 Found 46 hits with Last Name = 'marotti' and Initial = 'kr'
Found 46 hits with Last Name = 'marotti' and Initial = 'kr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205450
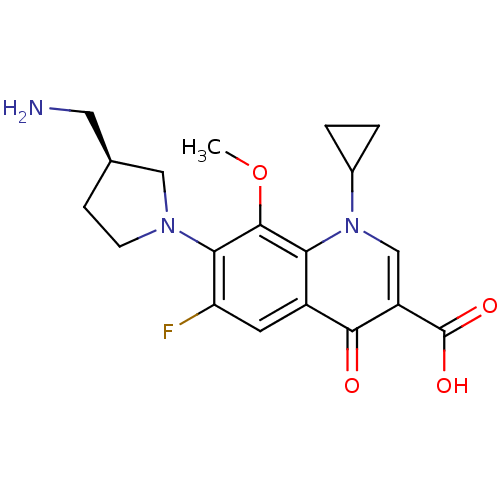
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(N2CC[C@@H](CN)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C19H22FN3O4/c1-27-18-15-12(6-14(20)16(18)22-5-4-10(7-21)8-22)17(24)13(19(25)26)9-23(15)11-2-3-11/h6,9-11H,2-5,7-8,21H2,1H3,(H,25,26)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205452
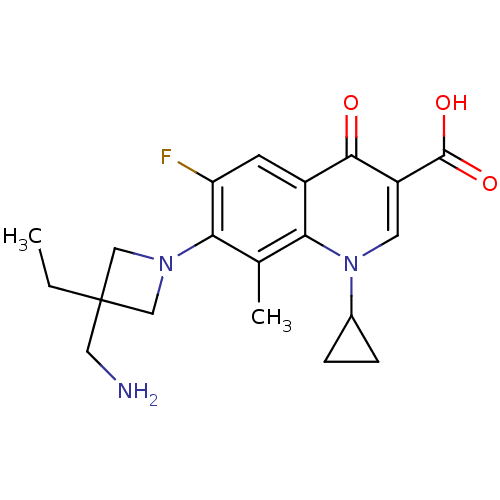
(7-(3-(aminomethyl)-3-ethylazetidin-1-yl)-1-cyclopr...)Show SMILES CCC1(CN)CN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O3/c1-3-20(8-22)9-23(10-20)17-11(2)16-13(6-15(17)21)18(25)14(19(26)27)7-24(16)12-4-5-12/h6-7,12H,3-5,8-10,22H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205465
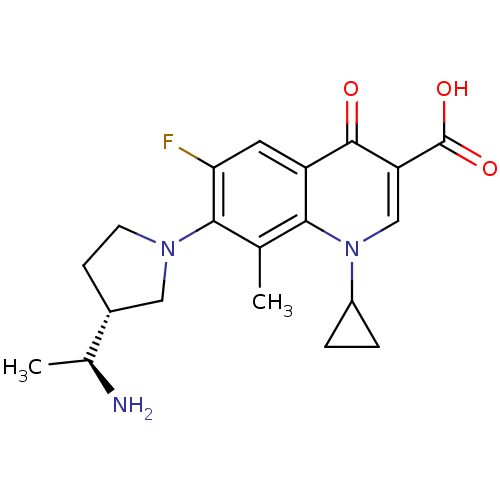
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES C[C@H](N)[C@@H]1CCN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O3/c1-10-17-14(19(25)15(20(26)27)9-24(17)13-3-4-13)7-16(21)18(10)23-6-5-12(8-23)11(2)22/h7,9,11-13H,3-6,8,22H2,1-2H3,(H,26,27)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205451
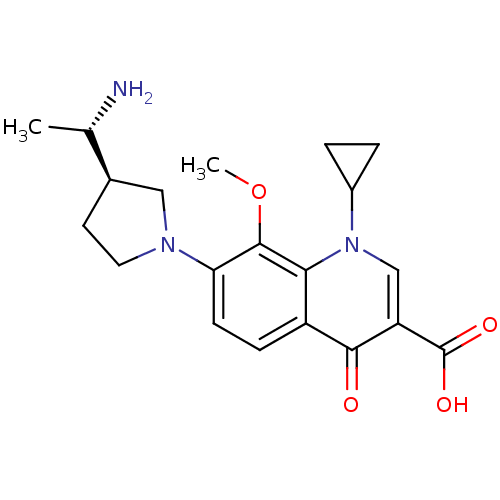
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@H](C)N Show InChI InChI=1S/C20H25N3O4/c1-11(21)12-7-8-22(9-12)16-6-5-14-17(19(16)27-2)23(13-3-4-13)10-15(18(14)24)20(25)26/h5-6,10-13H,3-4,7-9,21H2,1-2H3,(H,25,26)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205466
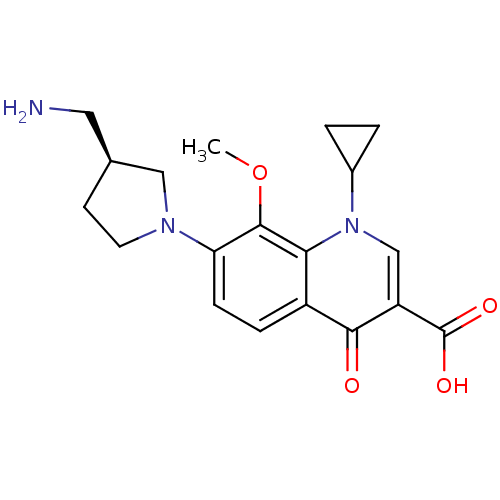
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@@H](CN)C1 Show InChI InChI=1S/C19H23N3O4/c1-26-18-15(21-7-6-11(8-20)9-21)5-4-13-16(18)22(12-2-3-12)10-14(17(13)23)19(24)25/h4-5,10-12H,2-3,6-9,20H2,1H3,(H,24,25)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205457

