 Found 239 hits with Last Name = 'marmorstein' and Initial = 'r'
Found 239 hits with Last Name = 'marmorstein' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25391
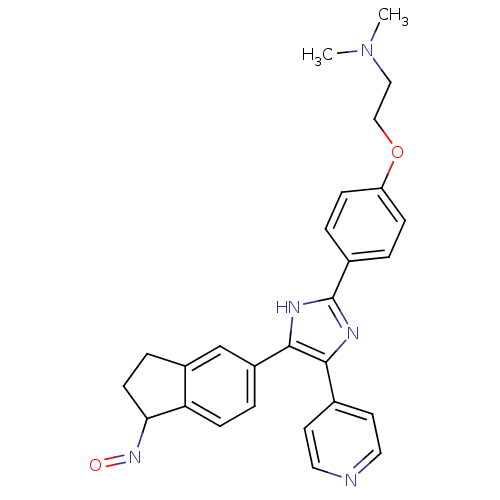
(CHEMBL200622 | SB-590885 | SB590885 | [2-(4-{4-[(1...)Show SMILES CN(C)CCOc1ccc(cc1)-c1nc(c([nH]1)-c1ccc2C(CCc2c1)N=O)-c1ccncc1 Show InChI InChI=1S/C27H27N5O2/c1-32(2)15-16-34-22-7-3-19(4-8-22)27-29-25(18-11-13-28-14-12-18)26(30-27)21-5-9-23-20(17-21)6-10-24(23)31-33/h3-5,7-9,11-14,17,24H,6,10,15-16H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Pennsylvania
| Assay Description
BRAF kinase activity was quantified using an ELISA-based MEK phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curve f... |
J Med Chem 51: 6121-7 (2008)
Article DOI: 10.1021/jm800539g
BindingDB Entry DOI: 10.7270/Q2BC3WVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581153

(CHEMBL5081275)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human NatD using various concentration of human H4 peptide and fixed [14C]acetyl-CoA as substrate measured after 13 mins ra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581153

(CHEMBL5081275)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148928

(CHEMBL3770909)Show SMILES CCNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(C[C@H]3CC[C@H](N)CC3)c2n1 |r,wU:27.28,wD:30.32,(-6.42,1.38,;-5.35,.76,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;2.68,5.39,;4.01,6.15,;5.34,5.38,;6.41,5.99,;5.34,3.84,;4,3.07,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30ClN7O/c1-3-31-27-32-13-19-10-22(21-9-6-18(11-23(21)28)24-14-30-12-16(2)33-24)26(36)35(25(19)34-27)15-17-4-7-20(29)8-5-17/h6,9-14,17,20H,3-5,7-8,15,29H2,1-2H3,(H,31,32,34)/t17-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148920
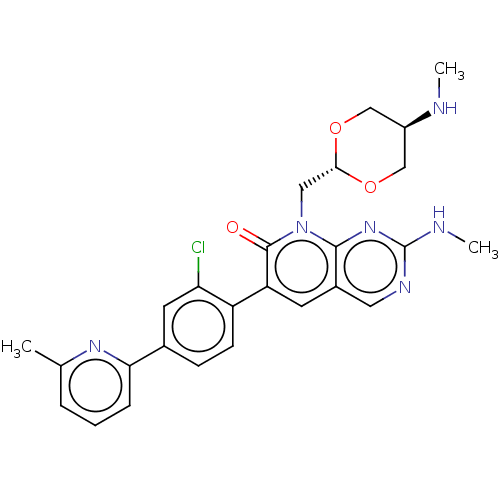
(CHEMBL3769478)Show SMILES CN[C@H]1CO[C@H](Cn2c3nc(NC)ncc3cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c2=O)OC1 |r,wU:2.1,wD:5.5,(6.68,-7.38,;6.68,-6.15,;5.34,-5.38,;4.01,-6.15,;2.68,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-4.01,-1.54,;-4.01,-2.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;4,1.54,;5.33,.77,;6.66,1.53,;6.67,3.07,;5.33,3.85,;4,3.08,;2.93,3.7,;8,3.84,;9.34,3.07,;10.67,3.84,;10.67,5.38,;9.34,6.15,;9.34,7.39,;8,5.38,;2.66,-.77,;3.73,-1.38,;4,-3.07,;5.34,-3.84,)| Show InChI InChI=1S/C26H27ClN6O3/c1-15-5-4-6-22(31-15)16-7-8-19(21(27)10-16)20-9-17-11-30-26(29-3)32-24(17)33(25(20)34)12-23-35-13-18(28-2)14-36-23/h4-11,18,23,28H,12-14H2,1-3H3,(H,29,30,32)/t18-,23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581149

