 Found 326 hits with Last Name = 'martin' and Initial = 'lj'
Found 326 hits with Last Name = 'martin' and Initial = 'lj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135249

((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2cc(F)ccc2s1 Show InChI InChI=1S/C26H29FN2O2S/c1-16-10-22-23(28-16)4-3-5-24(22)31-15-21(30)14-29-9-8-18(11-17(29)2)26-13-19-12-20(27)6-7-25(19)32-26/h3-7,10,12-13,17-18,21,28,30H,8-9,11,14-15H2,1-2H3/t17-,18+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130163

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590329
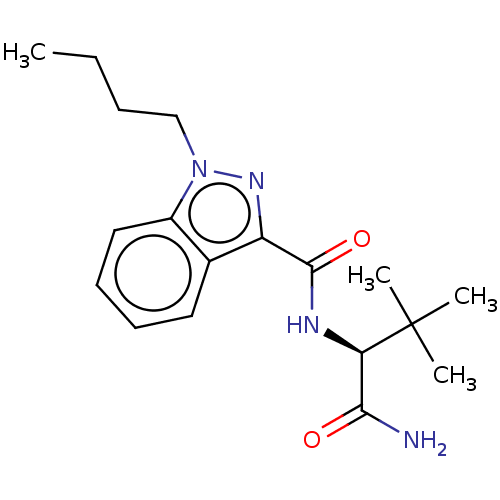
(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590329