(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES C[C@H](NCCC#N)[C@@H]1CCN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O3/c1-13-20-17(22(29)18(23(30)31)12-28(20)16-4-5-16)10-19(24)21(13)27-9-6-15(11-27)14(2)26-8-3-7-25/h10,12,14-16,26H,3-6,8-9,11H2,1-2H3,(H,30,31)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205453
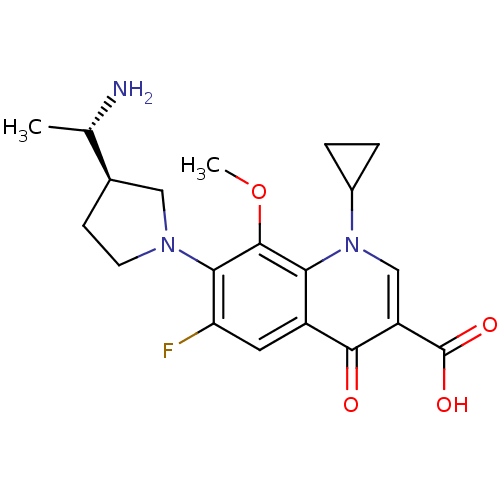
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES COc1c(N2CC[C@H](C2)[C@H](C)N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O4/c1-10(22)11-5-6-23(8-11)17-15(21)7-13-16(19(17)28-2)24(12-3-4-12)9-14(18(13)25)20(26)27/h7,9-12H,3-6,8,22H2,1-2H3,(H,26,27)/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205446
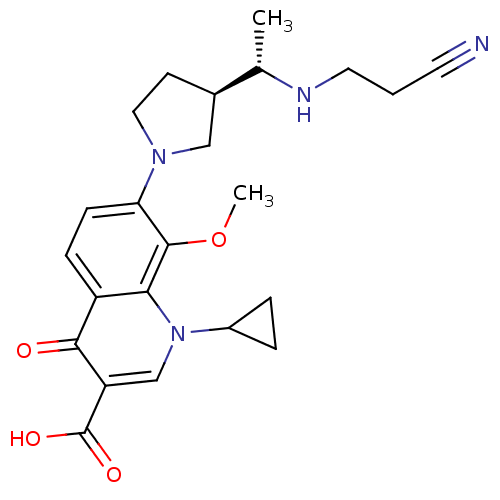
(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@H](C)NCCC#N Show InChI InChI=1S/C23H28N4O4/c1-14(25-10-3-9-24)15-8-11-26(12-15)19-7-6-17-20(22(19)31-2)27(16-4-5-16)13-18(21(17)28)23(29)30/h6-7,13-16,25H,3-5,8,10-12H2,1-2H3,(H,29,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205447

(10-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-9-flu...)Show SMILES C[C@H](N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:17.19| Show InChI InChI=1S/C19H22FN3O4/c1-9-8-27-18-15-12(17(24)13(19(25)26)7-23(9)15)5-14(20)16(18)22-4-3-11(6-22)10(2)21/h5,7,9-11H,3-4,6,8,21H2,1-2H3,(H,25,26)/t9?,10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205461
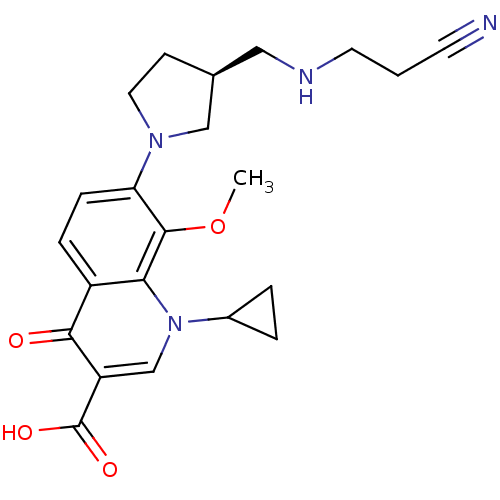
((S)-7-(3-((2-cyanoethylamino)methyl)pyrrolidin-1-y...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@@H](CNCCC#N)C1 Show InChI InChI=1S/C22H26N4O4/c1-30-21-18(25-10-7-14(12-25)11-24-9-2-8-23)6-5-16-19(21)26(15-3-4-15)13-17(20(16)27)22(28)29/h5-6,13-15,24H,2-4,7,9-12H2,1H3,(H,28,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205459
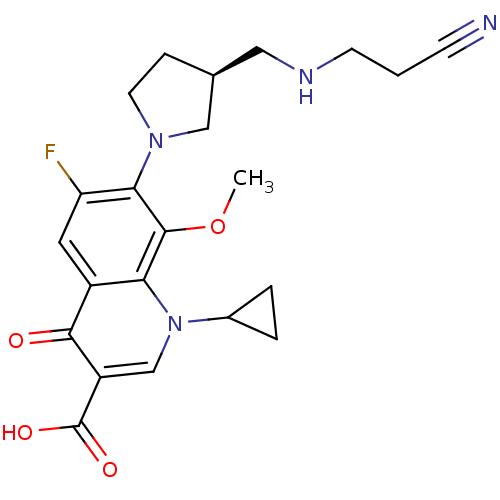