(CHEMBL5077025)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148927

(CHEMBL3770588)Show SMILES CCNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(C[C@H]3CC[C@H](N)CC3)c2n1 |r,wU:27.28,wD:30.32,(-6.42,1.38,;-5.35,.76,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;2.68,5.39,;4.01,6.15,;5.34,5.38,;6.41,5.99,;5.34,3.84,;4,3.07,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H31ClN6O/c1-3-31-28-32-15-20-13-23(22-12-9-19(14-24(22)29)25-6-4-5-17(2)33-25)27(36)35(26(20)34-28)16-18-7-10-21(30)11-8-18/h4-6,9,12-15,18,21H,3,7-8,10-11,16,30H2,1-2H3,(H,31,32,34)/t18-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148921

(CHEMBL3770443)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:26.27,wD:29.31,(-5.08,.92,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;2.68,5.39,;4.01,6.15,;5.34,5.38,;6.41,5.99,;5.34,3.84,;4,3.07,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H25ClN6O3/c1-14-4-3-5-21(30-14)15-6-7-18(20(26)9-15)19-8-16-10-29-25(28-2)31-23(16)32(24(19)33)11-22-34-12-17(27)13-35-22/h3-10,17,22H,11-13,27H2,1-2H3,(H,28,29,31)/t17-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581154
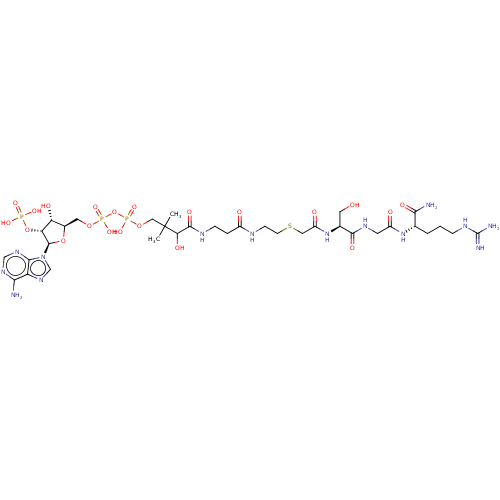
(CHEMBL5090533)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148919
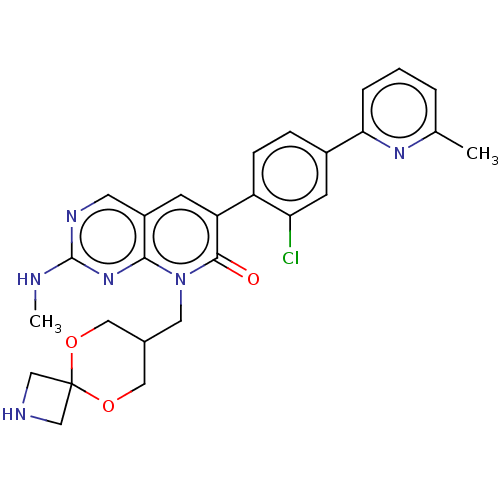
(CHEMBL3770363)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(CC3COC4(CNC4)OC3)c2n1 Show InChI InChI=1S/C27H27ClN6O3/c1-16-4-3-5-23(32-16)18-6-7-20(22(28)9-18)21-8-19-10-31-26(29-2)33-24(19)34(25(21)35)11-17-12-36-27(37-13-17)14-30-15-27/h3-10,17,30H,11-15H2,1-2H3,(H,29,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581152
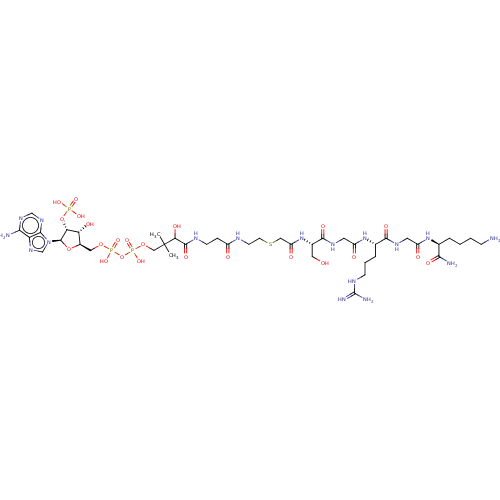
(CHEMBL5093444)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148930

(CHEMBL3769748)Show SMILES CCNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(CC3CCNCC3)c2n1 Show InChI InChI=1S/C26H28ClN7O/c1-3-30-26-31-13-19-10-21(25(35)34(24(19)33-26)15-17-6-8-28-9-7-17)20-5-4-18(11-22(20)27)23-14-29-12-16(2)32-23/h4-5,10-14,17,28H,3,6-9,15H2,1-2H3,(H,30,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148929

