

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50087713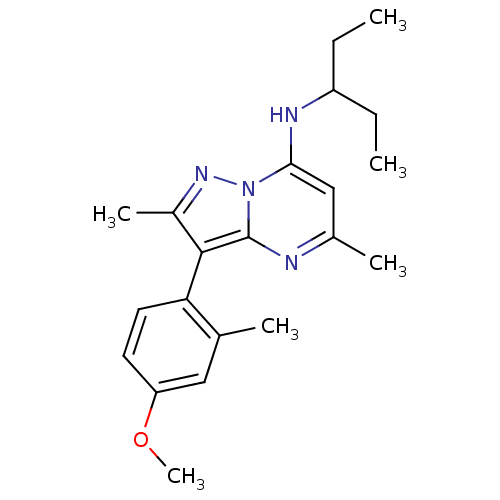 ((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163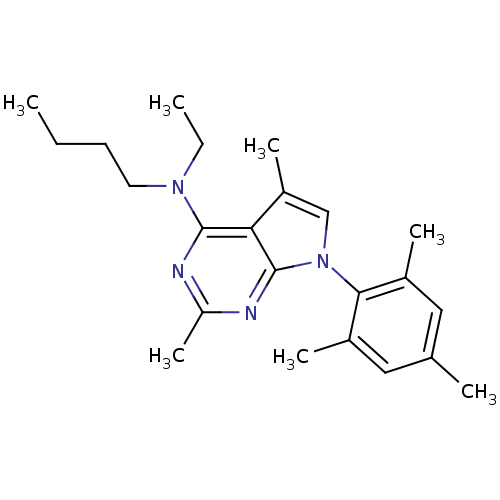 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50087713 ((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86190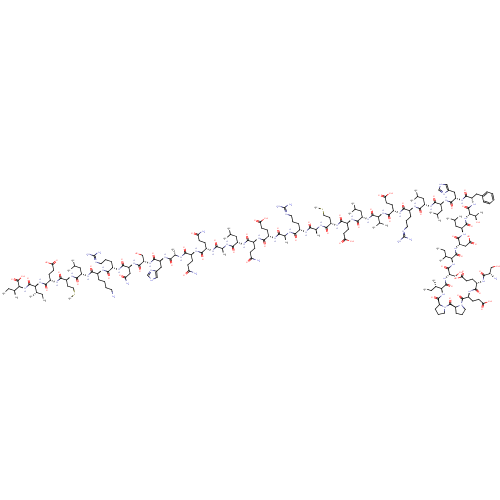 (CAS_0 | NSC_0 | hrCRF) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875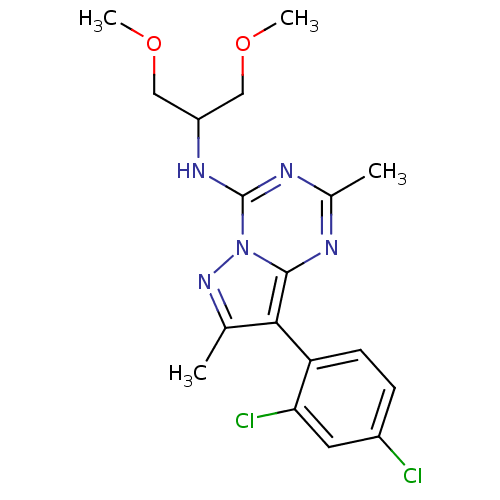 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86189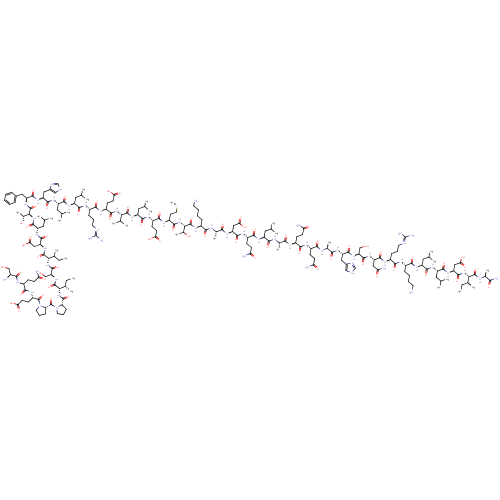 (CAS_0 | NSC_0 | OCRF) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50001498 (1-Butyl-3,7-dihydro-purine-2,6-dione | CHEMBL68278) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-I-AB-MECA binding to human Adenosine A3 receptor | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86192 (CAS_197801-88-0 | CHEMBL2165203 | SN003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86192 (CAS_197801-88-0 | CHEMBL2165203 | SN003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021454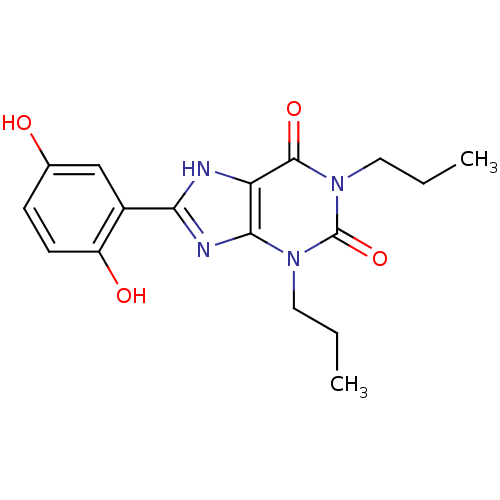 (8-(2,5-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50042211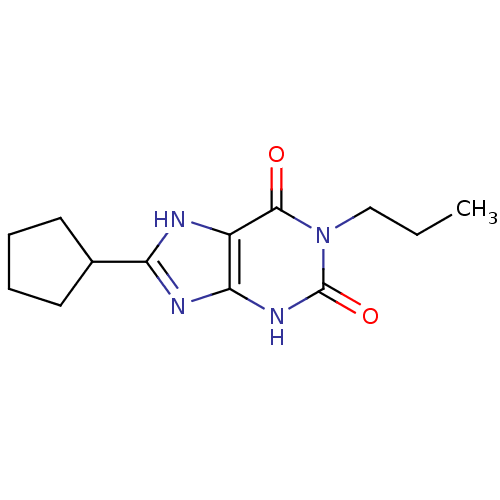 (8-Cyclopentyl-1-propyl-3,7-dihydro-purine-2,6-dion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50299277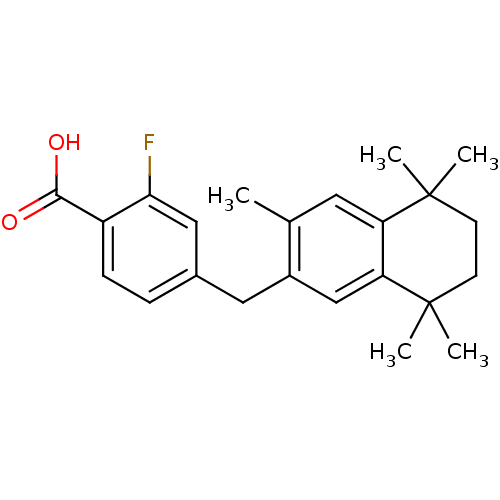 (2-Fluoro-4-(1-(1,2,3,4-tetrahydro-1,1,4,4,6-pentam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM50086170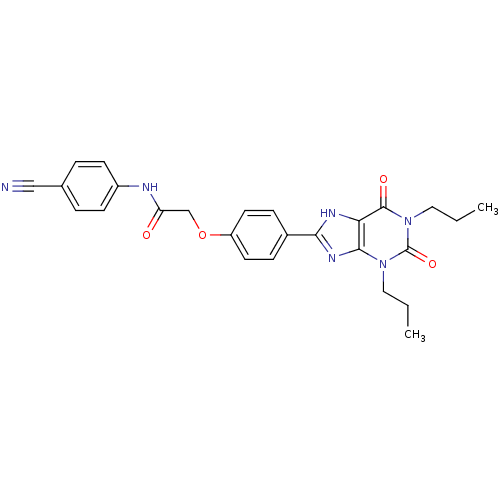 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-APOBX binding to rat Adenosine A2B receptor expressed in HEK cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86190 (CAS_0 | NSC_0 | hrCRF) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50086170 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50021449 (8-(2,4-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86191 (CAS_0 | D-PheCRF | NSC_0) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM86189 (CAS_0 | NSC_0 | OCRF) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50113238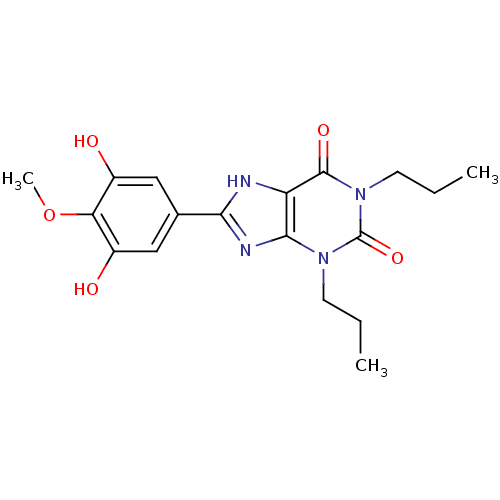 (8-(3,5-Dihydroxy-4-methoxy-phenyl)-1,3-dipropyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50042211 (8-Cyclopentyl-1-propyl-3,7-dihydro-purine-2,6-dion...