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(N2CC[C@@H](CNCCC#N)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O4/c1-31-21-18-15(20(28)16(22(29)30)12-27(18)14-3-4-14)9-17(23)19(21)26-8-5-13(11-26)10-25-7-2-6-24/h9,12-14,25H,2-5,7-8,10-11H2,1H3,(H,29,30)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205455
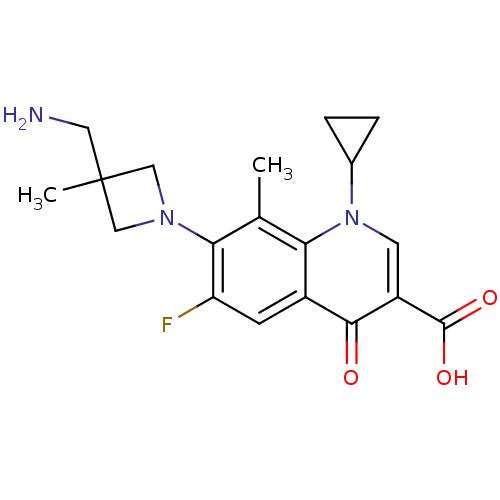
(7-(3-(aminomethyl)-3-methylazetidin-1-yl)-1-cyclop...)Show SMILES Cc1c(N2CC(C)(CN)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C19H22FN3O3/c1-10-15-12(5-14(20)16(10)22-8-19(2,7-21)9-22)17(24)13(18(25)26)6-23(15)11-3-4-11/h5-6,11H,3-4,7-9,21H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205463
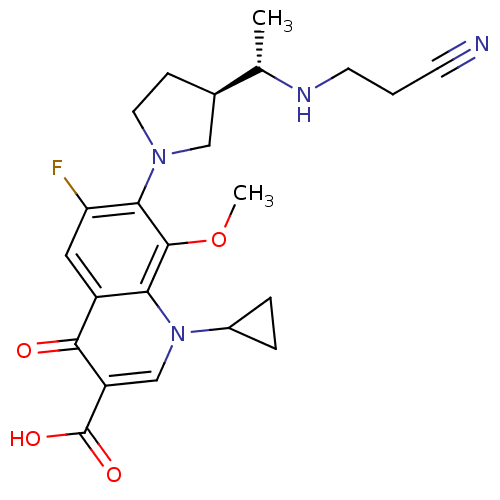
(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES COc1c(N2CC[C@H](C2)[C@H](C)NCCC#N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O4/c1-13(26-8-3-7-25)14-6-9-27(11-14)20-18(24)10-16-19(22(20)32-2)28(15-4-5-15)12-17(21(16)29)23(30)31/h10,12-15,26H,3-6,8-9,11H2,1-2H3,(H,30,31)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50131445
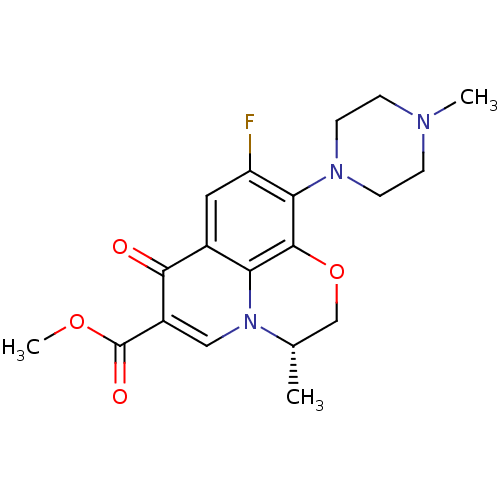
((3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)...)Show SMILES COC(=O)c1cn2[C@@H](C)COc3c(N4CCN(C)CC4)c(F)cc(c23)c1=O Show InChI InChI=1S/C19H22FN3O4/c1-11-10-27-18-15-12(17(24)13(9-23(11)15)19(25)26-3)8-14(20)16(18)22-6-4-21(2)5-7-22/h8-9,11H,4-7,10H2,1-3H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205458
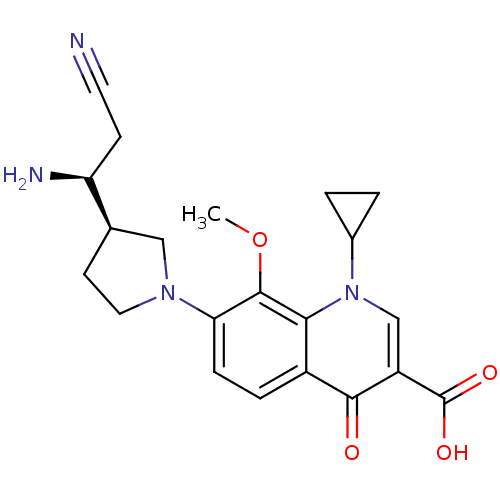
(7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@@H](N)CC#N Show InChI InChI=1S/C21H24N4O4/c1-29-20-17(24-9-7-12(10-24)16(23)6-8-22)5-4-14-18(20)25(13-2-3-13)11-15(19(14)26)21(27)28/h4-5,11-13,16H,2-3,6-7,9-10,23H2,1H3,(H,27,28)/t12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205460
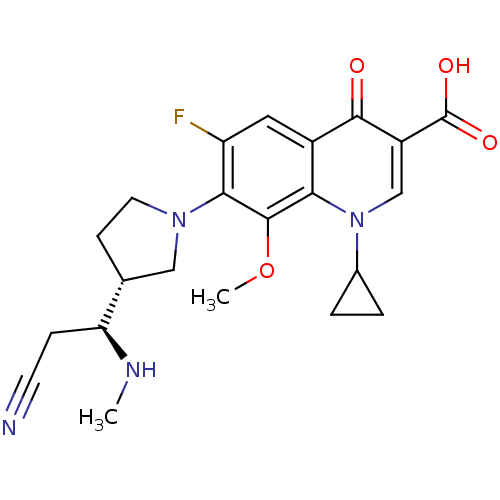