(CHEMBL3770369)Show SMILES CCNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(CCCCN)c2n1 Show InChI InChI=1S/C24H26ClN7O/c1-3-28-24-29-13-17-10-19(23(33)32(22(17)31-24)9-5-4-8-26)18-7-6-16(11-20(18)25)21-14-27-12-15(2)30-21/h6-7,10-14H,3-5,8-9,26H2,1-2H3,(H,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581150
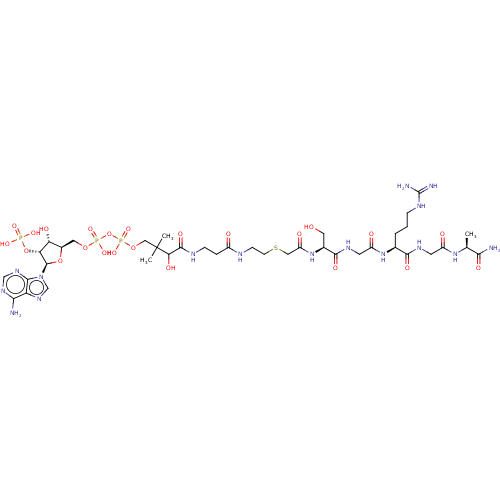
(CHEMBL5075935)Show SMILES C[C@H](NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CO)NC(=O)CSCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148926
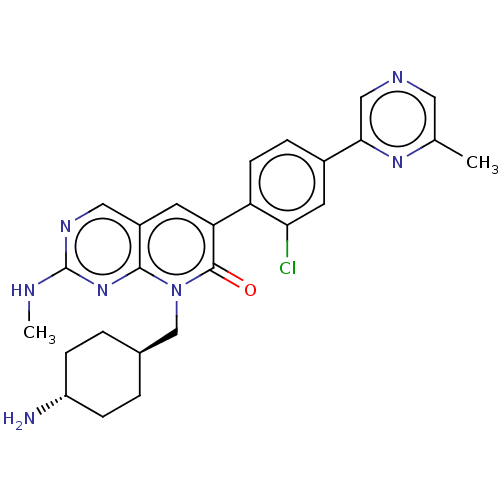
(CHEMBL3769456)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(C[C@H]3CC[C@H](N)CC3)c2n1 |r,wU:29.31,wD:26.27,(-5.08,.92,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;4,3.07,;5.34,3.84,;5.34,5.38,;6.41,5.99,;4.01,6.15,;2.68,5.39,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H28ClN7O/c1-15-11-30-13-23(32-15)17-5-8-20(22(27)10-17)21-9-18-12-31-26(29-2)33-24(18)34(25(21)35)14-16-3-6-19(28)7-4-16/h5,8-13,16,19H,3-4,6-7,14,28H2,1-2H3,(H,29,31,33)/t16-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148918
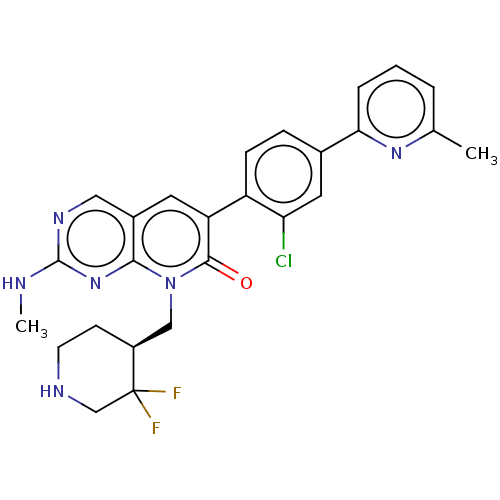
(CHEMBL3769552)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(C[C@@H]3CCNCC3(F)F)c2n1 |r| Show InChI InChI=1S/C26H25ClF2N6O/c1-15-4-3-5-22(33-15)16-6-7-19(21(27)11-16)20-10-17-12-32-25(30-2)34-23(17)35(24(20)36)13-18-8-9-31-14-26(18,28)29/h3-7,10-12,18,31H,8-9,13-14H2,1-2H3,(H,30,32,34)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148922