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50113242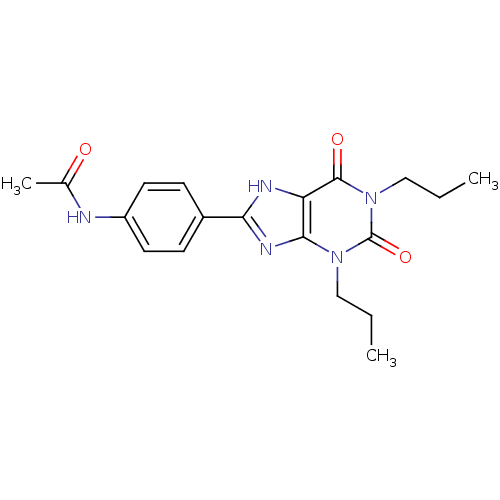 (CHEMBL66337 | N-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50020960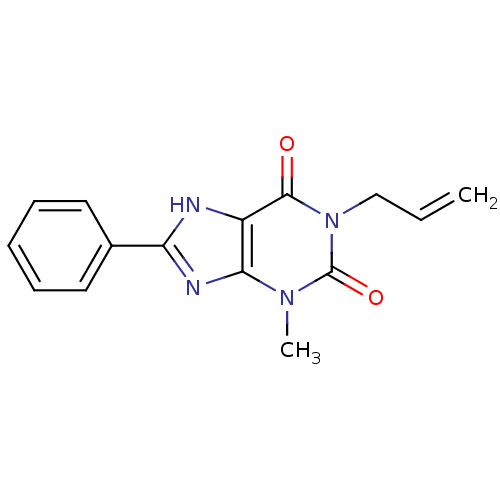 (1-Allyl-3-methyl-8-phenyl-3,7-dihydro-purine-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021457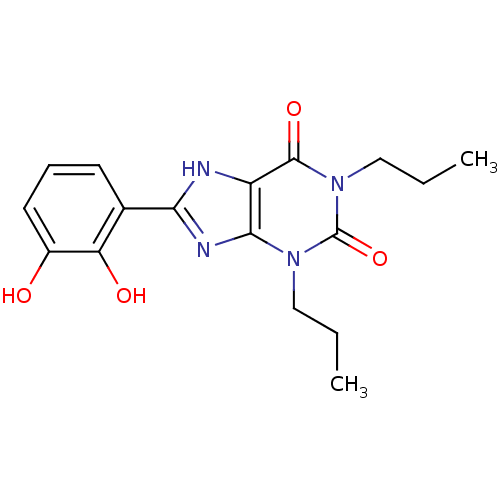 (8-(2,3-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50113242 (CHEMBL66337 | N-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50008405 (2-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50021448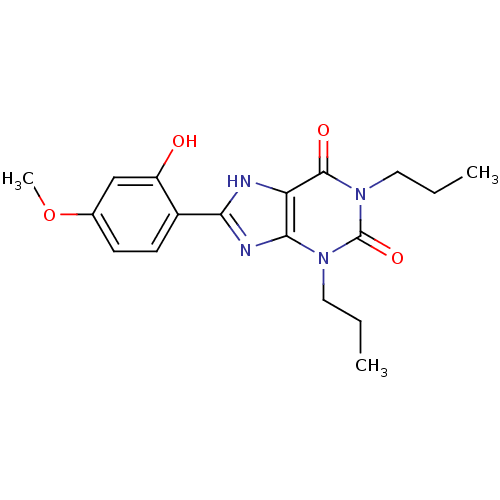 (8-(2-Hydroxy-4-methoxy-phenyl)-1,3-dipropyl-3,7-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50113238 (8-(3,5-Dihydroxy-4-methoxy-phenyl)-1,3-dipropyl-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 59.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM82012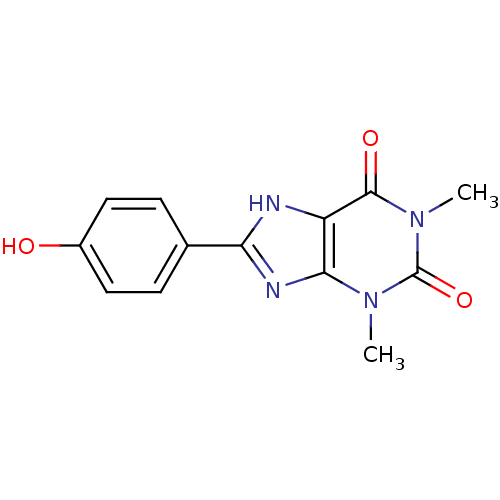 (8-(4-Hydroxy-phenyl)-1,3-dimethyl-3,7-dihydro-puri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM81971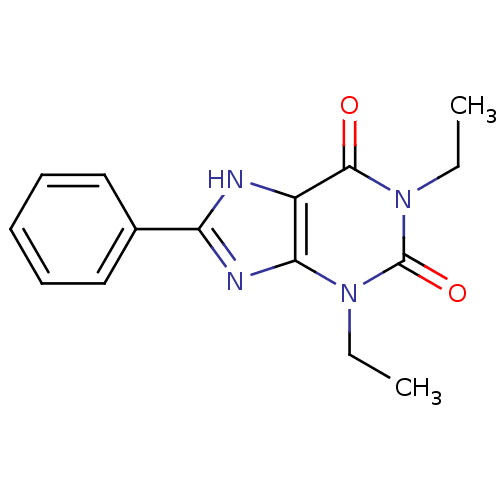 (1,3-Diethyl-8-phenyl-3,7-dihydro-purine-2,6-dione ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 63.