 Found 112 hits with Last Name = 'marzinzik' and Initial = 'al'
Found 112 hits with Last Name = 'marzinzik' and Initial = 'al' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213578
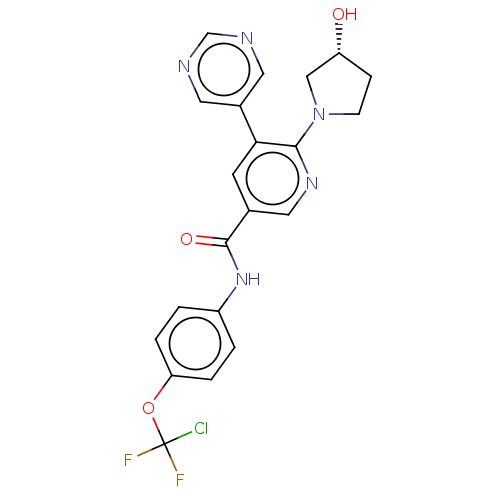
(US9278981, 170)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C21H18ClF2N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213594

(US9278981, 186)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O2S/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213656

(US9278981, 248)Show SMILES O[C@H]1CN(C[C@@H]1O)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C21H18ClF2N5O4/c22-21(23,24)33-15-3-1-14(2-4-15)28-20(32)12-5-16(13-6-25-11-26-7-13)19(27-8-12)29-9-17(30)18(31)10-29/h1-8,11,17-18,30-31H,9-10H2,(H,28,32)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50459091
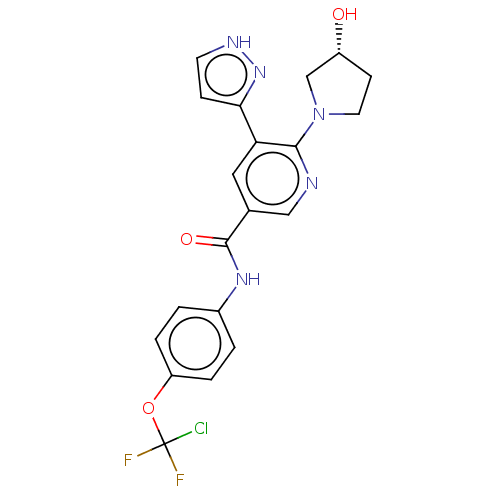
(ABL-001 | ABL001 | ABL001-NX | Asciminib | NVP-ABL...)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cc[nH]n1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C20H18ClF2N5O3/c21-20(22,23)31-15-3-1-13(2-4-15)26-19(30)12-9-16(17-5-7-25-27-17)18(24-10-12)28-8-6-14(29)11-28/h1-5,7,9-10,14,29H,6,8,11H2,(H,25,27)(H,26,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50459090
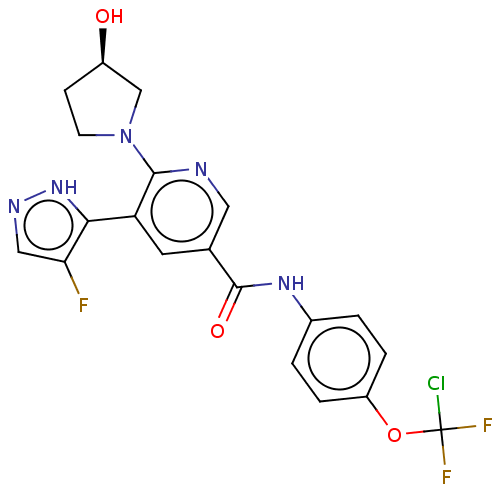