(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130152
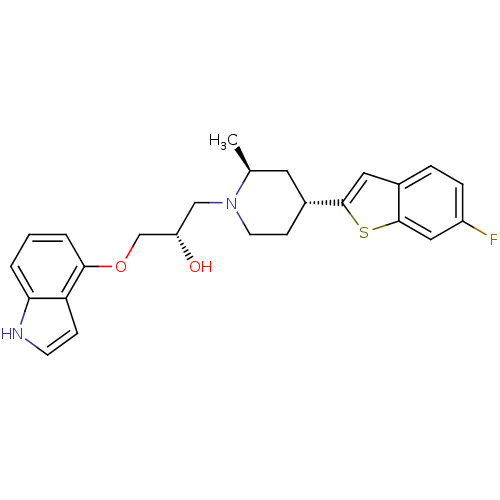
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C25H27FN2O2S/c1-16-11-18(24-12-17-5-6-19(26)13-25(17)31-24)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16,18,20,27,29H,8,10-11,14-15H2,1H3/t16-,18+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130168
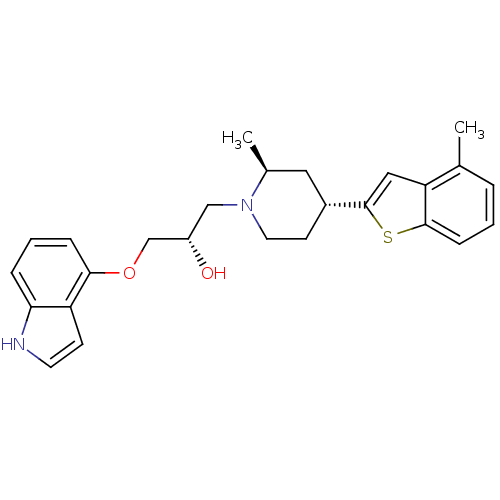
((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2c(C)cccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-5-3-8-25-22(17)14-26(31-25)19-10-12-28(18(2)13-19)15-20(29)16-30-24-7-4-6-23-21(24)9-11-27-23/h3-9,11,14,18-20,27,29H,10,12-13,15-16H2,1-2H3/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130157
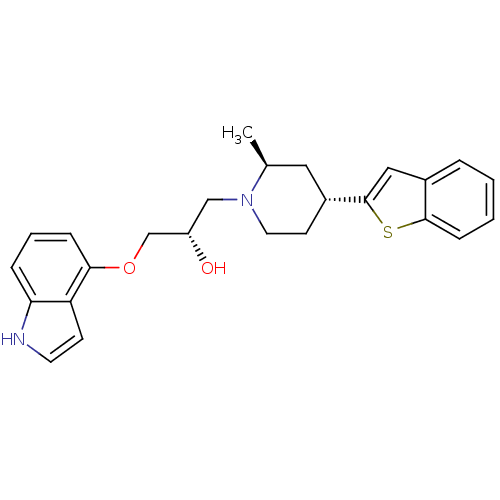
((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccccc2s1 Show InChI InChI=1S/C25H28N2O2S/c1-17-13-19(25-14-18-5-2-3-8-24(18)30-25)10-12-27(17)15-20(28)16-29-23-7-4-6-22-21(23)9-11-26-22/h2-9,11,14,17,19-20,26,28H,10,12-13,15-16H2,1H3/t17-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135246
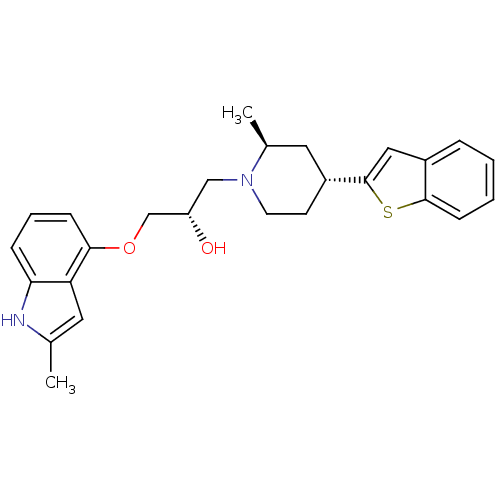
((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2ccccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-12-22-23(27-17)7-5-8-24(22)30-16-21(29)15-28-11-10-20(13-18(28)2)26-14-19-6-3-4-9-25(19)31-26/h3-9,12,14,18,20-21,27,29H,10-11,13,15-16H2,1-2H3/t18-,20+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130169
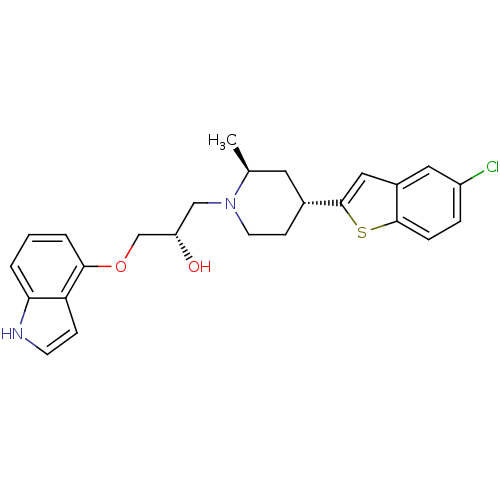
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2cc(Cl)ccc2s1 Show InChI InChI=1S/C25H27ClN2O2S/c1-16-11-17(25-13-18-12-19(26)5-6-24(18)31-25)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16-17,20,27,29H,8,10-11,14-15H2,1H3/t16-,17+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135256
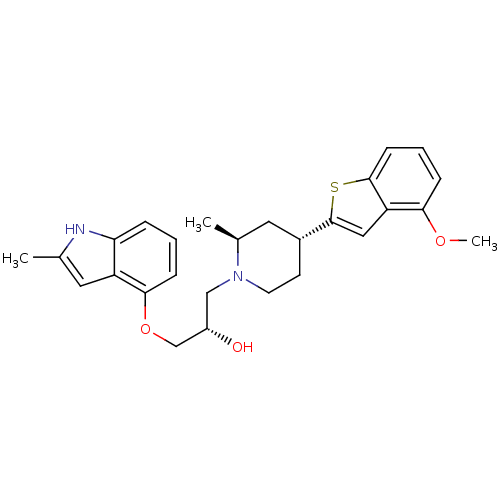
((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)[C@@H](C)C1 Show InChI InChI=1S/C27H32N2O3S/c1-17-12-21-23(28-17)6-4-8-25(21)32-16-20(30)15-29-11-10-19(13-18(29)2)27-14-22-24(31-3)7-5-9-26(22)33-27/h4-9,12,14,18-20,28,30H,10-11,13,15-16H2,1-3H3/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590329