(CHEMBL3770295)Show SMILES CCNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:30.32,wD:27.28,(-6.42,1.38,;-5.35,.76,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;4,3.07,;5.34,3.84,;5.34,5.38,;6.41,5.99,;4.01,6.15,;2.68,5.39,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H26ClN7O3/c1-3-29-25-30-9-16-6-19(18-5-4-15(7-20(18)26)21-10-28-8-14(2)31-21)24(34)33(23(16)32-25)11-22-35-12-17(27)13-36-22/h4-10,17,22H,3,11-13,27H2,1-2H3,(H,29,30,32)/t17-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581155
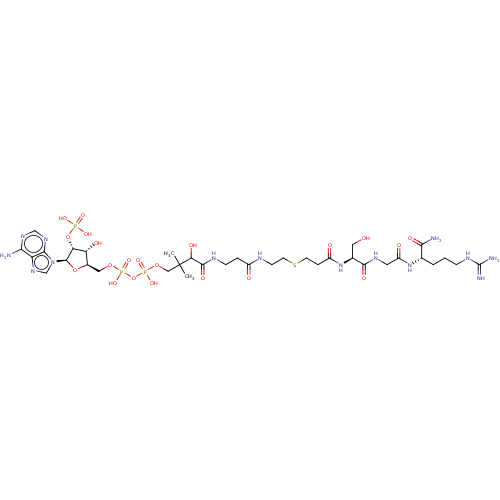
(CHEMBL5083117)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581151
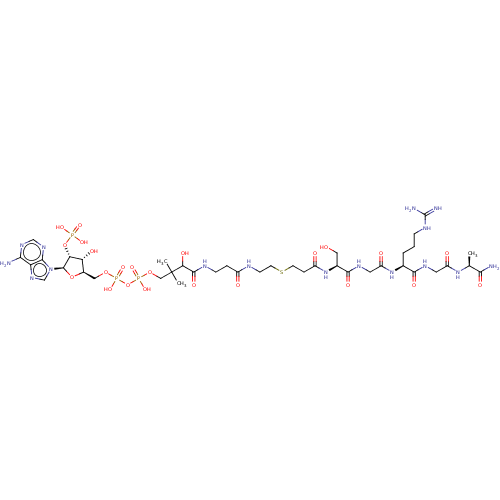
(CHEMBL5077510)Show SMILES C[C@H](NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CO)NC(=O)CCSCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148923
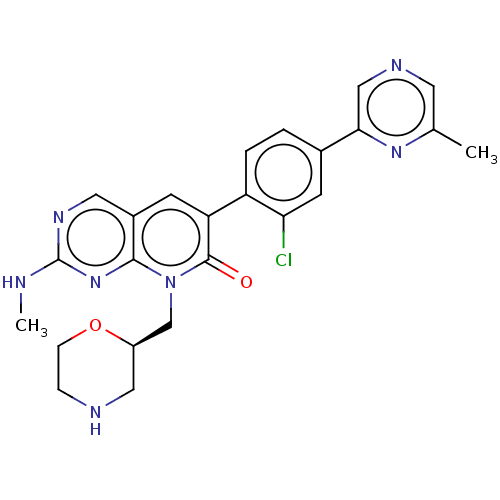
(CHEMBL3770943)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(C[C@H]3CNCCO3)c2n1 |r| Show InChI InChI=1S/C24H24ClN7O2/c1-14-9-28-12-21(30-14)15-3-4-18(20(25)8-15)19-7-16-10-29-24(26-2)31-22(16)32(23(19)33)13-17-11-27-5-6-34-17/h3-4,7-10,12,17,27H,5-6,11,13H2,1-2H3,(H,26,29,31)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148931
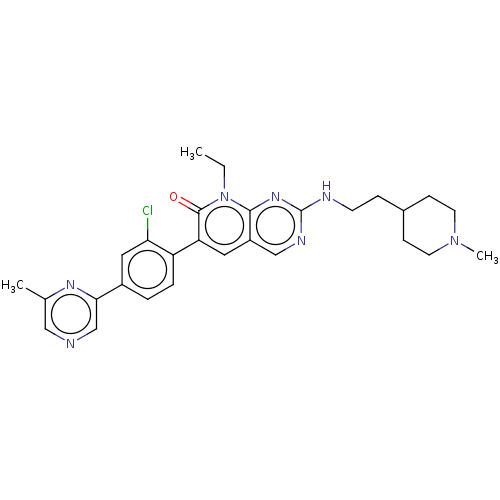
(CHEMBL3770186)Show SMILES CCn1c2nc(NCCC3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncc(C)n2)c1=O Show InChI InChI=1S/C28H32ClN7O/c1-4-36-26-21(16-32-28(34-26)31-10-7-19-8-11-35(3)12-9-19)13-23(27(36)37)22-6-5-20(14-24(22)29)25-17-30-15-18(2)33-25/h5-6,13-17,19H,4,7-12H2,1-3H3,(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 38 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Pennsylvania
| Assay Description
BRAF kinase activity was quantified using an ELISA-based MEK phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curve f... |
J Med Chem 51: 6121-7 (2008)
Article DOI: 10.1021/jm800539g
BindingDB Entry DOI: 10.7270/Q2BC3WVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148925
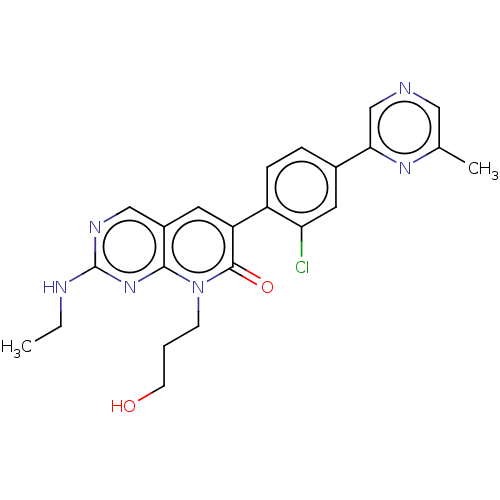
(CHEMBL3770806)Show SMILES CCNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(CCCO)c2n1 Show InChI InChI=1S/C23H23ClN6O2/c1-3-26-23-27-12-16-9-18(22(32)30(7-4-8-31)21(16)29-23)17-6-5-15(10-19(17)24)20-13-25-11-14(2)28-20/h5-6,9-13,31H,3-4,7-8H2,1-2H3,(H,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148924