(7-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrroli...)Show SMILES CN[C@@H](CC#N)[C@@H]1CCN(C1)c1c(F)cc2c(c1OC)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O4/c1-25-17(5-7-24)12-6-8-26(10-12)19-16(23)9-14-18(21(19)31-2)27(13-3-4-13)11-15(20(14)28)22(29)30/h9,11-13,17,25H,3-6,8,10H2,1-2H3,(H,29,30)/t12-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205464
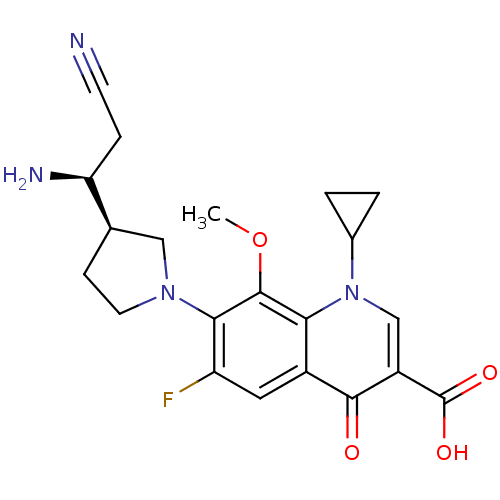
(7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...)Show SMILES COc1c(N2CC[C@H](C2)[C@@H](N)CC#N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C21H23FN4O4/c1-30-20-17-13(19(27)14(21(28)29)10-26(17)12-2-3-12)8-15(22)18(20)25-7-5-11(9-25)16(24)4-6-23/h8,10-12,16H,2-5,7,9,24H2,1H3,(H,28,29)/t11-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205467
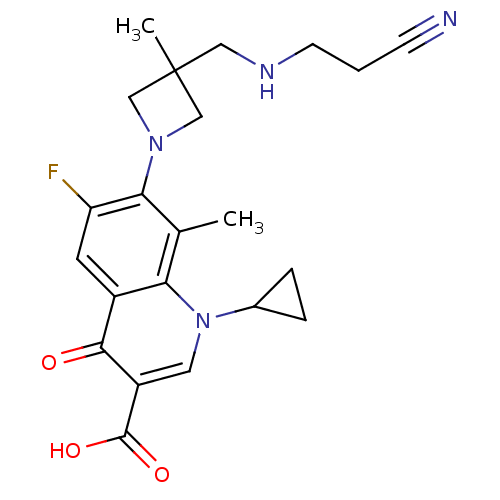
(7-(3-((2-cyanoethylamino)methyl)-3-methylazetidin-...)Show SMILES Cc1c(N2CC(C)(CNCCC#N)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O3/c1-13-18-15(20(28)16(21(29)30)9-27(18)14-4-5-14)8-17(23)19(13)26-11-22(2,12-26)10-25-7-3-6-24/h8-9,14,25H,3-5,7,10-12H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205448
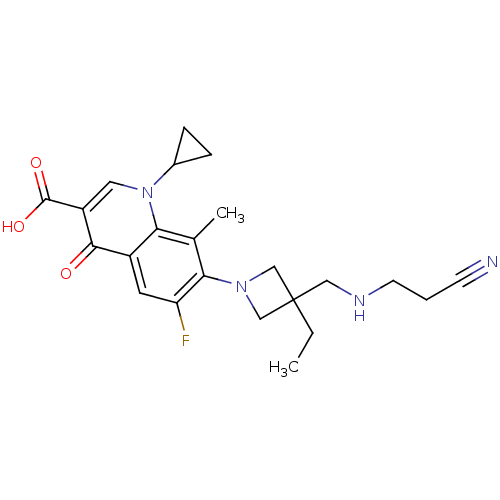
(7-(3-((2-cyanoethylamino)methyl)-3-ethylazetidin-1...)Show SMILES CCC1(CNCCC#N)CN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O3/c1-3-23(11-26-8-4-7-25)12-27(13-23)20-14(2)19-16(9-18(20)24)21(29)17(22(30)31)10-28(19)15-5-6-15/h9-10,15,26H,3-6,8,11-13H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205462
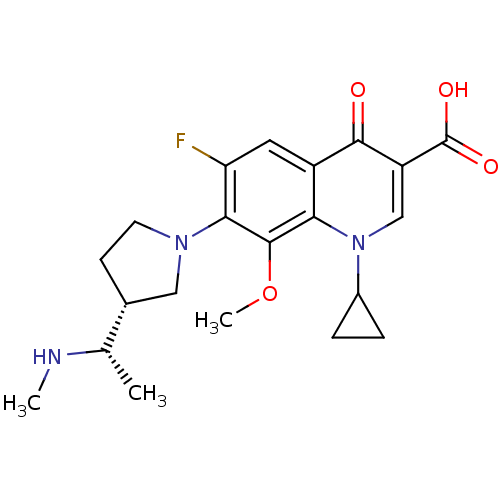
(1-cyclopropyl-6-fluoro-8-methoxy-7-((R)-3-((S)-1-(...)Show SMILES CN[C@@H](C)[C@@H]1CCN(C1)c1c(F)cc2c(c1OC)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C21H26FN3O4/c1-11(23-2)12-6-7-24(9-12)18-16(22)8-14-17(20(18)29-3)25(13-4-5-13)10-15(19(14)26)21(27)28/h8,10-13,23H,4-7,9H2,1-3H3,(H,27,28)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205456
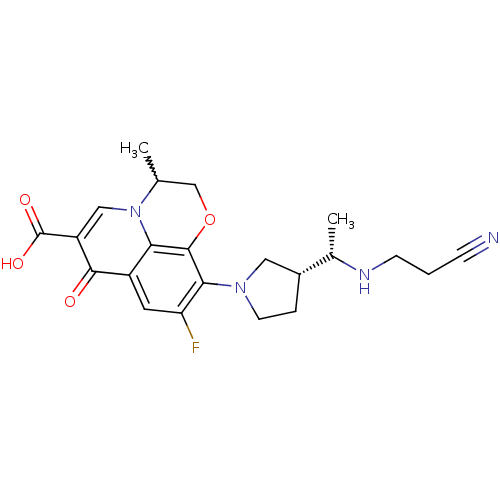