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50023354 (1,3-Dipropyl-8-pyrazin-2-yl-3,7-dihydro-purine-2,6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50299279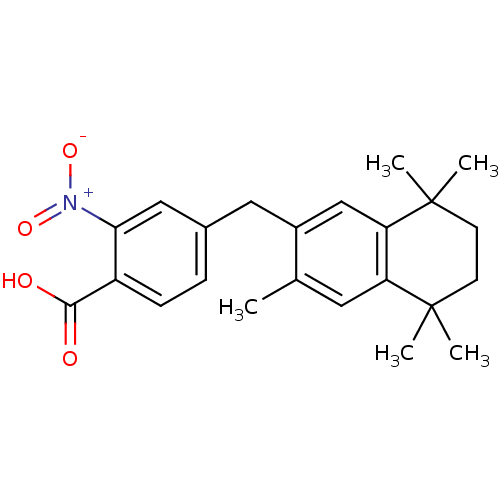 (4-(1-(1,2,3,4-Tetrahydro-1,1,4,4,6-pentamethylnaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021448 (8-(2-Hydroxy-4-methoxy-phenyl)-1,3-dipropyl-3,7-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 97.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50021449 (8-(2,4-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at rat Adenosine A2A receptor by [3H]-CGS- 21680 displacement. | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50021457 (8-(2,3-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50021454 (8-(2,5-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50299278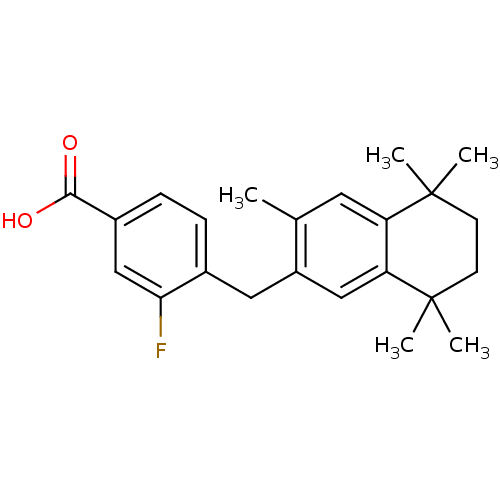 (3-Fluoro-4-(1-(1,2,3,4-tetrahydro-1,1,4,4,6-pentam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid form human RXRalpha expressed in human Caco2 cells after 16 hrs | J Med Chem 52: 5950-66 (2009) Article DOI: 10.1021/jm900496b BindingDB Entry DOI: 10.7270/Q2Q81D41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM50020960 (1-Allyl-3-methyl-8-phenyl-3,7-dihydro-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-APOBX binding to rat Adenosine A2B receptor expressed in HEK cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]-APOBX binding to rat Adenosine A2B receptor expressed in HEK cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50023353 (CHEMBL156178 | [4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021449 (8-(2,4-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50020972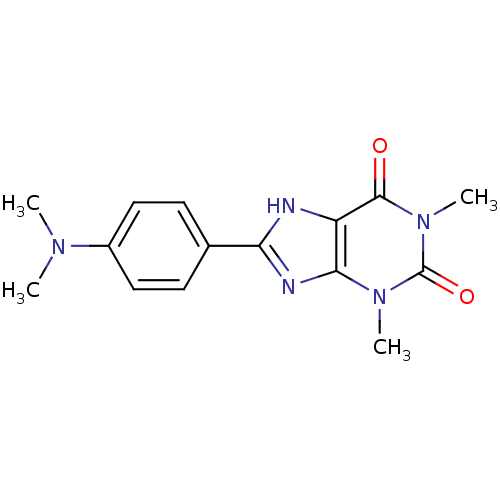 (8-(4-Dimethylamino-phenyl)-1,3-dimethyl-3,7-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50020960 (1-Allyl-3-methyl-8-phenyl-3,7-dihydro-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50113245 (8-(2,6-Difluoro-phenyl)-1,3-dipropyl-3,7-dihydro-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50113237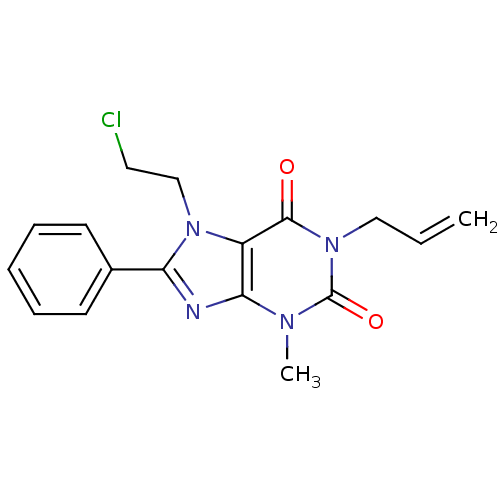 (1-Allyl-7-(2-chloro-ethyl)-3-methyl-8-phenyl-3,7-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at human Adenosine A2B receptor expressed in HEK-293 cells, using [125I]-ABOPX as radioligand | J Med Chem 45: 2131-8 (2002) BindingDB Entry DOI: 10.7270/Q2V1243G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 811 total ) | Next | Last >> |