(CHEMBL3769981)Show SMILES CCNc1ncc2cc(-c3ccc(cc3Cl)-c3cncc(C)n3)c(=O)n(CC3CCNC(=O)C3)c2n1 Show InChI InChI=1S/C26H26ClN7O2/c1-3-29-26-31-12-18-9-20(19-5-4-17(10-21(19)27)22-13-28-11-15(2)32-22)25(36)34(24(18)33-26)14-16-6-7-30-23(35)8-16/h4-5,9-13,16H,3,6-8,14H2,1-2H3,(H,30,35)(H,29,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate preincubated for 10 mins followed by ... |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25381
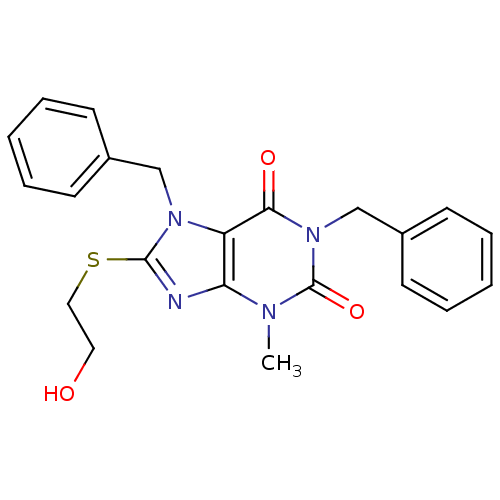
(1,7-dibenzyl-8-[(2-hydroxyethyl)sulfanyl]-3-methyl...)Show SMILES Cn1c2nc(SCCO)n(Cc3ccccc3)c2c(=O)n(Cc2ccccc2)c1=O Show InChI InChI=1S/C22H22N4O3S/c1-24-19-18(20(28)26(22(24)29)15-17-10-6-3-7-11-17)25(21(23-19)30-13-12-27)14-16-8-4-2-5-9-16/h2-11,27H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | -41.4 | 1.72E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Pennsylvania
| Assay Description
BRAF kinase activity was quantified using an ELISA-based MEK phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curve f... |
J Med Chem 51: 6121-7 (2008)
Article DOI: 10.1021/jm800539g
BindingDB Entry DOI: 10.7270/Q2BC3WVM |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304357
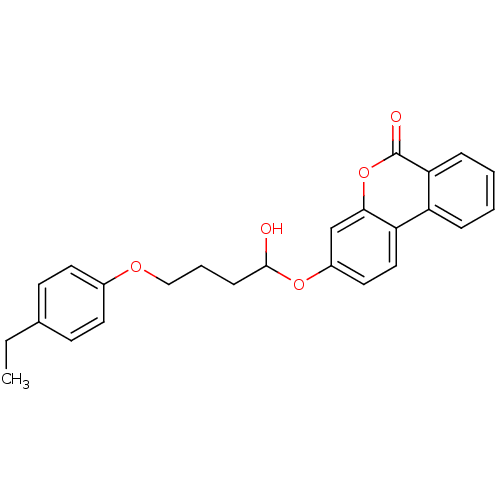
(4-(4-ethylphenoxy)-butyric acid 6-oxo-6H-benzo[c]c...)Show SMILES CCc1ccc(OCCCC(O)Oc2ccc3c(c2)oc(=O)c2ccccc32)cc1 Show InChI InChI=1S/C25H24O5/c1-2-17-9-11-18(12-10-17)28-15-5-8-24(26)29-19-13-14-21-20-6-3-4-7-22(20)25(27)30-23(21)16-19/h3-4,6-7,9-14,16,24,26H,2,5,8,15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of yeast Hst2 using NAD+ substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304355

(3,4,6-trichloro-2-(2,5-dichloro-6-hydroxy-3-methyl...)Show InChI InChI=1S/C14H9Cl5O2/c1-5-2-9(16)13(20)6(11(5)18)3-7-12(19)8(15)4-10(17)14(7)21/h2,4,20-21H,3H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of yeast Hst2 using NAD+ substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304355

(3,4,6-trichloro-2-(2,5-dichloro-6-hydroxy-3-methyl...)Show InChI InChI=1S/C14H9Cl5O2/c1-5-2-9(16)13(20)6(11(5)18)3-7-12(19)8(15)4-10(17)14(7)21/h2,4,20-21H,3H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Mixed inhibition of yeast Hst2 using Acetyl-lysine substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581148
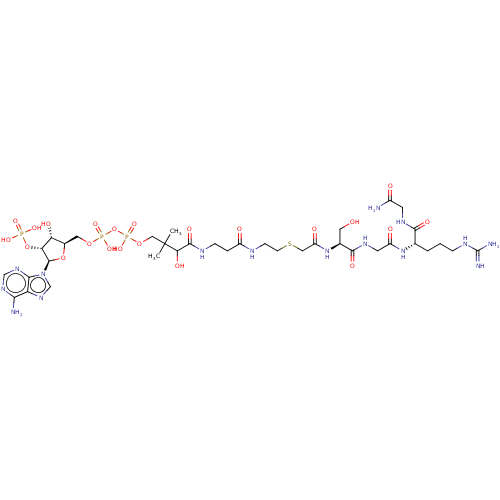
(CHEMBL5075404)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at Km value and AcCoA by Morrison's quadratic equation analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304357

