 Found 37 hits with Last Name = 'marshall' and Initial = 'w'
Found 37 hits with Last Name = 'marshall' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM26810
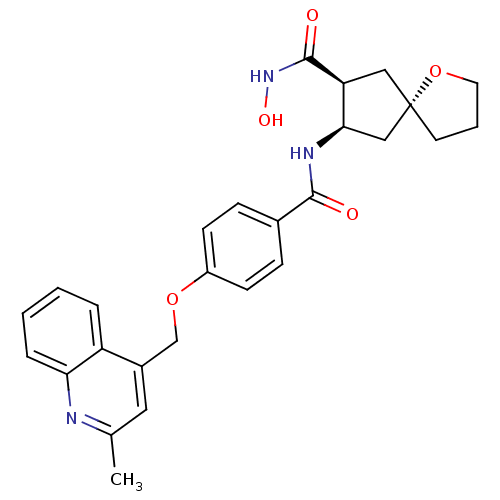
((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM26809
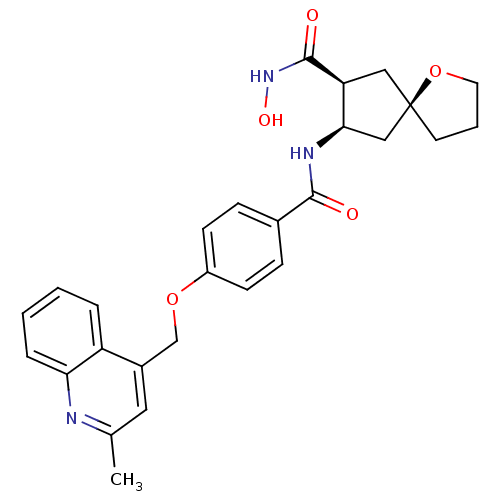
((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM26809

((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM26810

((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM26808
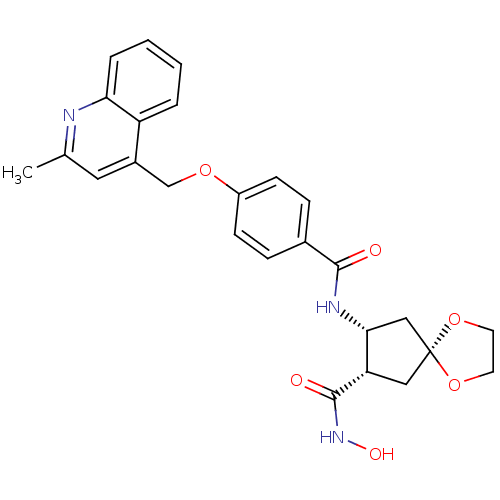
((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.13E+3 | >-32.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM26808

((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM26809

((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM26808

((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM26810

((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26809

((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26808

((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26810

((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50084636
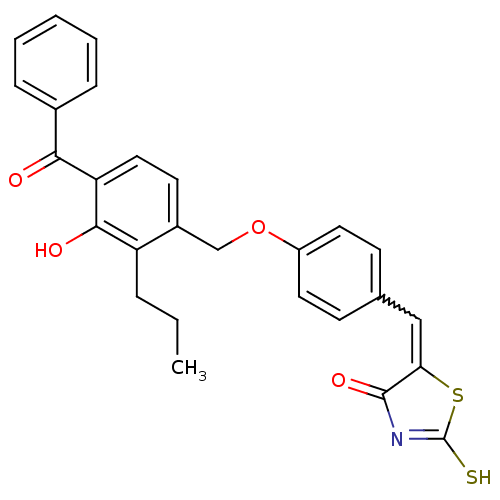
(5-[1-[4-(4-Benzoyl-3-hydroxy-2-propyl-benzyloxy)-p...)Show SMILES CCCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1 |w:16.16,c:21| Show InChI InChI=1S/C27H23NO4S2/c1-2-6-21-19(11-14-22(25(21)30)24(29)18-7-4-3-5-8-18)16-32-20-12-9-17(10-13-20)15-23-26(31)28-27(33)34-23/h3-5,7-15,30H,2,6,16H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50080991
