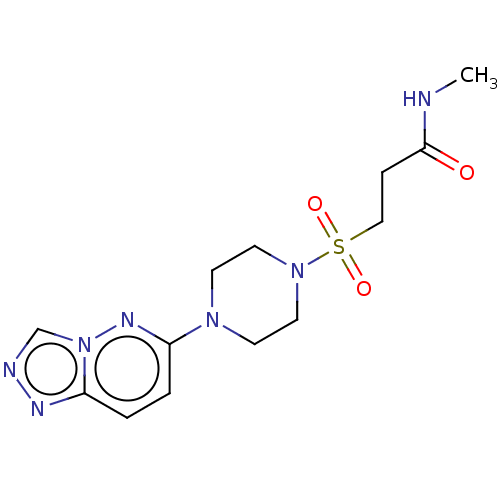 (CHEMBL4779026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546798 (CHEMBL4779026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546799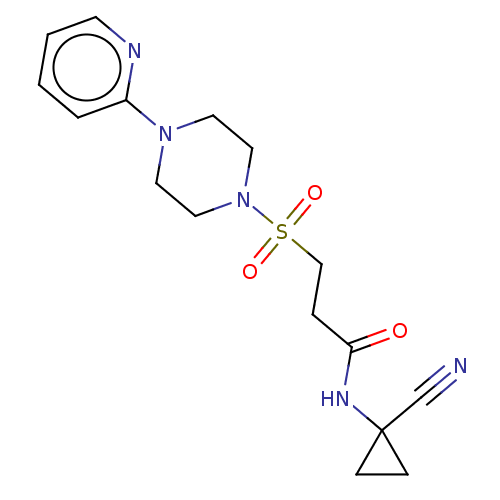 (CHEMBL4755678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546799 (CHEMBL4755678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458775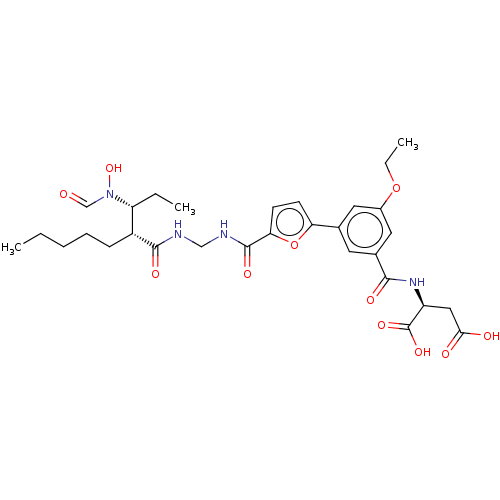 (CHEMBL4218415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged PI4K-alpha (1 to 2044) (unknown origin) using D-myo-phosphatidylinositol as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458774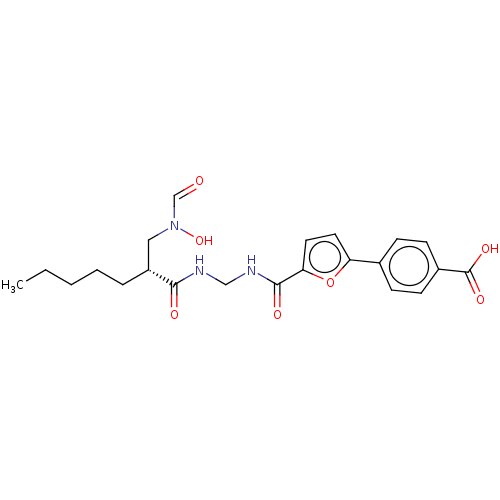 (CHEMBL4202714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458773 (CHEMBL4204567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458765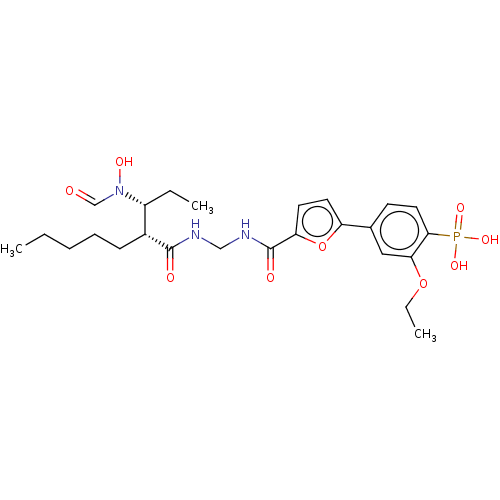 (CHEMBL4205697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458764 (CHEMBL4209125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458763 (CHEMBL4202848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439218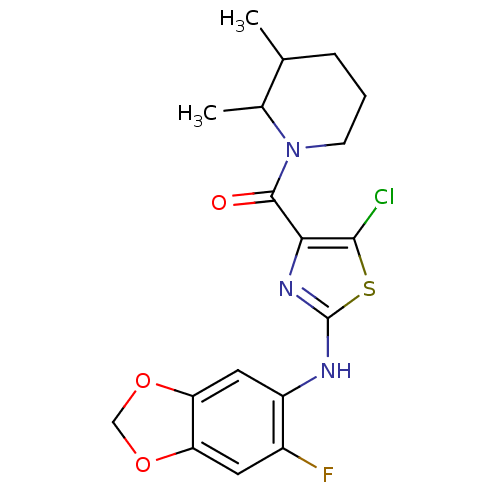 (CHEMBL2418809) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294706 (4-((2-chlorobenzyl)(3-methyl-1-(1-methyl-1H-tetraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM50296010 (2-isobutyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of SGK1 by fluorescence polarization assay | Bioorg Med Chem Lett 19: 4441-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.051 BindingDB Entry DOI: 10.7270/Q2WQ03VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458758 (CHEMBL4207907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298210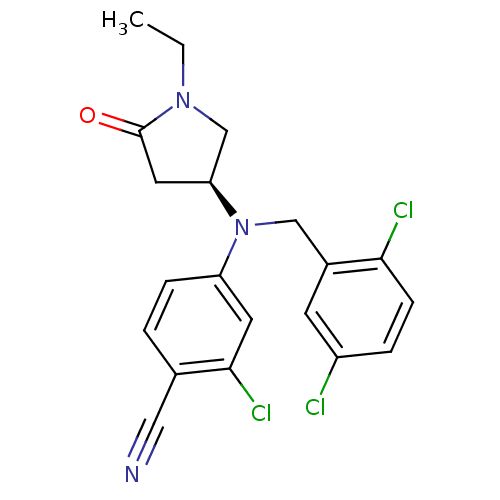 ((S)-2-chloro-4-((2,5-dichlorobenzyl)(1-ethyl-5-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298207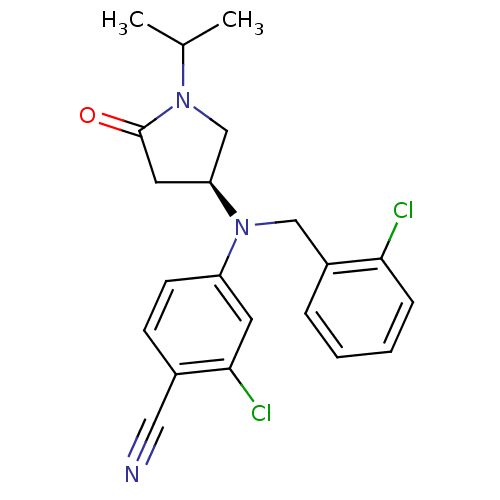 ((S)-2-chloro-4-((2-chlorobenzyl)(1-isopropyl-5-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298208 ((S)-2-chloro-4-((2-chloro-5-fluorobenzyl)(1-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294715 (4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294702 (4-((2-chlorobenzyl)(1-(1-methyl-1H-tetrazol-5-yl)e...