(CHEMBL4213152)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1[nH]ncc1F)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C20H17ClF3N5O3/c21-20(23,24)32-14-3-1-12(2-4-14)27-19(31)11-7-15(17-16(22)9-26-28-17)18(25-8-11)29-6-5-13(30)10-29/h1-4,7-9,13,30H,5-6,10H2,(H,26,28)(H,27,31)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50112349
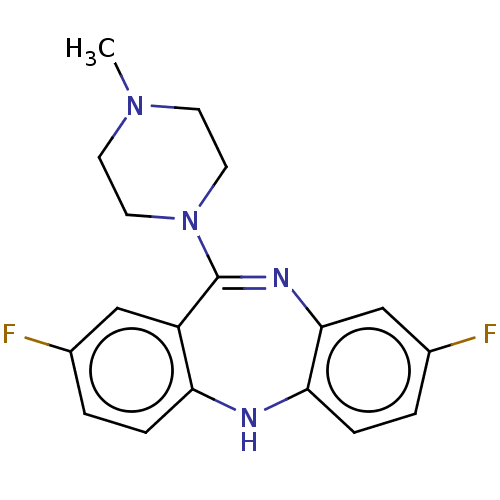
(CHEMBL3609328)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2Nc2ccc(F)cc12 |t:8| Show InChI InChI=1S/C18H18F2N4/c1-23-6-8-24(9-7-23)18-14-10-12(19)2-4-15(14)21-16-5-3-13(20)11-17(16)22-18/h2-5,10-11,21H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human histamine H1 receptor |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50459089
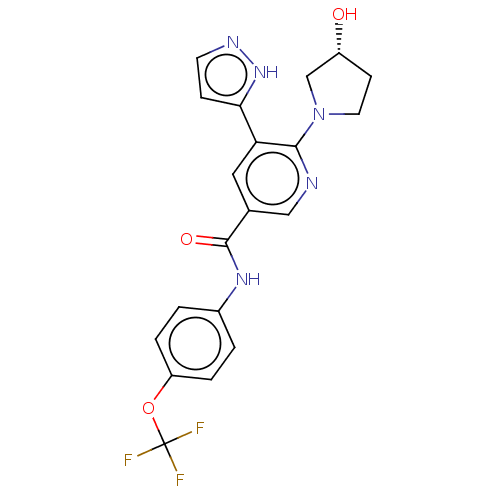
(CHEMBL4217559)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1ccn[nH]1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C20H18F3N5O3/c21-20(22,23)31-15-3-1-13(2-4-15)26-19(30)12-9-16(17-5-7-25-27-17)18(24-10-12)28-8-6-14(29)11-28/h1-5,7,9-10,14,29H,6,8,11H2,(H,25,27)(H,26,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM230760
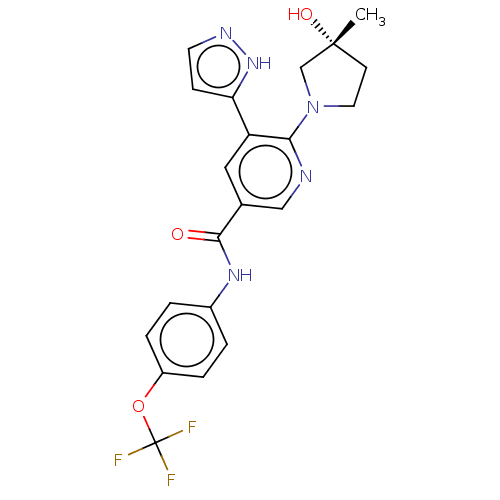
(US9340537, 14 | US9896444, Example 14)Show SMILES C[C@@]1(O)CCN(C1)c1ncc(cc1-c1ccn[nH]1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213441
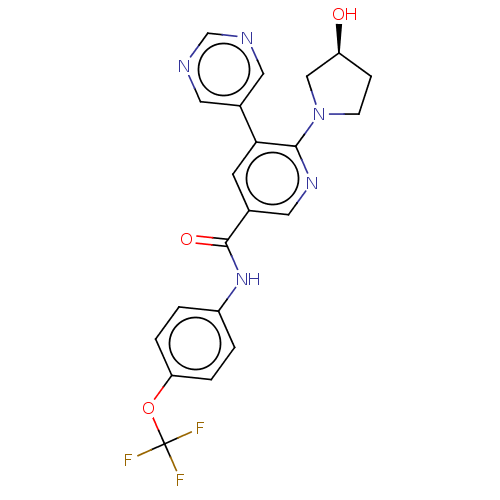
(US9278981, 33)Show SMILES O[C@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213443
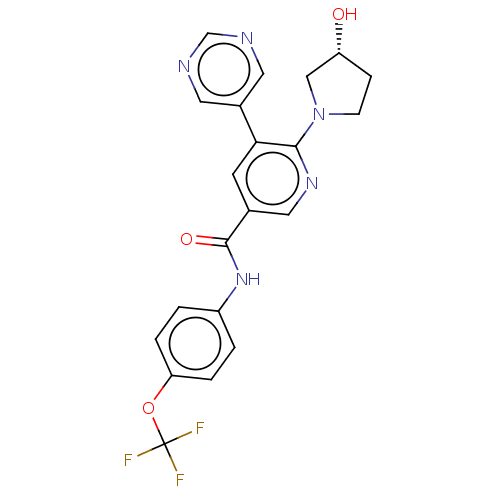
(US9278981, 35)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112348