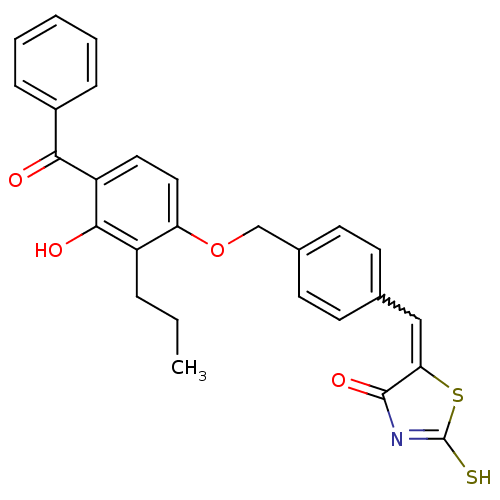
(5-[1-[4-(4-Benzoyl-3-hydroxy-2-propyl-phenoxymethy...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1 |w:16.16,c:21| Show InChI InChI=1S/C27H23NO4S2/c1-2-6-20-22(14-13-21(25(20)30)24(29)19-7-4-3-5-8-19)32-16-18-11-9-17(10-12-18)15-23-26(31)28-27(33)34-23/h3-5,7-15,30H,2,6,16H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287763
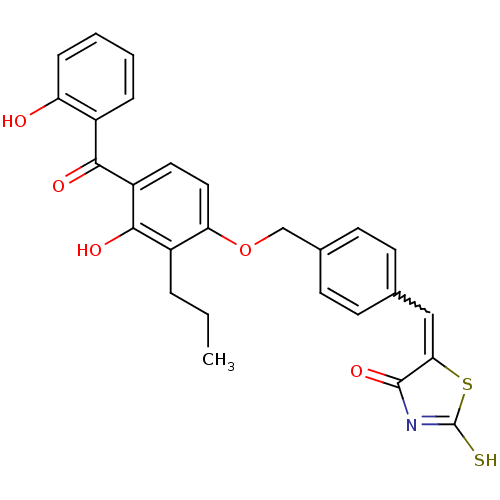
(5-[1-{4-[3-Hydroxy-4-(2-hydroxy-benzoyl)-2-propyl-...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1O |w:16.16,c:21| Show InChI InChI=1S/C27H23NO5S2/c1-2-5-19-22(13-12-20(25(19)31)24(30)18-6-3-4-7-21(18)29)33-15-17-10-8-16(9-11-17)14-23-26(32)28-27(34)35-23/h3-4,6-14,29,31H,2,5,15H2,1H3,(H,28,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287749
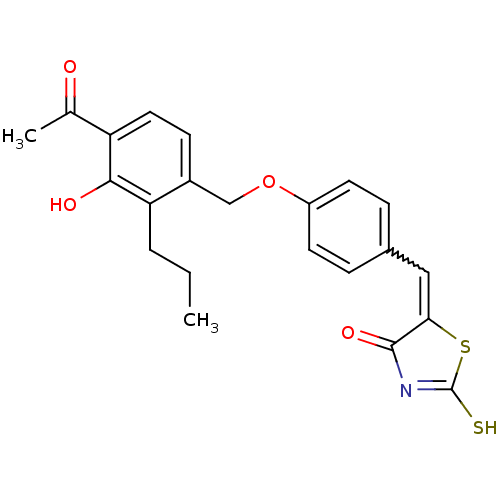
(5-[1-[4-(4-Acetyl-3-hydroxy-2-propyl-benzyloxy)-ph...)Show SMILES CCCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(C)=O |w:16.16,c:21| Show InChI InChI=1S/C22H21NO4S2/c1-3-4-18-15(7-10-17(13(2)24)20(18)25)12-27-16-8-5-14(6-9-16)11-19-21(26)23-22(28)29-19/h5-11,25H,3-4,12H2,1-2H3,(H,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287758
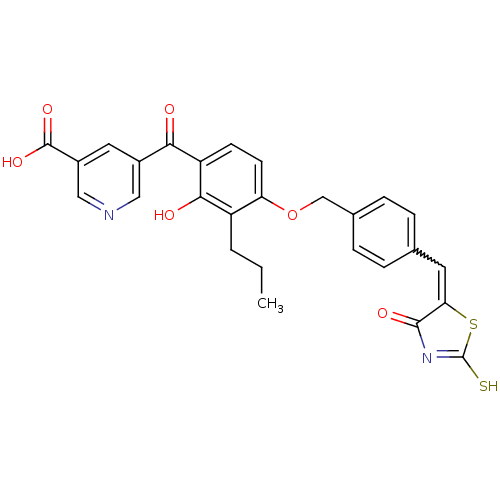