(4-(4-ethylphenoxy)-butyric acid 6-oxo-6H-benzo[c]c...)Show SMILES CCc1ccc(OCCCC(O)Oc2ccc3c(c2)oc(=O)c2ccccc32)cc1 Show InChI InChI=1S/C25H24O5/c1-2-17-9-11-18(12-10-17)28-15-5-8-24(26)29-19-13-14-21-20-6-3-4-7-22(20)25(27)30-23(21)16-19/h3-4,6-7,9-14,16,24,26H,2,5,8,15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of yeast Hst2 using Acetyl-lysine substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304354

(3-(1-Oxo-1,3-dihydro-isoindol-2-yl)-propionic acid...)Show InChI InChI=1S/C11H11NO3/c13-10(14)5-6-12-7-8-3-1-2-4-9(8)11(12)15/h1-4H,5-7H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of yeast Hst2 using NAD+ substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304354

(3-(1-Oxo-1,3-dihydro-isoindol-2-yl)-propionic acid...)Show InChI InChI=1S/C11H11NO3/c13-10(14)5-6-12-7-8-3-1-2-4-9(8)11(12)15/h1-4H,5-7H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of yeast Hst2 using Acetyl-lysine substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304351

(6-methoxy-1-(3-methoxy-prop-1-ynyl)-2-methyl-1,2,3...)Show InChI InChI=1S/C15H19NO3/c1-16-7-6-11-9-15(19-3)14(17)10-12(11)13(16)5-4-8-18-2/h9-10,13,17H,6-8H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Mixed inhibition of yeast Hst2 using NAD+ substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase HST2
(Saccharomyces cerevisiae) | BDBM50304351

(6-methoxy-1-(3-methoxy-prop-1-ynyl)-2-methyl-1,2,3...)Show InChI InChI=1S/C15H19NO3/c1-16-7-6-11-9-15(19-3)14(17)10-12(11)13(16)5-4-8-18-2/h9-10,13,17H,6-8H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of yeast Hst2 using Acetyl-lysine substrate |
Bioorg Med Chem 17: 7031-41 (2009)
Article DOI: 10.1016/j.bmc.2009.07.073
BindingDB Entry DOI: 10.7270/Q21V5FWG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM36523
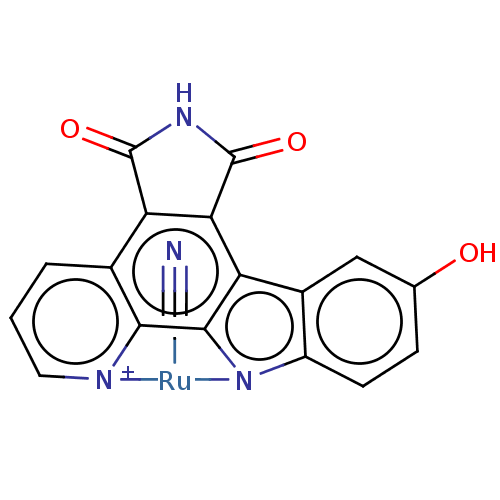
(DW12)Show SMILES Oc1ccc2n3[Ru](C#N)[n+]4cccc5c6C(=O)NC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C17H10N3O3.CO.Ru/c21-7-3-4-10-9(6-7)11-13-12(16(22)20-17(13)23)8-2-1-5-18-14(8)15(11)19-10;1-2;/h1-7H,(H3,18,19,20,21,22,23);;/q;;+2/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Pennsylvania
| Assay Description
Fluorescence polarization-based kinase in vitro assay using human PI3K alpha purchased from Echelon Biosciences and p110 alpha/p85 alpha from Upstate... |
ACS Chem Biol 3: 305-16 (2008)
Article DOI: 10.1021/cb800039y
BindingDB Entry DOI: 10.7270/Q26D5RB3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM99471

(US8497274, 32)Show SMILES Fc1ccc(Oc2ccc3nc(NC(=O)C4CC4)sc3c2C#N)cc1NC(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H18F4N4O3S/c28-19-7-6-17(12-21(19)33-23(36)11-14-2-1-3-16(10-14)27(29,30)31)38-22-9-8-20-24(18(22)13-32)39-26(34-20)35-25(37)15-4-5-15/h1-3,6-10,12,15H,4-5,11H2,(H,33,36)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6x-His-tagged BRAF kinase domain (unknown origin) expressed in baculovirus infected Sf9 insect cells co-expressing mouse p50... |
J Med Chem 61: 5034-5046 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00499
BindingDB Entry DOI: 10.7270/Q2R49TDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581153