(CHEMBL3609372)Show SMILES CC(C)NC(=O)N1CC[C@@H](C1)NC1=Nc2cc(F)ccc2N(CC(F)F)c2ccc(Cl)cc12 |r,t:13| Show InChI InChI=1S/C23H25ClF3N5O/c1-13(2)28-23(33)31-8-7-16(11-31)29-22-17-9-14(24)3-5-19(17)32(12-21(26)27)20-6-4-15(25)10-18(20)30-22/h3-6,9-10,13,16,21H,7-8,11-12H2,1-2H3,(H,28,33)(H,29,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50112349

(CHEMBL3609328)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2Nc2ccc(F)cc12 |t:8| Show InChI InChI=1S/C18H18F2N4/c1-23-6-8-24(9-7-23)18-14-10-12(19)2-4-15(14)21-16-5-3-13(20)11-17(16)22-18/h2-5,10-11,21H,6-9H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human muscarinic M1 receptor |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213434

(US9278981, 26)Show SMILES CN1CCN(CC1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C22H21F3N6O2/c1-30-6-8-31(9-7-30)20-19(16-11-26-14-27-12-16)10-15(13-28-20)21(32)29-17-2-4-18(5-3-17)33-22(23,24)25/h2-5,10-14H,6-9H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112347
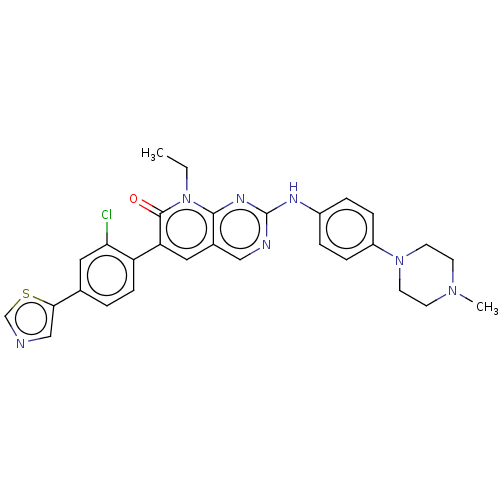
(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PAK1 by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112355
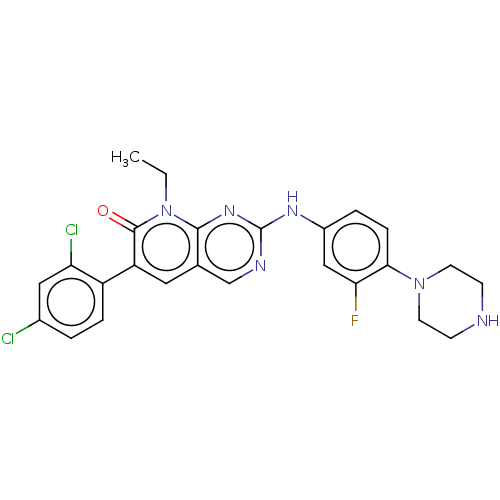
(CHEMBL3609326)Show SMILES CCn1c2nc(Nc3ccc(N4CCNCC4)c(F)c3)ncc2cc(-c2ccc(Cl)cc2Cl)c1=O Show InChI InChI=1S/C25H23Cl2FN6O/c1-2-34-23-15(11-19(24(34)35)18-5-3-16(26)12-20(18)27)14-30-25(32-23)31-17-4-6-22(21(28)13-17)33-9-7-29-8-10-33/h3-6,11-14,29H,2,7-10H2,1H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length PAK1 (unknown origin) by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50325999
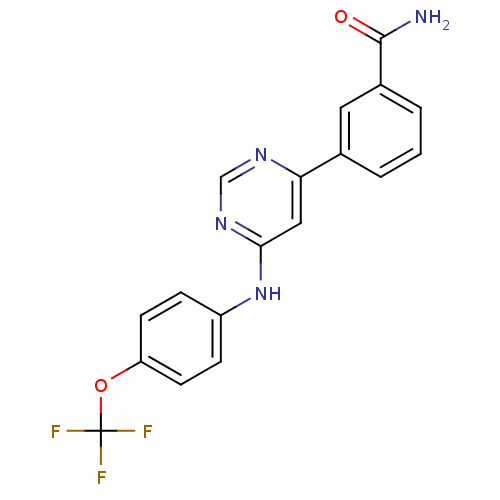
(3-(6-(4-(trifluoromethoxy)phenylamino)pyrimidin-4-...)Show SMILES NC(=O)c1cccc(c1)-c1cc(Nc2ccc(OC(F)(F)F)cc2)ncn1 Show InChI InChI=1S/C18H13F3N4O2/c19-18(20,21)27-14-6-4-13(5-7-14)25-16-9-15(23-10-24-16)11-2-1-3-12(8-11)17(22)26/h1-10H,(H2,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human c-ABL SH3/SH2/SH1 domain (46 to 531 residues) expressed in sf9 insect cells after 30 mins in presence of [gamma-32P]A... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM222504