(5-(2-Hydroxy-4-{4-[4-oxo-2-thioxo-thiazolidin-(5Z)...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1cncc(c1)C(O)=O |w:16.16,c:21| Show InChI InChI=1S/C27H22N2O6S2/c1-2-3-19-21(9-8-20(24(19)31)23(30)17-11-18(26(33)34)13-28-12-17)35-14-16-6-4-15(5-7-16)10-22-25(32)29-27(36)37-22/h4-13,31H,2-3,14H2,1H3,(H,33,34)(H,29,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287743
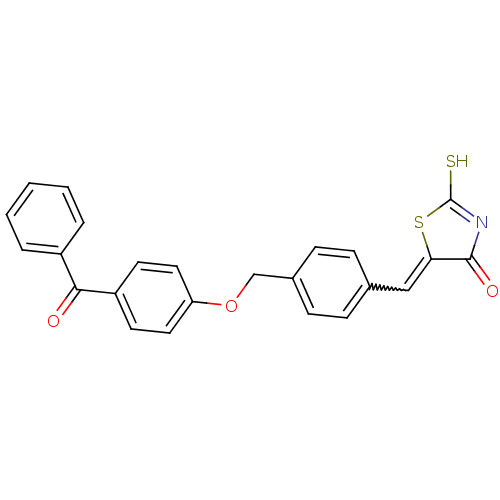
(5-[1-[4-(4-Benzoyl-phenoxymethyl)-phenyl]-meth-(Z)...)Show SMILES SC1=NC(=O)C(S1)=Cc1ccc(COc2ccc(cc2)C(=O)c2ccccc2)cc1 |w:7.8,t:1| Show InChI InChI=1S/C24H17NO3S2/c26-22(18-4-2-1-3-5-18)19-10-12-20(13-11-19)28-15-17-8-6-16(7-9-17)14-21-23(27)25-24(29)30-21/h1-14H,15H2,(H,25,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287747
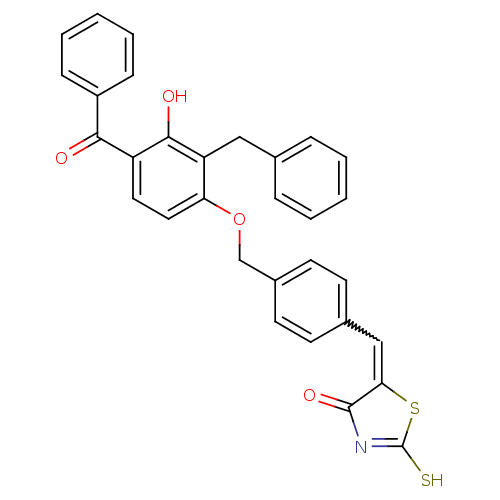
(5-[1-[4-(4-Benzoyl-2-benzyl-3-hydroxy-phenoxymethy...)Show SMILES Oc1c(Cc2ccccc2)c(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc1C(=O)c1ccccc1 |w:17.17,c:22| Show InChI InChI=1S/C31H23NO4S2/c33-28(23-9-5-2-6-10-23)24-15-16-26(25(29(24)34)17-20-7-3-1-4-8-20)36-19-22-13-11-21(12-14-22)18-27-30(35)32-31(37)38-27/h1-16,18,34H,17,19H2,(H,32,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50080991