(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590329

(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130167
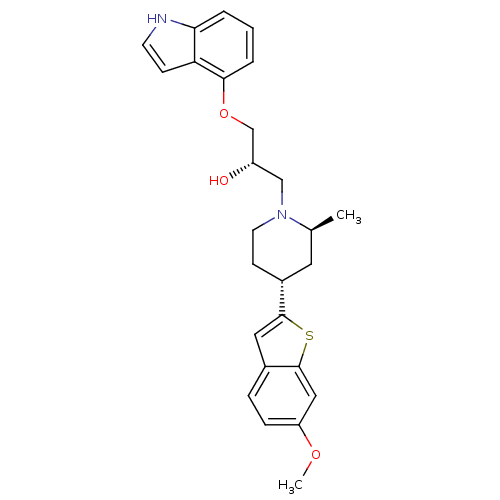
((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(6-methoxy-b...)Show SMILES COc1ccc2cc(sc2c1)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-12-19(25-13-18-6-7-21(30-2)14-26(18)32-25)9-11-28(17)15-20(29)16-31-24-5-3-4-23-22(24)8-10-27-23/h3-8,10,13-14,17,19-20,27,29H,9,11-12,15-16H2,1-2H3/t17-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130165
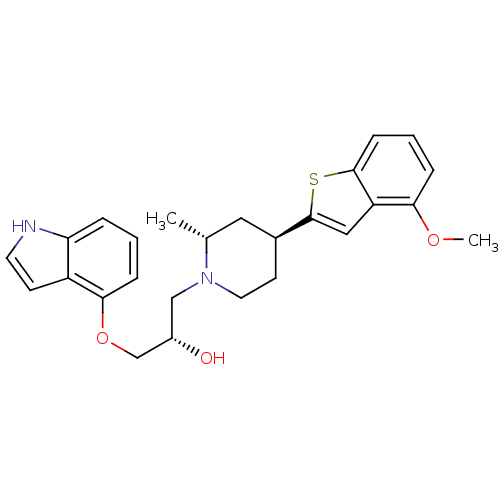
((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128368
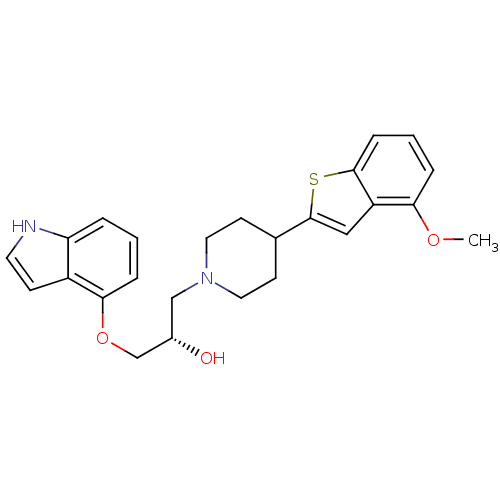
((S)-1-(1H-Indol-4-yloxy)-3-[4-(4-methoxy-benzo[b]t...)Show SMILES COc1cccc2sc(cc12)C1CCN(C[C@H](O)COc2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C25H28N2O3S/c1-29-22-5-3-7-24-20(22)14-25(31-24)17-9-12-27(13-10-17)15-18(28)16-30-23-6-2-4-21-19(23)8-11-26-21/h2-8,11,14,17-18,26,28H,9-10,12-13,15-16H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50128367
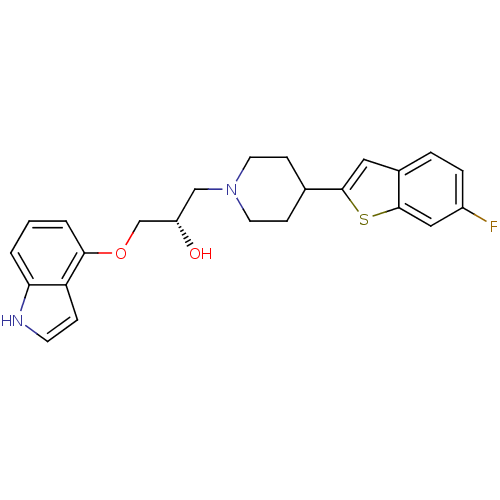
((S)-1-(1H-indol-4-yloxy)-3-(4-(6-fluorobenzo[b]thi...)Show SMILES O[C@H](COc1cccc2[nH]ccc12)CN1CCC(CC1)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C24H25FN2O2S/c25-18-5-4-17-12-23(30-24(17)13-18)16-7-10-27(11-8-16)14-19(28)15-29-22-3-1-2-21-20(22)6-9-26-21/h1-6,9,12-13,16,19,26,28H,7-8,10-11,14-15H2/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130161