(US9315489, 40)Show SMILES FC(F)(F)Oc1ccc(NC(=O)c2ccc(OCCN3CCCC3)c(c2)-c2cncnc2)cc1 Show InChI InChI=1S/C24H23F3N4O3/c25-24(26,27)34-20-6-4-19(5-7-20)30-23(32)17-3-8-22(33-12-11-31-9-1-2-10-31)21(13-17)18-14-28-16-29-15-18/h3-8,13-16H,1-2,9-12H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by high throughput assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM222494
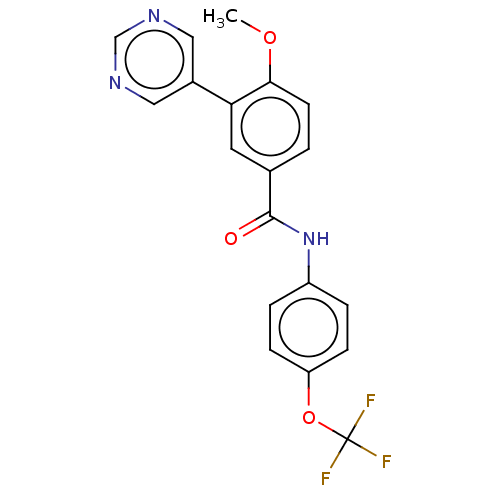
(US9315489, 30)Show SMILES COc1ccc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C19H14F3N3O3/c1-27-17-7-2-12(8-16(17)13-9-23-11-24-10-13)18(26)25-14-3-5-15(6-4-14)28-19(20,21)22/h2-11H,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM50112347

(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PAK2 by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM101618
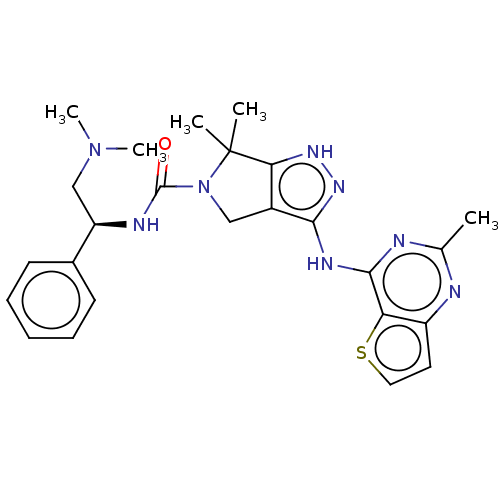
(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) using Syntide2 peptide as substrate by pyruvate kinase/lactate dehydrogenase coupled assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 6
(Homo sapiens (Human)) | BDBM101618

(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK6 (unknown origin) using peptide 7 as substrate by pyruvate kinase/lactate dehydrogenase coupled assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50328152
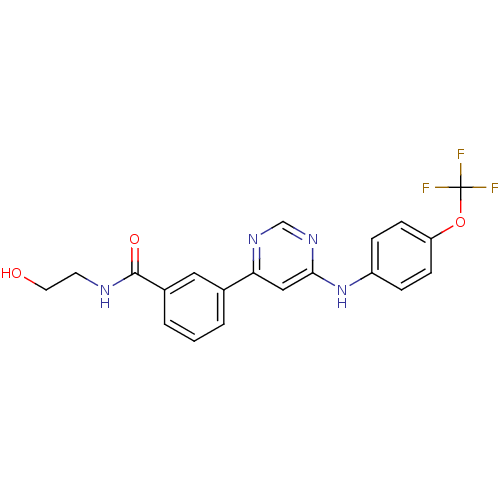
(CHEMBL1257423 | N-(2-hydroxyethyl)-3-(6-(4-(triflu...)Show SMILES OCCNC(=O)c1cccc(c1)-c1cc(Nc2ccc(OC(F)(F)F)cc2)ncn1 Show InChI InChI=1S/C20H17F3N4O3/c21-20(22,23)30-16-6-4-15(5-7-16)27-18-11-17(25-12-26-18)13-2-1-3-14(10-13)19(29)24-8-9-28/h1-7,10-12,28H,8-9H2,(H,24,29)(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human c-ABL SH3/SH2/SH1 domain (46 to 515 residues) expressed in bacterial expression system using EAIYAAPFAKKK as substrat... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM101618