(5-[1-[4-(4-Benzoyl-3-hydroxy-2-propyl-phenoxymethy...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1 |w:16.16,c:21| Show InChI InChI=1S/C27H23NO4S2/c1-2-6-20-22(14-13-21(25(20)30)24(29)19-7-4-3-5-8-19)32-16-18-11-9-17(10-12-18)15-23-26(31)28-27(33)34-23/h3-5,7-15,30H,2,6,16H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of cathepsin D at an enzyme level of 50 ng/mL |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287761
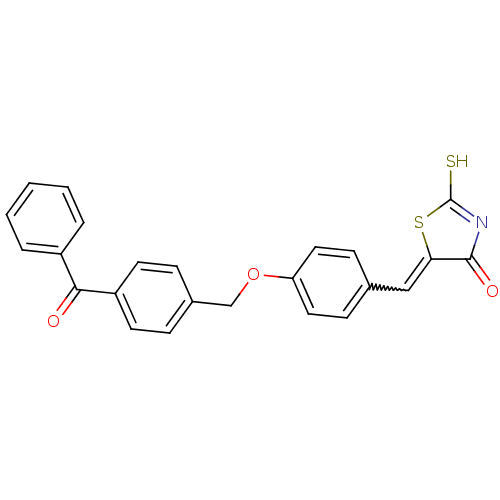
(5-[1-[4-(4-Benzoyl-benzyloxy)-phenyl]-meth-(Z)-yli...)Show SMILES SC1=NC(=O)C(S1)=Cc1ccc(OCc2ccc(cc2)C(=O)c2ccccc2)cc1 |w:7.8,t:1| Show InChI InChI=1S/C24H17NO3S2/c26-22(18-4-2-1-3-5-18)19-10-6-17(7-11-19)15-28-20-12-8-16(9-13-20)14-21-23(27)25-24(29)30-21/h1-14H,15H2,(H,25,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287759
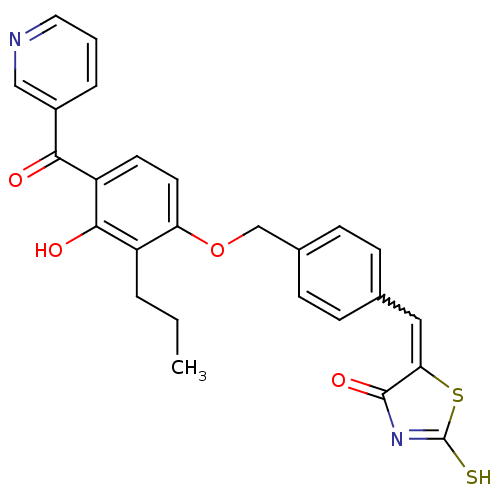
(5-[1-{4-[3-Hydroxy-2-propyl-4-(pyridine-3-carbonyl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1cccnc1 |w:16.16,c:21| Show InChI InChI=1S/C26H22N2O4S2/c1-2-4-19-21(11-10-20(24(19)30)23(29)18-5-3-12-27-14-18)32-15-17-8-6-16(7-9-17)13-22-25(31)28-26(33)34-22/h3,5-14,30H,2,4,15H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287742
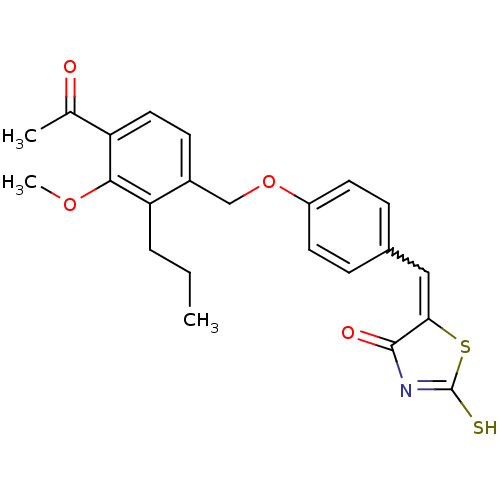
(5-[1-[4-(4-Acetyl-3-methoxy-2-propyl-benzyloxy)-ph...)Show SMILES CCCc1c(COc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC |w:11.10,c:15| Show InChI InChI=1S/C23H23NO4S2/c1-4-5-19-16(8-11-18(14(2)25)21(19)27-3)13-28-17-9-6-15(7-10-17)12-20-22(26)24-23(29)30-20/h6-12H,4-5,13H2,1-3H3,(H,24,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287750