((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130157

((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccccc2s1 Show InChI InChI=1S/C25H28N2O2S/c1-17-13-19(25-14-18-5-2-3-8-24(18)30-25)10-12-27(17)15-20(28)16-29-23-7-4-6-22-21(23)9-11-26-22/h2-9,11,14,17,19-20,26,28H,10,12-13,15-16H2,1H3/t17-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130163

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128380

((S)-1-(1H-indol-4-yloxy)-3-(4-(benzo[b]thiophen-2-...)Show SMILES O[C@H](COc1cccc2[nH]ccc12)CN1CCC(CC1)c1cc2ccccc2s1 Show InChI InChI=1S/C24H26N2O2S/c27-19(16-28-22-6-3-5-21-20(22)8-11-25-21)15-26-12-9-17(10-13-26)24-14-18-4-1-2-7-23(18)29-24/h1-8,11,14,17,19,25,27H,9-10,12-13,15-16H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50515386
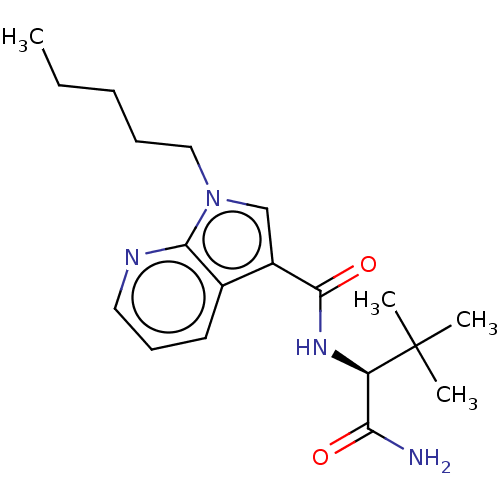
(CHEMBL4476642)Show SMILES CCCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-6-7-11-23-12-14(13-9-8-10-21-17(13)23)18(25)22-15(16(20)24)19(2,3)4/h8-10,12,15H,5-7,11H2,1-4H3,(H2,20,24)(H,22,25)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50515386

(CHEMBL4476642)Show SMILES CCCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-6-7-11-23-12-14(13-9-8-10-21-17(13)23)18(25)22-15(16(20)24)19(2,3)4/h8-10,12,15H,5-7,11H2,1-4H3,(H2,20,24)(H,22,25)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128370
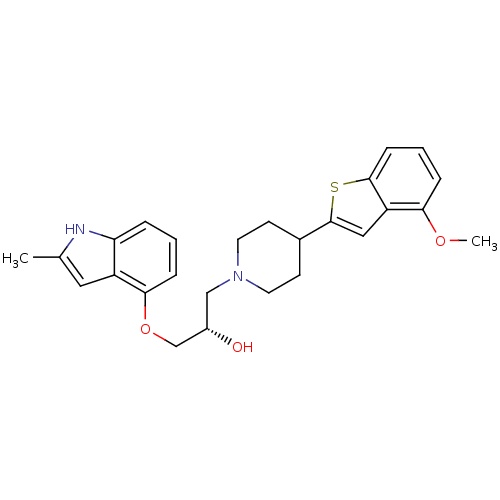
((S)-1-(4-(4-methoxybenzo[b]thiophen-2-yl)piperidin...)Show SMILES COc1cccc2sc(cc12)C1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)CC1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-20-22(27-17)5-3-7-24(20)31-16-19(29)15-28-11-9-18(10-12-28)26-14-21-23(30-2)6-4-8-25(21)32-26/h3-8,13-14,18-19,27,29H,9-12,15-16H2,1-2H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130152