(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK5 (unknown origin) using peptide 7 as substrate by pyruvate kinase/lactate dehydrogenase coupled assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM222465
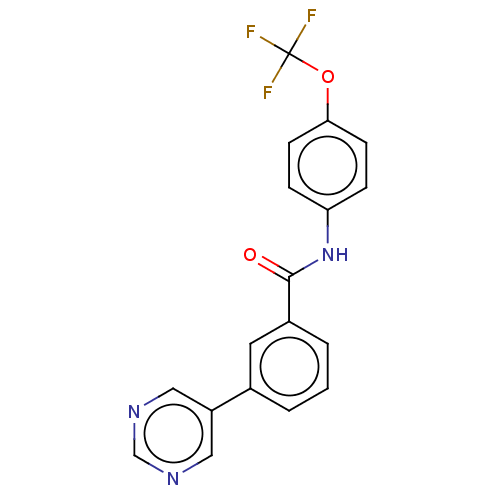
(US9315489, 1)Show SMILES FC(F)(F)Oc1ccc(NC(=O)c2cccc(c2)-c2cncnc2)cc1 Show InChI InChI=1S/C18H12F3N3O2/c19-18(20,21)26-16-6-4-15(5-7-16)24-17(25)13-3-1-2-12(8-13)14-9-22-11-23-10-14/h1-11H,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112352
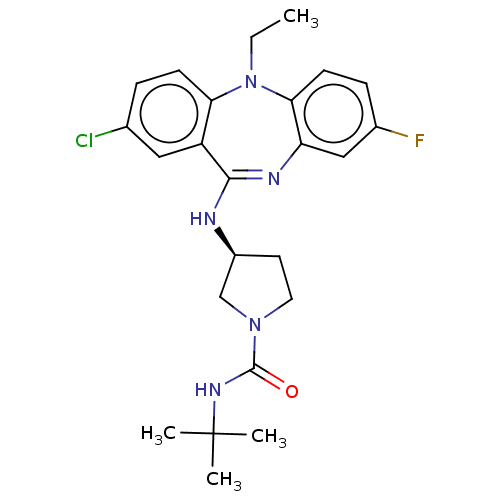
(CHEMBL3609371)Show SMILES CCN1c2ccc(F)cc2N=C(N[C@H]2CCN(C2)C(=O)NC(C)(C)C)c2cc(Cl)ccc12 |r,t:11| Show InChI InChI=1S/C24H29ClFN5O/c1-5-31-20-8-6-15(25)12-18(20)22(28-19-13-16(26)7-9-21(19)31)27-17-10-11-30(14-17)23(32)29-24(2,3)4/h6-9,12-13,17H,5,10-11,14H2,1-4H3,(H,27,28)(H,29,32)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213569
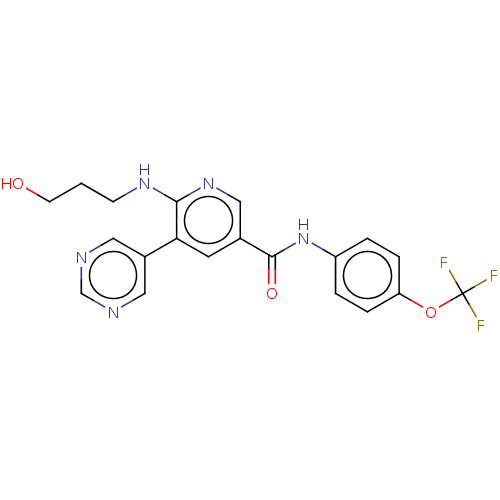
(US9278981, 161)Show SMILES OCCCNc1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C20H18F3N5O3/c21-20(22,23)31-16-4-2-15(3-5-16)28-19(30)13-8-17(14-9-24-12-25-10-14)18(27-11-13)26-6-1-7-29/h2-5,8-12,29H,1,6-7H2,(H,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM101618