(10-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolid...)Show SMILES C[C@H](NCCC#N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:21.23| Show InChI InChI=1S/C22H25FN4O4/c1-12-11-31-21-18-15(20(28)16(22(29)30)10-27(12)18)8-17(23)19(21)26-7-4-14(9-26)13(2)25-6-3-5-24/h8,10,12-14,25H,3-4,6-7,9,11H2,1-2H3,(H,29,30)/t12?,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205454
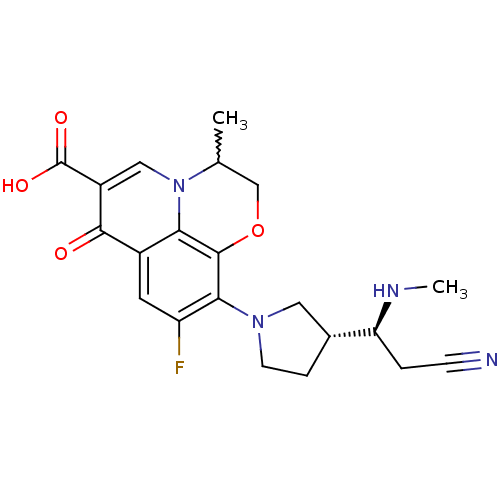
(10-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrrol...)Show SMILES CN[C@@H](CC#N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:20.22| Show InChI InChI=1S/C21H23FN4O4/c1-11-10-30-20-17-13(19(27)14(21(28)29)9-26(11)17)7-15(22)18(20)25-6-4-12(8-25)16(24-2)3-5-23/h7,9,11-12,16,24H,3-4,6,8,10H2,1-2H3,(H,28,29)/t11?,12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205449
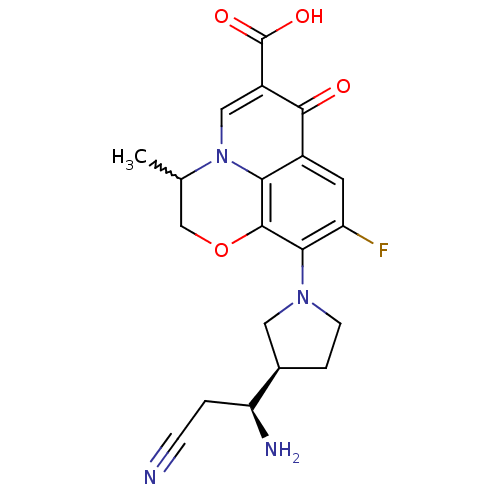
(10-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-y...)Show SMILES CC1COc2c(N3CC[C@H](C3)[C@@H](N)CC#N)c(F)cc3c2n1cc(C(O)=O)c3=O |w:1.0| Show InChI InChI=1S/C20H21FN4O4/c1-10-9-29-19-16-12(18(26)13(20(27)28)8-25(10)16)6-14(21)17(19)24-5-3-11(7-24)15(23)2-4-22/h6,8,10-11,15H,2-3,5,7,9,23H2,1H3,(H,27,28)/t10?,11-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102059

((3R,4R)-3-[2-(5-Bromo-2-chloro-phenyl)-ethyl]-4-fl...)Show InChI InChI=1S/C13H16BrClFN/c14-11-3-4-12(15)9(7-11)1-2-10-8-17-6-5-13(10)16/h3-4,7,10,13,17H,1-2,5-6,8H2/t10-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102064
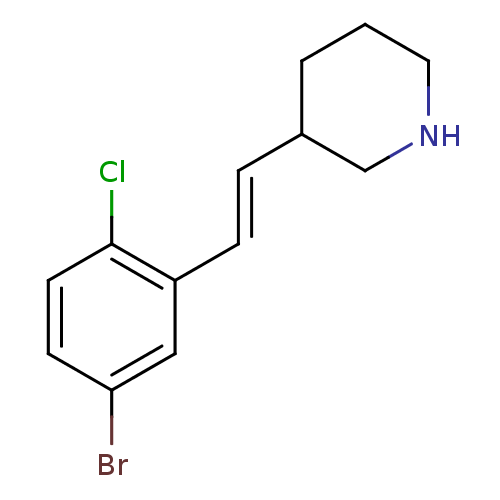
(3-[2-(5-Bromo-2-chloro-phenyl)-vinyl]-piperidine; ...)Show InChI InChI=1S/C13H15BrClN/c14-12-5-6-13(15)11(8-12)4-3-10-2-1-7-16-9-10/h3-6,8,10,16H,1-2,7,9H2/b4-3+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
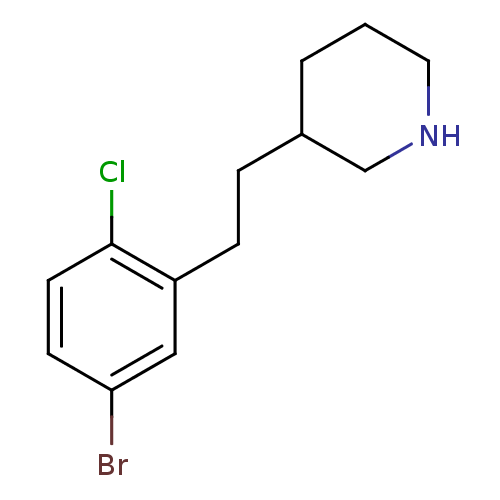
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102057