(CHEMBL5081275)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at 4 times Km value and AcCoA measured after 30 min incubation by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM99471

(US8497274, 32)Show SMILES Fc1ccc(Oc2ccc3nc(NC(=O)C4CC4)sc3c2C#N)cc1NC(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H18F4N4O3S/c28-19-7-6-17(12-21(19)33-23(36)11-14-2-1-3-16(10-14)27(29,30)31)38-22-9-8-20-24(18(22)13-32)39-26(34-20)35-25(37)15-4-5-15/h1-3,6-10,12,15H,4-5,11H2,(H,33,36)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6x-His-tagged BRAF V600E mutant (unknown origin) expressed in baculovirus infected Sf9 insect cells co-expressing mouse p50c... |
J Med Chem 61: 5034-5046 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00499
BindingDB Entry DOI: 10.7270/Q2R49TDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50462458
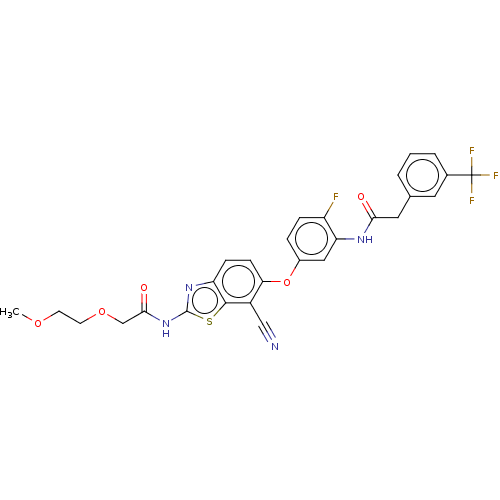
(CHEMBL4237480)Show SMILES COCCOCC(=O)Nc1nc2ccc(Oc3ccc(F)c(NC(=O)Cc4cccc(c4)C(F)(F)F)c3)c(C#N)c2s1 Show InChI InChI=1S/C28H22F4N4O5S/c1-39-9-10-40-15-25(38)36-27-35-21-7-8-23(19(14-33)26(21)42-27)41-18-5-6-20(29)22(13-18)34-24(37)12-16-3-2-4-17(11-16)28(30,31)32/h2-8,11,13H,9-10,12,15H2,1H3,(H,34,37)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6x-His-tagged BRAF kinase domain (unknown origin) expressed in baculovirus infected Sf9 insect cells co-expressing mouse p50... |
J Med Chem 61: 5034-5046 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00499
BindingDB Entry DOI: 10.7270/Q2R49TDJ |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581149

(CHEMBL5077025)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at 4 times Km value and AcCoA measured after 30 min incubation by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1 [249-545,K299R]
(Homo sapiens (Human)) | BDBM50112347
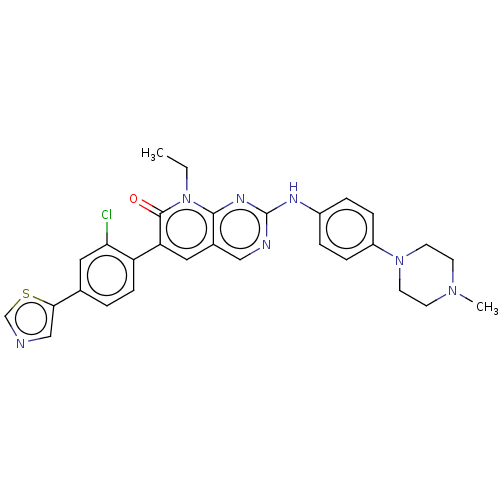
(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| Assay Description
IC50 values were determined using a 10 concentration point, non-radioactive, functional assay that employs a fluorescence-based, coupled enzyme forma... |
J Biol Chem 288: 29105-14 (2013)
Article DOI: 10.1074/jbc.M113.510933
BindingDB Entry DOI: 10.7270/Q2B27T4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase SIK2
(Homo sapiens (Human)) | BDBM50148921

(CHEMBL3770443)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:26.27,wD:29.31,(-5.08,.92,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;2.68,5.39,;4.01,6.15,;5.34,5.38,;6.41,5.99,;5.34,3.84,;4,3.07,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H25ClN6O3/c1-14-4-3-5-21(30-14)15-6-7-18(20(26)9-15)19-8-16-10-29-25(28-2)31-23(16)32(24(19)33)11-22-34-12-17(27)13-35-22/h3-10,17,22H,11-13,27H2,1-2H3,(H,28,29,31)/t17-,22- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human SIK2 |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 5
(Homo sapiens (Human)) | BDBM50148921