(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant human PAK4 kinase domain (300 to 591) using peptide 7 as substrate by pyruvate kinase/lactate dehydr... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 3
(Homo sapiens (Human)) | BDBM50112347

(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PAK3 by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM222504

(US9315489, 40)Show SMILES FC(F)(F)Oc1ccc(NC(=O)c2ccc(OCCN3CCCC3)c(c2)-c2cncnc2)cc1 Show InChI InChI=1S/C24H23F3N4O3/c25-24(26,27)34-20-6-4-19(5-7-20)30-23(32)17-3-8-22(33-12-11-31-9-1-2-10-31)21(13-17)18-14-28-16-29-15-18/h3-8,13-16H,1-2,9-12H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM222506
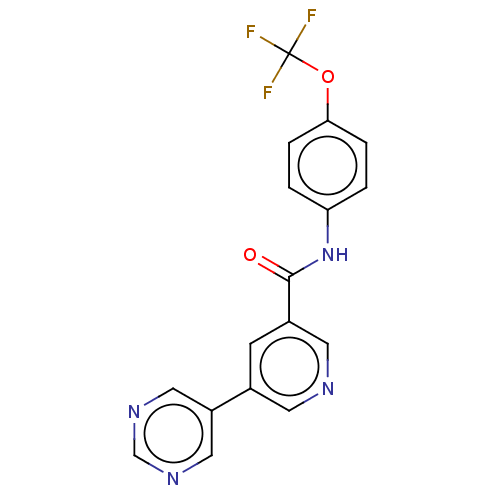
(US9315489, 42)Show SMILES FC(F)(F)Oc1ccc(NC(=O)c2cncc(c2)-c2cncnc2)cc1 Show InChI InChI=1S/C17H11F3N4O2/c18-17(19,20)26-15-3-1-14(2-4-15)24-16(25)12-5-11(6-21-7-12)13-8-22-10-23-9-13/h1-10H,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM50112355

(CHEMBL3609326)Show SMILES CCn1c2nc(Nc3ccc(N4CCNCC4)c(F)c3)ncc2cc(-c2ccc(Cl)cc2Cl)c1=O Show InChI InChI=1S/C25H23Cl2FN6O/c1-2-34-23-15(11-19(24(34)35)18-5-3-16(26)12-20(18)27)14-30-25(32-23)31-17-4-6-22(21(28)13-17)33-9-7-29-8-10-33/h3-6,11-14,29H,2,7-10H2,1H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length PAK2 (unknown origin) by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 3
(Homo sapiens (Human)) | BDBM50112355

(CHEMBL3609326)Show SMILES CCn1c2nc(Nc3ccc(N4CCNCC4)c(F)c3)ncc2cc(-c2ccc(Cl)cc2Cl)c1=O Show InChI InChI=1S/C25H23Cl2FN6O/c1-2-34-23-15(11-19(24(34)35)18-5-3-16(26)12-20(18)27)14-30-25(32-23)31-17-4-6-22(21(28)13-17)33-9-7-29-8-10-33/h3-6,11-14,29H,2,7-10H2,1H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length PAK3 (unknown origin) by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 3
(Homo sapiens (Human)) | BDBM101618

(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK3 (unknown origin) |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM101618

(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK2 (unknown origin) |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112350
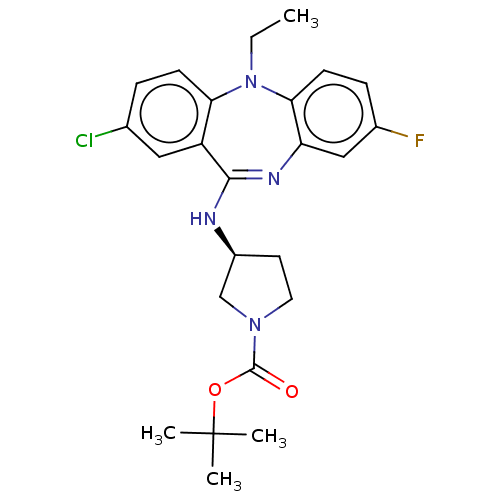
(CHEMBL3609370)Show SMILES CCN1c2ccc(F)cc2N=C(N[C@H]2CCN(C2)C(=O)OC(C)(C)C)c2cc(Cl)ccc12 |r,t:11| Show InChI InChI=1S/C24H28ClFN4O2/c1-5-30-20-8-6-15(25)12-18(20)22(28-19-13-16(26)7-9-21(19)30)27-17-10-11-29(14-17)23(31)32-24(2,3)4/h6-9,12-13,17H,5,10-11,14H2,1-4H3,(H,27,28)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM213434