(5-[1-[4-(4-Acetyl-3-hydroxy-2-propyl-phenoxymethyl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(C)=O |w:16.16,c:21| Show InChI InChI=1S/C22H21NO4S2/c1-3-4-17-18(10-9-16(13(2)24)20(17)25)27-12-15-7-5-14(6-8-15)11-19-21(26)23-22(28)29-19/h5-11,25H,3-4,12H2,1-2H3,(H,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287757
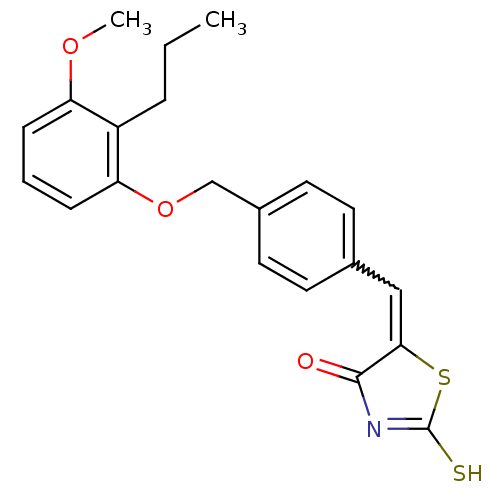
(5-[1-[4-(3-Methoxy-2-propyl-phenoxymethyl)-phenyl]...)Show SMILES CCCc1c(OC)cccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1 |w:17.17,c:22| Show InChI InChI=1S/C21H21NO3S2/c1-3-5-16-17(24-2)6-4-7-18(16)25-13-15-10-8-14(9-11-15)12-19-20(23)22-21(26)27-19/h4,6-12H,3,5,13H2,1-2H3,(H,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287762
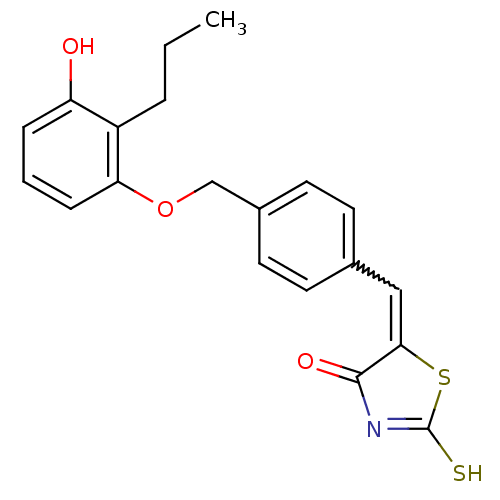
(5-[1-[4-(3-Hydroxy-2-propyl-phenoxymethyl)-phenyl]...)Show SMILES CCCc1c(O)cccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1 |w:16.16,c:21| Show InChI InChI=1S/C20H19NO3S2/c1-2-4-15-16(22)5-3-6-17(15)24-12-14-9-7-13(8-10-14)11-18-19(23)21-20(25)26-18/h3,5-11,22H,2,4,12H2,1H3,(H,21,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287752
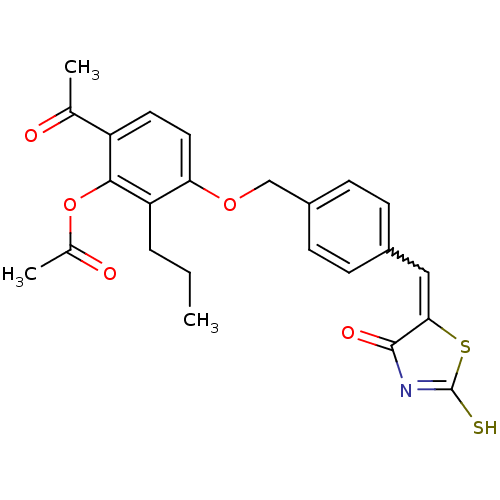
(Acetic acid 6-acetyl-3-{4-[4-oxo-2-thioxo-thiazoli...)Show SMILES CCCc1c(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC(C)=O |w:11.10,c:15| Show InChI InChI=1S/C24H23NO5S2/c1-4-5-19-20(11-10-18(14(2)26)22(19)30-15(3)27)29-13-17-8-6-16(7-9-17)12-21-23(28)25-24(31)32-21/h6-12H,4-5,13H2,1-3H3,(H,25,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287745