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294703 (4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM50296011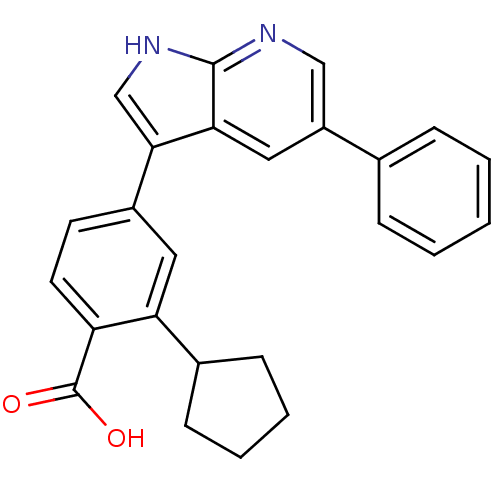 (2-cyclopentyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of SGK1 by fluorescence polarization assay | Bioorg Med Chem Lett 19: 4441-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.051 BindingDB Entry DOI: 10.7270/Q2WQ03VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294705 (4-((2-chlorobenzyl)(1-(1-methyl-1H-tetrazol-5-yl)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294696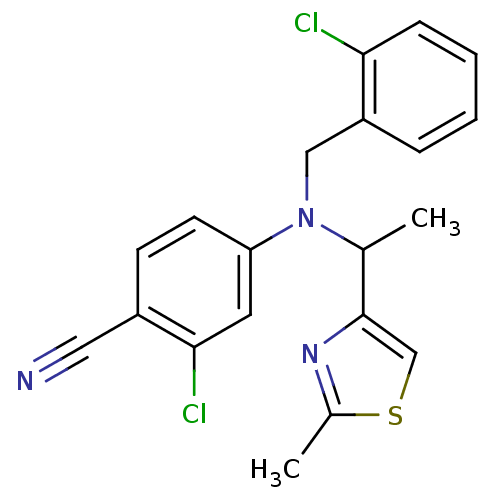 (4-((2-chlorobenzyl)(1-(2-methylthiazol-4-yl)ethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298219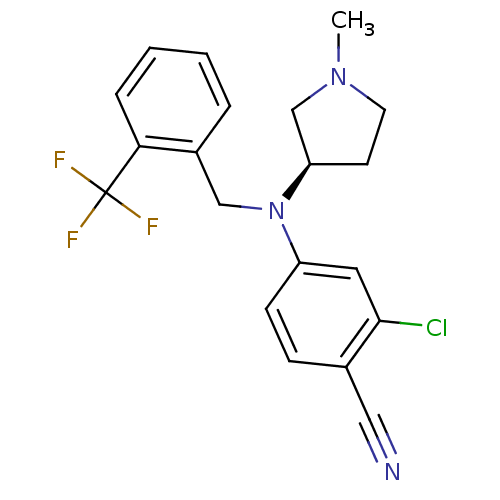 ((R)-2-chloro-4-((1-methylpyrrolidin-3-yl)(2-(trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298209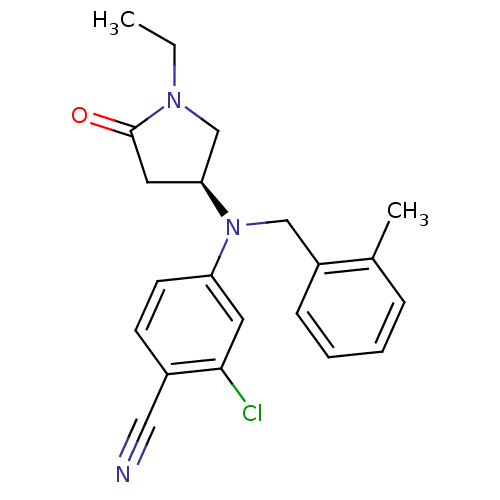 ((S)-2-chloro-4-((1-ethyl-5-oxopyrrolidin-3-yl)(2-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439217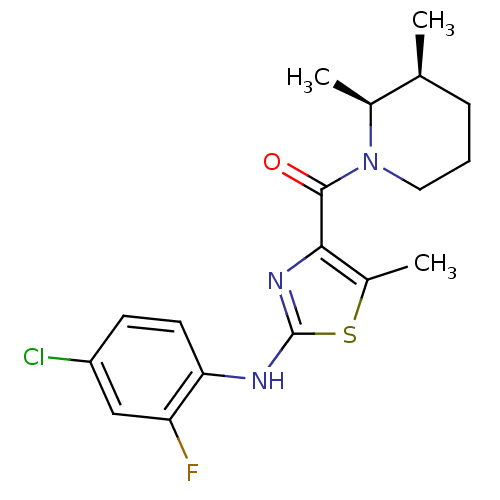 (CHEMBL2418811) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50439220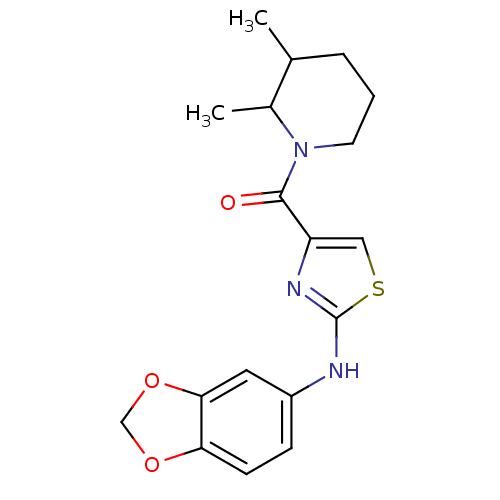 (CHEMBL2418807) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assay | Bioorg Med Chem Lett 23: 4979-84 (2013) Article DOI: 10.1016/j.bmcl.2013.06.047 BindingDB Entry DOI: 10.7270/Q2319X9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294707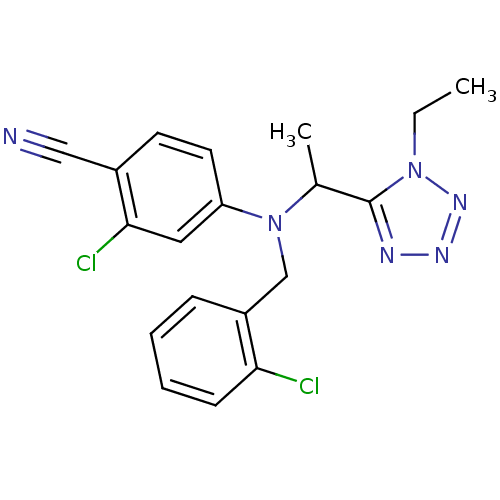 (4-((2-chlorobenzyl)(1-(1-ethyl-1H-tetrazol-5-yl)et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294710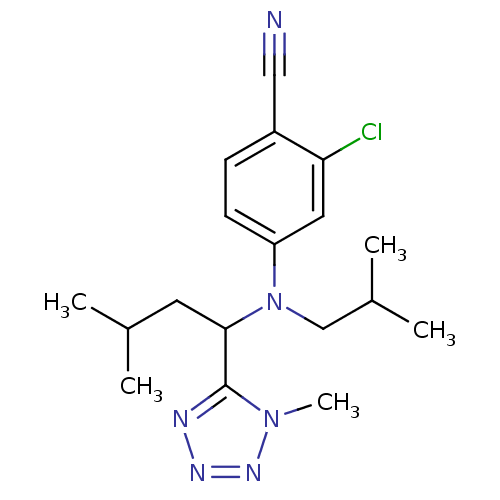 (2-chloro-4-(isobutyl(3-methyl-1-(1-methyl-1H-tetra...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294712 ((S)-2-chloro-4-((cyclobutylmethyl)(3-methyl-1-(1-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294714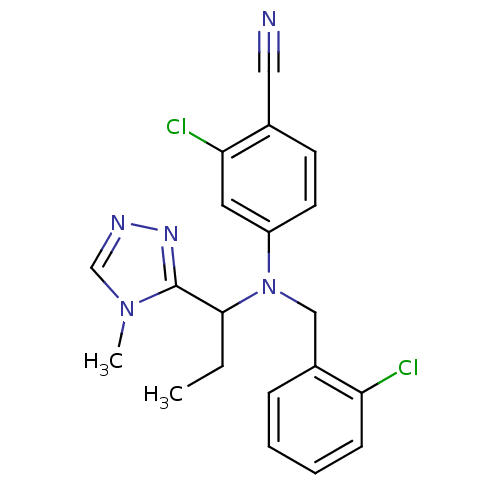 (4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM50296009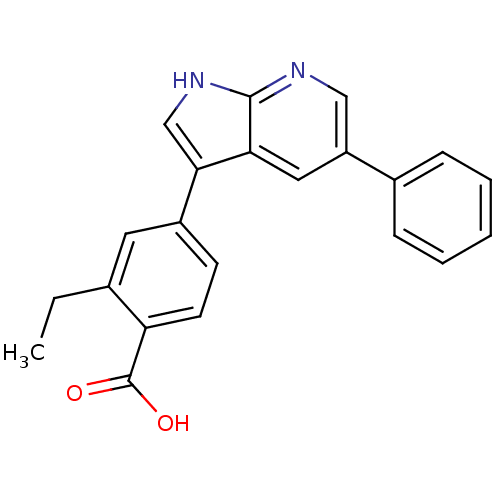 (2-ethyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of SGK1 by fluorescence polarization assay | Bioorg Med Chem Lett 19: 4441-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.051 BindingDB Entry DOI: 10.7270/Q2WQ03VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 515 total ) | Next | Last >> |