(3-[2-(5-Bromo-2-chloro-phenyl)-ethyl]-piperidine; ...)Show InChI InChI=1S/C13H17BrClN/c14-12-5-6-13(15)11(8-12)4-3-10-2-1-7-16-9-10/h5-6,8,10,16H,1-4,7,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
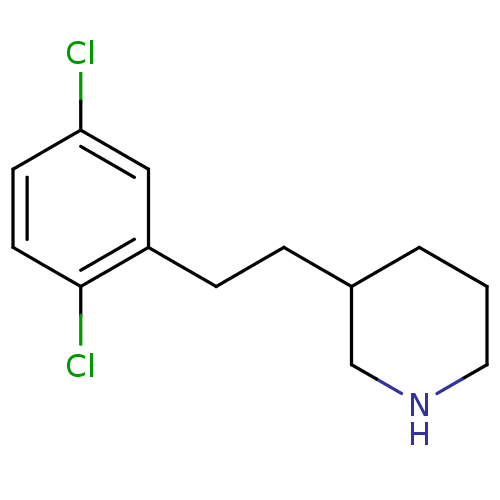
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102061
(3-[2-(2,5-Dichloro-phenyl)-ethyl]-piperidine | CHE...)Show InChI InChI=1S/C13H17Cl2N/c14-12-5-6-13(15)11(8-12)4-3-10-2-1-7-16-9-10/h5-6,8,10,16H,1-4,7,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102051
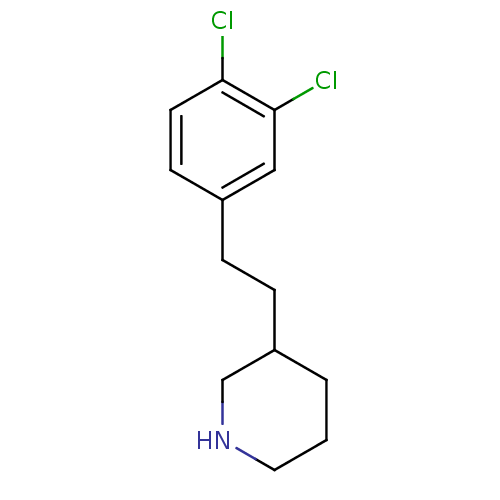
(3-[2-(3,4-Dichloro-phenyl)-ethyl]-piperidine | CHE...)Show InChI InChI=1S/C13H17Cl2N/c14-12-6-5-10(8-13(12)15)3-4-11-2-1-7-16-9-11/h5-6,8,11,16H,1-4,7,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102060
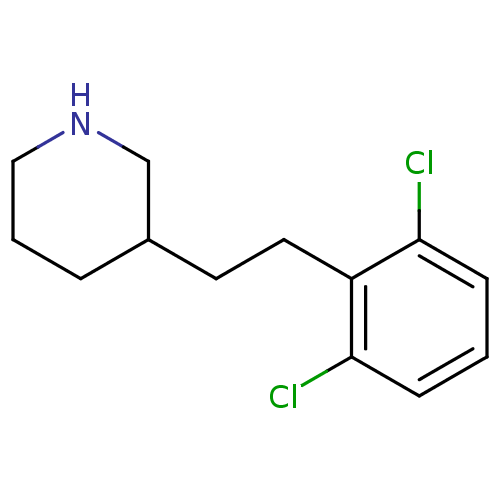
(3-[2-(2,6-Dichloro-phenyl)-ethyl]-piperidine | CHE...)Show InChI InChI=1S/C13H17Cl2N/c14-12-4-1-5-13(15)11(12)7-6-10-3-2-8-16-9-10/h1,4-5,10,16H,2-3,6-9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102056
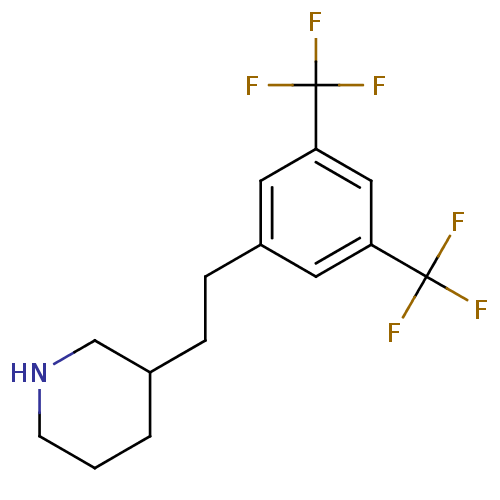
(3-[2-(3,5-Bis-trifluoromethyl-phenyl)-ethyl]-piper...)Show InChI InChI=1S/C15H17F6N/c16-14(17,18)12-6-11(7-13(8-12)15(19,20)21)4-3-10-2-1-5-22-9-10/h6-8,10,22H,1-5,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102067

((3S,4S)-3-[3-(5-Bromo-2-chloro-phenyl)-propyl]-4-f...)Show InChI InChI=1S/C14H18BrClFN/c15-12-4-5-13(16)10(8-12)2-1-3-11-9-18-7-6-14(11)17/h4-5,8,11,14,18H,1-3,6-7,9H2/t11-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102052

(5-(5-bromo-2-chlorobenzyl)-4-fluoro-1,2,3,6-tetrah...)Show InChI InChI=1S/C12H12BrClFN/c13-10-1-2-11(14)8(6-10)5-9-7-16-4-3-12(9)15/h1-2,6,16H,3-5,7H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102049

(5-(5-Bromo-2-chloro-benzyl)-1,2,3,6-tetrahydro-pyr...)Show InChI InChI=1S/C12H13BrClN/c13-11-3-4-12(14)10(7-11)6-9-2-1-5-15-8-9/h2-4,7,15H,1,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102053
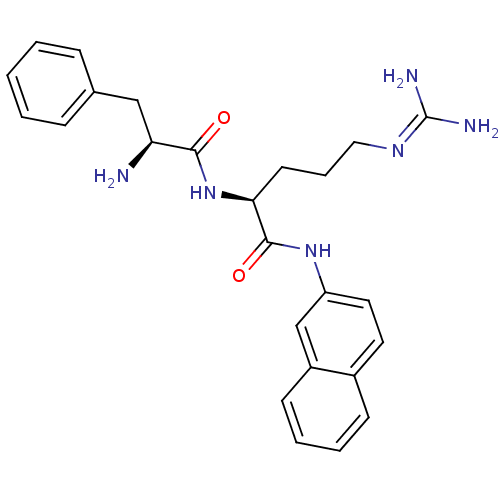