(US9278981, 26)Show SMILES CN1CCN(CC1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C22H21F3N6O2/c1-30-6-8-31(9-7-30)20-19(16-11-26-14-27-12-16)10-15(13-28-20)21(32)29-17-2-4-18(5-3-17)33-22(23,24)25/h2-5,10-14H,6-9H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by high throughput assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112351
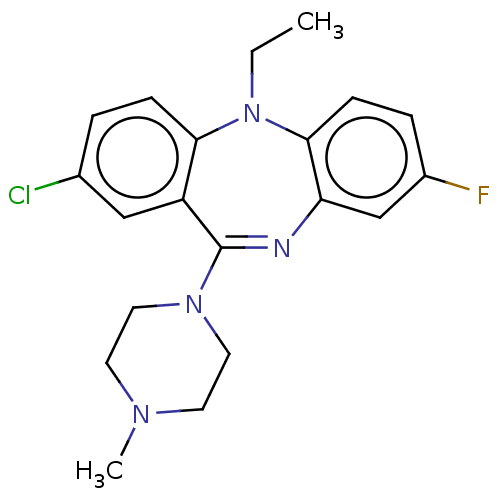
(CHEMBL3609330)Show SMILES CCN1c2ccc(F)cc2N=C(N2CCN(C)CC2)c2cc(Cl)ccc12 |t:11| Show InChI InChI=1S/C20H22ClFN4/c1-3-26-18-6-4-14(21)12-16(18)20(25-10-8-24(2)9-11-25)23-17-13-15(22)5-7-19(17)26/h4-7,12-13H,3,8-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50459086
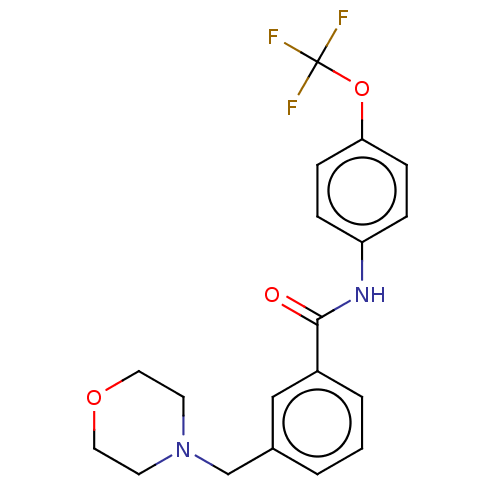
(CHEMBL4217428)Show SMILES FC(F)(F)Oc1ccc(NC(=O)c2cccc(CN3CCOCC3)c2)cc1 Show InChI InChI=1S/C19H19F3N2O3/c20-19(21,22)27-17-6-4-16(5-7-17)23-18(25)15-3-1-2-14(12-15)13-24-8-10-26-11-9-24/h1-7,12H,8-11,13H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50112355

(CHEMBL3609326)Show SMILES CCn1c2nc(Nc3ccc(N4CCNCC4)c(F)c3)ncc2cc(-c2ccc(Cl)cc2Cl)c1=O Show InChI InChI=1S/C25H23Cl2FN6O/c1-2-34-23-15(11-19(24(34)35)18-5-3-16(26)12-20(18)27)14-30-25(32-23)31-17-4-6-22(21(28)13-17)33-9-7-29-8-10-33/h3-6,11-14,29H,2,7-10H2,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 779 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length PAK4 (unknown origin) by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112353
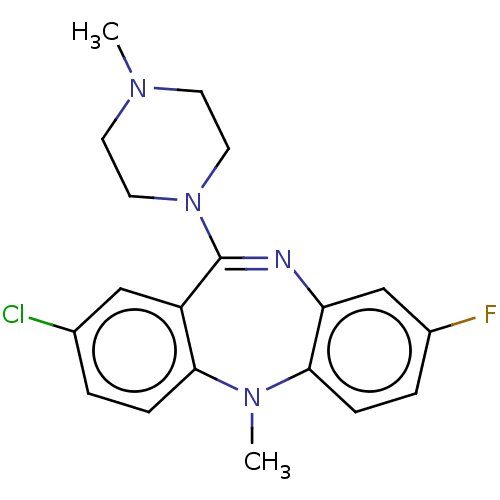
(CHEMBL3609329)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2N(C)c2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C19H20ClFN4/c1-23-7-9-25(10-8-23)19-15-11-13(20)3-5-17(15)24(2)18-6-4-14(21)12-16(18)22-19/h3-6,11-12H,7-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112353