(CHEMBL3770443)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:26.27,wD:29.31,(-5.08,.92,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;2.68,5.39,;4.01,6.15,;5.34,5.38,;6.41,5.99,;5.34,3.84,;4,3.07,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H25ClN6O3/c1-14-4-3-5-21(30-14)15-6-7-18(20(26)9-15)19-8-16-10-29-25(28-2)31-23(16)32(24(19)33)11-22-34-12-17(27)13-35-22/h3-10,17,22H,11-13,27H2,1-2H3,(H,28,29,31)/t17-,22- | NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KHS1 |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM50148921

(CHEMBL3770443)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:26.27,wD:29.31,(-5.08,.92,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;2.68,5.39,;4.01,6.15,;5.34,5.38,;6.41,5.99,;5.34,3.84,;4,3.07,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H25ClN6O3/c1-14-4-3-5-21(30-14)15-6-7-18(20(26)9-15)19-8-16-10-29-25(28-2)31-23(16)32(24(19)33)11-22-34-12-17(27)13-35-22/h3-10,17,22H,11-13,27H2,1-2H3,(H,28,29,31)/t17-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK2 |
ACS Med Chem Lett 6: 1241-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00398
BindingDB Entry DOI: 10.7270/Q2MP554V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM50112347

(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| Assay Description
IC50 values were determined using a 10 concentration point, non-radioactive, functional assay that employs a fluorescence-based, coupled enzyme forma... |
J Biol Chem 288: 29105-14 (2013)
Article DOI: 10.1074/jbc.M113.510933
BindingDB Entry DOI: 10.7270/Q2B27T4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581154

(CHEMBL5090533)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at 4 times Km value and AcCoA measured after 30 min incubation by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 3
(Homo sapiens (Human)) | BDBM50112347

(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 19.3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| Assay Description
IC50 values were determined using a 10 concentration point, non-radioactive, functional assay that employs a fluorescence-based, coupled enzyme forma... |
J Biol Chem 288: 29105-14 (2013)
Article DOI: 10.1074/jbc.M113.510933
BindingDB Entry DOI: 10.7270/Q2B27T4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50462457
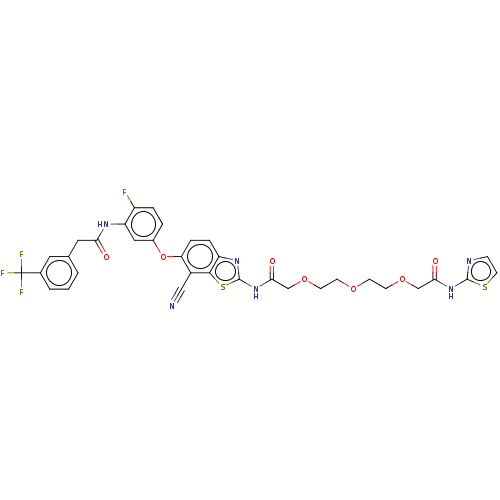
(CHEMBL4241641)Show SMILES Fc1ccc(Oc2ccc3nc(NC(=O)COCCOCCOCC(=O)Nc4nccs4)sc3c2C#N)cc1NC(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C34H28F4N6O7S2/c35-24-5-4-22(16-26(24)41-28(45)15-20-2-1-3-21(14-20)34(36,37)38)51-27-7-6-25-31(23(27)17-39)53-33(42-25)44-30(47)19-50-12-10-48-9-11-49-18-29(46)43-32-40-8-13-52-32/h1-8,13-14,16H,9-12,15,18-19H2,(H,41,45)(H,40,43,46)(H,42,44,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6x-His-tagged BRAF kinase domain (unknown origin) expressed in baculovirus infected Sf9 insect cells co-expressing mouse p50... |
J Med Chem 61: 5034-5046 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00499
BindingDB Entry DOI: 10.7270/Q2R49TDJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1 [249-545,K299R]
(Homo sapiens (Human)) | BDBM213235

(FRAX414)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(Cl)cc2Cl)c1=O Show InChI InChI=1S/C26H26Cl2N6O/c1-3-34-24-17(14-22(25(34)35)21-9-4-18(27)15-23(21)28)16-29-26(31-24)30-19-5-7-20(8-6-19)33-12-10-32(2)11-13-33/h4-9,14-16H,3,10-13H2,1-2H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| Assay Description
IC50 values were determined using a 10 concentration point, non-radioactive, functional assay that employs a fluorescence-based, coupled enzyme forma... |
J Biol Chem 288: 29105-14 (2013)
Article DOI: 10.1074/jbc.M113.510933
BindingDB Entry DOI: 10.7270/Q2B27T4S |
More data for this
Ligand-Target Pair | |
N-alpha-acetyltransferase 40
(Homo sapiens) | BDBM50581152

(CHEMBL5093444)Show SMILES CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](OP(O)(O)=O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)C(=O)NCCC(=O)NCCSCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NatD using H4-8 peptide substrate at 4 times Km value and AcCoA measured after 30 min incubation by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00141
BindingDB Entry DOI: 10.7270/Q2XK8KFR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data