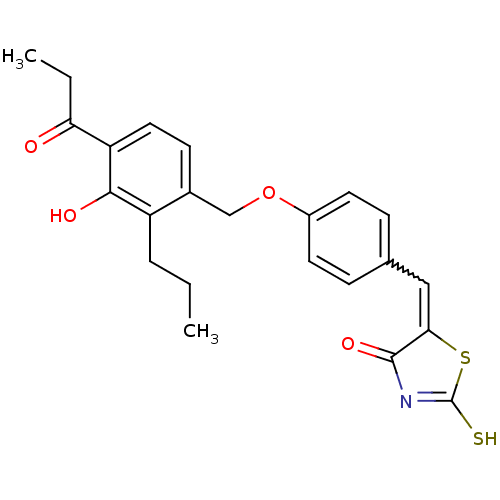
(5-[1-[4-(3-Hydroxy-4-propionyl-2-propyl-benzyloxy)...)Show SMILES CCCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)CC |w:16.16,c:21| Show InChI InChI=1S/C23H23NO4S2/c1-3-5-17-15(8-11-18(21(17)26)19(25)4-2)13-28-16-9-6-14(7-10-16)12-20-22(27)24-23(29)30-20/h6-12,26H,3-5,13H2,1-2H3,(H,24,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287760

(2-Hydroxy-4-{4-[4-oxo-2-thioxo-thiazolidin-(5Z)-yl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(O)=O |w:16.16,c:21| Show InChI InChI=1S/C21H19NO5S2/c1-2-3-14-16(9-8-15(18(14)23)20(25)26)27-11-13-6-4-12(5-7-13)10-17-19(24)22-21(28)29-17/h4-10,23H,2-3,11H2,1H3,(H,25,26)(H,22,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287751
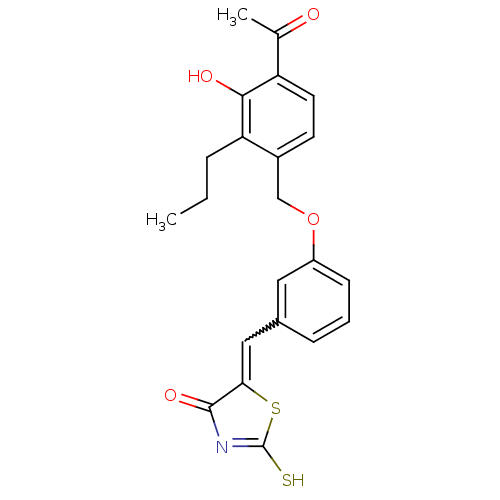
(5-[1-[3-(4-Acetyl-3-hydroxy-2-propyl-benzyloxy)-ph...)Show SMILES CCCc1c(O)c(ccc1COc1cccc(C=C2SC(S)=NC2=O)c1)C(C)=O |w:17.17,c:22| Show InChI InChI=1S/C22H21NO4S2/c1-3-5-18-15(8-9-17(13(2)24)20(18)25)12-27-16-7-4-6-14(10-16)11-19-21(26)23-22(28)29-19/h4,6-11,25H,3,5,12H2,1-2H3,(H,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287753
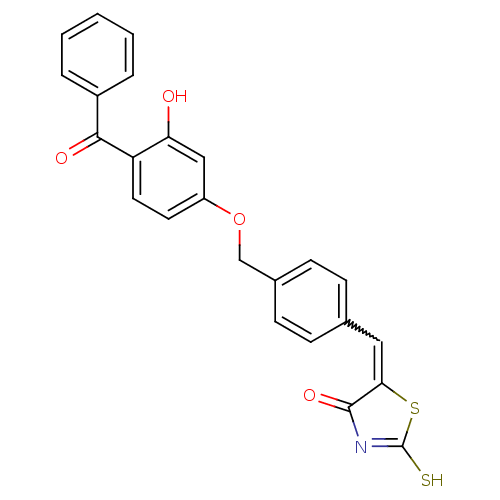