((S)-2-((S)-2-Amino-3-phenyl-propionylamino)-5-guan...)Show SMILES [#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C25H30N6O2/c26-21(15-17-7-2-1-3-8-17)23(32)31-22(11-6-14-29-25(27)28)24(33)30-20-13-12-18-9-4-5-10-19(18)16-20/h1-5,7-10,12-13,16,21-22H,6,11,14-15,26H2,(H,30,33)(H,31,32)(H4,27,28,29)/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102054

(3-[2-(2,5-Dimethyl-phenyl)-ethyl]-piperidine | CHE...)Show InChI InChI=1S/C15H23N/c1-12-5-6-13(2)15(10-12)8-7-14-4-3-9-16-11-14/h5-6,10,14,16H,3-4,7-9,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102050
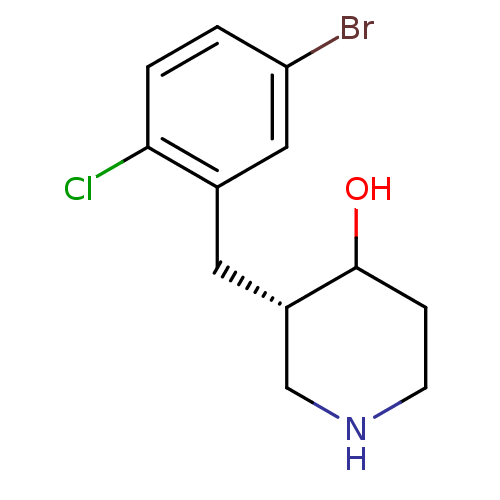
((S)-3-(5-Bromo-2-chloro-benzyl)-piperidin-4-ol; hy...)Show InChI InChI=1S/C12H15BrClNO/c13-10-1-2-11(14)8(6-10)5-9-7-15-4-3-12(9)16/h1-2,6,9,12,15-16H,3-5,7H2/t9-,12?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102058

((3S,4S)-3-(5-Bromo-2-chloro-benzyl)-piperidin-4-ol...)Show InChI InChI=1S/C12H15BrClNO/c13-10-1-2-11(14)8(6-10)5-9-7-15-4-3-12(9)16/h1-2,6,9,12,15-16H,3-5,7H2/t9-,12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102055

(4-(2-Piperidin-3-yl-ethyl)-benzonitrile | CHEMBL55...)Show InChI InChI=1S/C14H18N2/c15-10-13-6-3-12(4-7-13)5-8-14-2-1-9-16-11-14/h3-4,6-7,14,16H,1-2,5,8-9,11H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102062

(3-(5-Bromo-2-chloro-benzyl)-piperidin-4-one; hydro...)Show InChI InChI=1S/C12H13BrClNO/c13-10-1-2-11(14)8(6-10)5-9-7-15-4-3-12(9)16/h1-2,6,9,15H,3-5,7H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102068

(3-Phenethyl-piperidine | CHEMBL55554)Show InChI InChI=1S/C13H19N/c1-2-5-12(6-3-1)8-9-13-7-4-10-14-11-13/h1-3,5-6,13-14H,4,7-11H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102063

(3-[2-(4-Ethoxy-phenyl)-ethyl]-piperidine | CHEMBL5...)Show InChI InChI=1S/C15H23NO/c1-2-17-15-9-7-13(8-10-15)5-6-14-4-3-11-16-12-14/h7-10,14,16H,2-6,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102065