((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C25H27FN2O2S/c1-16-11-18(24-12-17-5-6-19(26)13-25(17)31-24)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16,18,20,27,29H,8,10-11,14-15H2,1H3/t16-,18+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50135249

((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2cc(F)ccc2s1 Show InChI InChI=1S/C26H29FN2O2S/c1-16-10-22-23(28-16)4-3-5-24(22)31-15-21(30)14-29-9-8-18(11-17(29)2)26-13-19-12-20(27)6-7-25(19)32-26/h3-7,10,12-13,17-18,21,28,30H,8-9,11,14-15H2,1-2H3/t17-,18+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130168

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2c(C)cccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-5-3-8-25-22(17)14-26(31-25)19-10-12-28(18(2)13-19)15-20(29)16-30-24-7-4-6-23-21(24)9-11-27-23/h3-9,11,14,18-20,27,29H,10,12-13,15-16H2,1-2H3/t18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50135246

((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2ccccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-12-22-23(27-17)7-5-8-24(22)30-16-21(29)15-28-11-10-20(13-18(28)2)26-14-19-6-3-4-9-25(19)31-26/h3-9,12,14,18,20-21,27,29H,10-11,13,15-16H2,1-2H3/t18-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130169

((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2cc(Cl)ccc2s1 Show InChI InChI=1S/C25H27ClN2O2S/c1-16-11-17(25-13-18-12-19(26)5-6-24(18)31-25)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16-17,20,27,29H,8,10-11,14-15H2,1H3/t16-,17+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130165

((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128367

((S)-1-(1H-indol-4-yloxy)-3-(4-(6-fluorobenzo[b]thi...)Show SMILES O[C@H](COc1cccc2[nH]ccc12)CN1CCC(CC1)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C24H25FN2O2S/c25-18-5-4-17-12-23(30-24(17)13-18)16-7-10-27(11-8-16)14-19(28)15-29-22-3-1-2-21-20(22)6-9-26-21/h1-6,9,12-13,16,19,26,28H,7-8,10-11,14-15H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590325
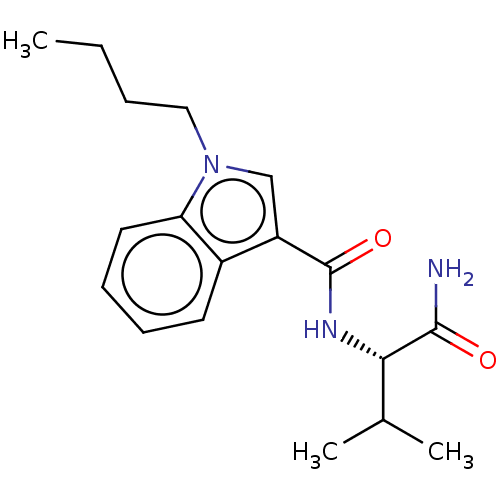
(CHEMBL5208741)Show SMILES CCCCn1cc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590325

(CHEMBL5208741)Show SMILES CCCCn1cc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50128368

((S)-1-(1H-Indol-4-yloxy)-3-[4-(4-methoxy-benzo[b]t...)Show SMILES COc1cccc2sc(cc12)C1CCN(C[C@H](O)COc2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C25H28N2O3S/c1-29-22-5-3-7-24-20(22)14-25(31-24)17-9-12-27(13-10-17)15-18(28)16-30-23-6-2-4-21-19(23)8-11-26-21/h2-8,11,14,17-18,26,28H,9-10,12-13,15-16H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130158

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6S)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data