(5-[1-[4-(4-Benzoyl-3-hydroxy-phenoxymethyl)-phenyl...)Show SMILES Oc1cc(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc1C(=O)c1ccccc1 |w:10.9,c:14| Show InChI InChI=1S/C24H17NO4S2/c26-20-13-18(10-11-19(20)22(27)17-4-2-1-3-5-17)29-14-16-8-6-15(7-9-16)12-21-23(28)25-24(30)31-21/h1-13,26H,14H2,(H,25,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287748
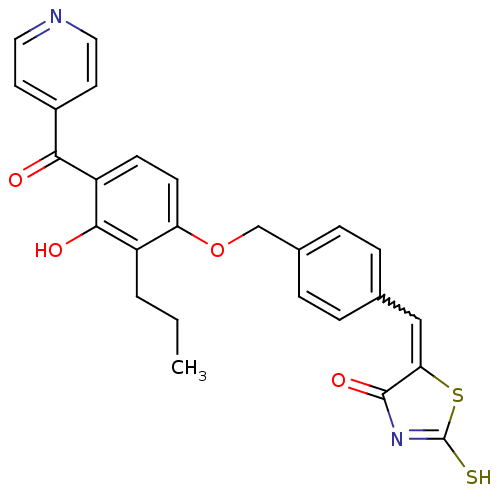
(5-[1-{4-[3-Hydroxy-2-propyl-4-(pyridine-4-carbonyl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccncc1 |w:16.16,c:21| Show InChI InChI=1S/C26H22N2O4S2/c1-2-3-19-21(9-8-20(24(19)30)23(29)18-10-12-27-13-11-18)32-15-17-6-4-16(5-7-17)14-22-25(31)28-26(33)34-22/h4-14,30H,2-3,15H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287746
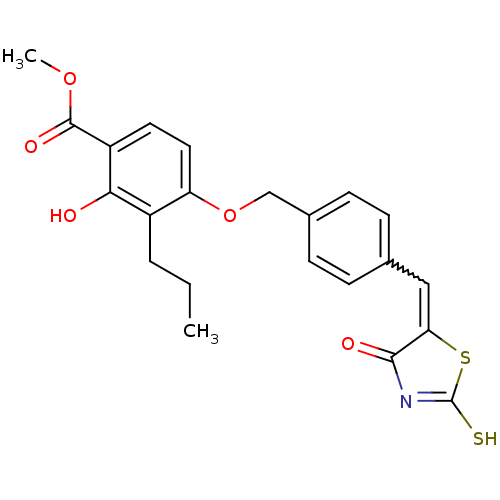
(2-Hydroxy-4-{4-[4-oxo-2-thioxo-thiazolidin-(5Z)-yl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)OC |w:16.16,c:21| Show InChI InChI=1S/C22H21NO5S2/c1-3-4-15-17(10-9-16(19(15)24)21(26)27-2)28-12-14-7-5-13(6-8-14)11-18-20(25)23-22(29)30-18/h5-11,24H,3-4,12H2,1-2H3,(H,23,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287755
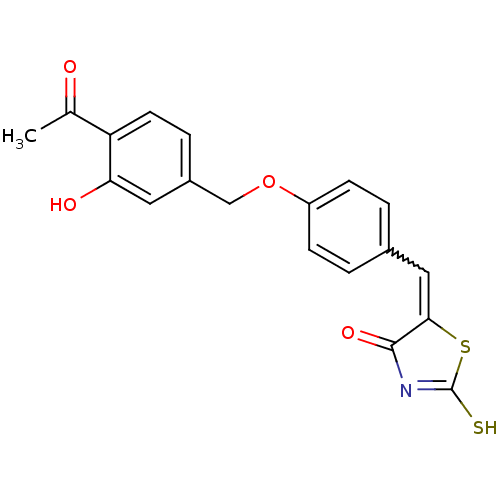
(5-[1-[4-(4-Acetyl-3-hydroxy-benzyloxy)-phenyl]-met...)Show SMILES CC(=O)c1ccc(COc2ccc(C=C3SC(S)=NC3=O)cc2)cc1O |w:13.12,c:17| Show InChI InChI=1S/C19H15NO4S2/c1-11(21)15-7-4-13(8-16(15)22)10-24-14-5-2-12(3-6-14)9-17-18(23)20-19(25)26-17/h2-9,22H,10H2,1H3,(H,20,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287754