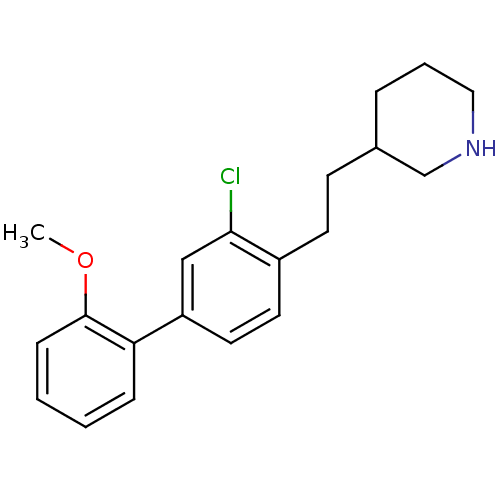
(3-[2-(3-Chloro-2'-methoxy-biphenyl-4-yl)-ethyl]-pi...)Show InChI InChI=1S/C20H24ClNO/c1-23-20-7-3-2-6-18(20)17-11-10-16(19(21)13-17)9-8-15-5-4-12-22-14-15/h2-3,6-7,10-11,13,15,22H,4-5,8-9,12,14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50102066

((3S,4S)-3-(5-Bromo-2-chloro-benzyl)-4-fluoro-piper...)Show InChI InChI=1S/C12H14BrClFN/c13-10-1-2-11(14)8(6-10)5-9-7-16-4-3-12(9)15/h1-2,6,9,12,16H,3-5,7H2/t9-,12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibitory activity against accumulation of [14C]-labeled Linezolid within wild-type Escherichia coli K12 cells |
Bioorg Med Chem Lett 11: 1903-6 (2001)
BindingDB Entry DOI: 10.7270/Q2251JPX |
More data for this
Ligand-Target Pair | |
Albumin
(Homo sapiens (Human)) | BDBM50209511

(5-cyano-2-(2-(3-(2-methoxyethoxy)propylthio)nicoti...)Show SMILES COCCOCCCSc1ccc(cn1)C(=O)Nc1ccc(cc1C(O)=O)C#N Show InChI InChI=1S/C20H21N3O5S/c1-27-8-9-28-7-2-10-29-18-6-4-15(13-22-18)19(24)23-17-5-3-14(12-21)11-16(17)20(25)26/h3-6,11,13H,2,7-10H2,1H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human serum albumin |
Bioorg Med Chem Lett 17: 3113-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.036
BindingDB Entry DOI: 10.7270/Q2SJ1K88 |
More data for this
Ligand-Target Pair | |
Albumin
(Homo sapiens (Human)) | BDBM50215044
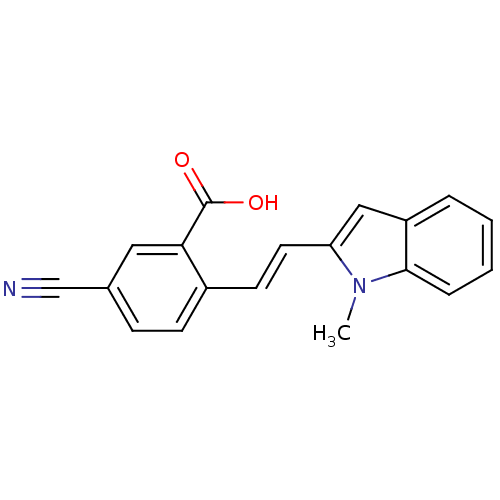
(5-cyano-2-(2-(1-methyl-1H-indol-2-yl)vinyl)benzoic...)Show InChI InChI=1S/C19H14N2O2/c1-21-16(11-15-4-2-3-5-18(15)21)9-8-14-7-6-13(12-20)10-17(14)19(22)23/h2-11H,1H3,(H,22,23)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Binding affinity at human serum albumin |
Bioorg Med Chem Lett 17: 4646-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.077
BindingDB Entry DOI: 10.7270/Q2N01662 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data