(CHEMBL3609329)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2N(C)c2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C19H20ClFN4/c1-23-7-9-25(10-8-23)19-15-11-13(20)3-5-17(15)24(2)18-6-4-14(21)12-16(18)22-19/h3-6,11-12H,7-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) in presence of 1.5 uM of ATP |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM222494

(US9315489, 30)Show SMILES COc1ccc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C19H14F3N3O3/c1-27-17-7-2-12(8-16(17)13-9-23-11-24-10-13)18(26)25-14-3-5-15(6-4-14)28-19(20,21)22/h2-11H,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by high throughput assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM213594

(US9278981, 186)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O2S/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM213569

(US9278981, 161)Show SMILES OCCCNc1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C20H18F3N5O3/c21-20(22,23)31-16-4-2-15(3-5-16)28-19(30)13-8-17(14-9-24-12-25-10-14)18(27-11-13)26-6-1-7-29/h2-5,8-12,29H,1,6-7H2,(H,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by high throughput assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM213578

(US9278981, 170)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C21H18ClF2N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50391732

(CHEMBL2147257)Show SMILES CNC(=O)[C@@H](Cc1c[nH]c2cc(Cl)ccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(cc1)C(=O)P(O)(O)O)NC(=O)[C@H]1CCCC[C@@H]1C(O)=O |r| Show InChI InChI=1S/C44H52ClN6O13P/c1-46-39(55)36(21-27-23-47-33-22-28(45)15-16-29(27)33)51-40(56)32(17-18-37(52)53)48-41(57)35(19-24-7-3-2-4-8-24)50-42(58)34(49-38(54)30-9-5-6-10-31(30)43(59)60)20-25-11-13-26(14-12-25)44(61)65(62,63)64/h2-4,7-8,11-16,22-23,30-32,34-36,47,62-65H,5-6,9-10,17-21H2,1H3,(H,46,55)(H,48,57)(H,49,54)(H,50,58)(H,51,56)(H,52,53)(H,59,60)/t30-,31-,32-,34-,35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of pre-activated human GST-His6-tagged IGF-1R expressed in Sf9 cells using Ac-EAEDEPEGDYFEWLE-NHMe as substrate after 25 mins by luminesce... |
Eur J Med Chem 57: 1-9 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.038
BindingDB Entry DOI: 10.7270/Q24J0G6V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112354
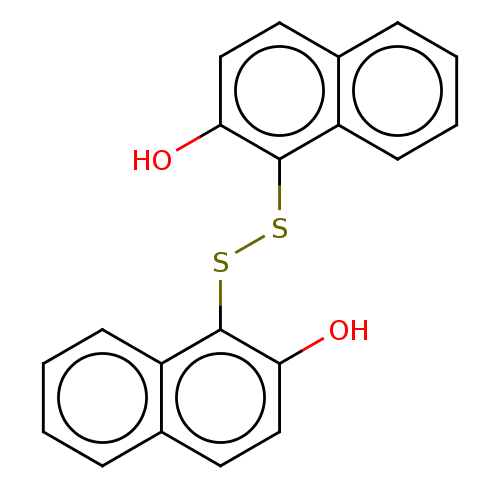
(CHEMBL472940)Show InChI InChI=1S/C20H14O2S2/c21-17-11-9-13-5-1-3-7-15(13)19(17)23-24-20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,21-22H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Non-ATP competitive inhibition of full-length human PAK1 assessed as phosphate incorporation onto MBP preincubated for 5 mins followed by Cdc42, MBP,... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM213578

(US9278981, 170)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C21H18ClF2N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by high throughput assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50459086

(CHEMBL4217428)Show SMILES FC(F)(F)Oc1ccc(NC(=O)c2cccc(CN3CCOCC3)c2)cc1 Show InChI InChI=1S/C19H19F3N2O3/c20-19(21,22)27-17-6-4-16(5-7-17)23-18(25)15-3-1-2-14(12-15)13-24-8-10-26-11-9-24/h1-7,12H,8-11,13H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by high throughput assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM213441

(US9278981, 33)Show SMILES O[C@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data