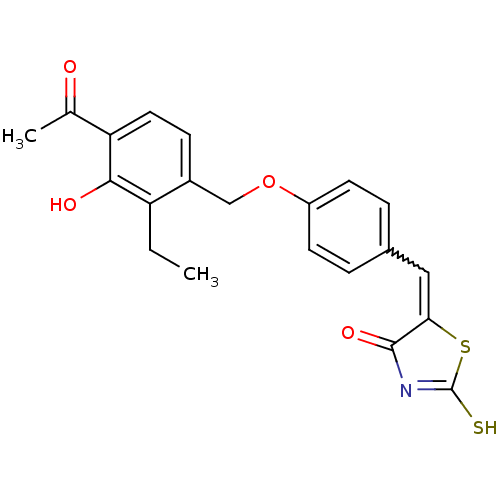
(5-[1-[4-(4-Acetyl-2-ethyl-3-hydroxy-benzyloxy)-phe...)Show SMILES CCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(C)=O |w:15.15,c:20| Show InChI InChI=1S/C21H19NO4S2/c1-3-16-14(6-9-17(12(2)23)19(16)24)11-26-15-7-4-13(5-8-15)10-18-20(25)22-21(27)28-18/h4-10,24H,3,11H2,1-2H3,(H,22,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287744
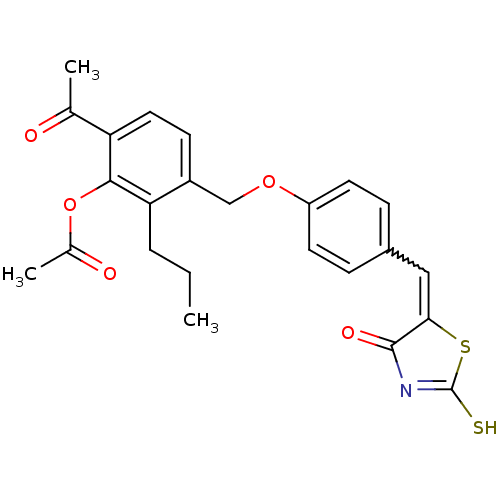
(Acetic acid 6-acetyl-3-{4-[4-oxo-2-thioxo-thiazoli...)Show SMILES CCCc1c(COc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC(C)=O |w:11.10,c:15| Show InChI InChI=1S/C24H23NO5S2/c1-4-5-20-17(8-11-19(14(2)26)22(20)30-15(3)27)13-29-18-9-6-16(7-10-18)12-21-23(28)25-24(31)32-21/h6-12H,4-5,13H2,1-3H3,(H,25,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of cathepsin D at concentration of 4.15 microg/mL |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287756
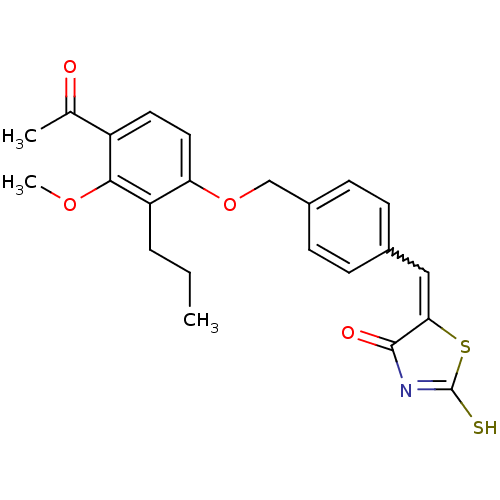
(5-[1-[4-(4-Acetyl-3-methoxy-2-propyl-phenoxymethyl...)Show SMILES CCCc1c(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC |w:11.10,c:15| Show InChI InChI=1S/C23H23NO4S2/c1-4-5-18-19(11-10-17(14(2)25)21(18)27-3)28-13-16-8-6-15(7-9-16)12-20-22(26)24-23(29)30-20/h6-12H,4-5,13H2,1-3H3,(H,24,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data