

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50472300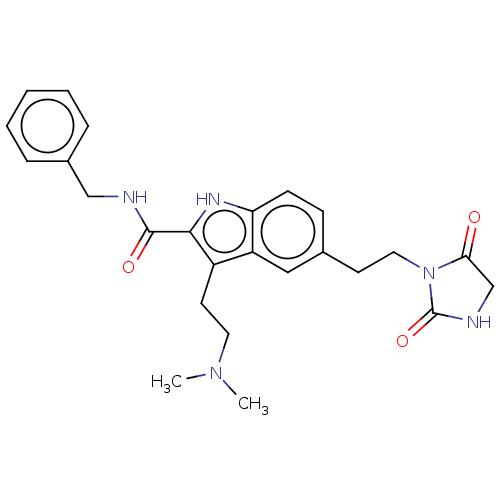 (CHEMBL84165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1D receptor in calf caudate homogenates. | J Med Chem 42: 2504-26 (1999) Article DOI: 10.1021/jm9706325 BindingDB Entry DOI: 10.7270/Q2VD7257 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50472300 (CHEMBL84165) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 2C receptor in rat cortex homogenates. | J Med Chem 42: 2504-26 (1999) Article DOI: 10.1021/jm9706325 BindingDB Entry DOI: 10.7270/Q2VD7257 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50472301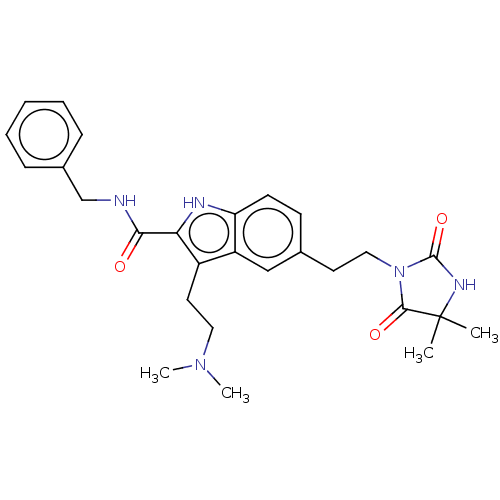 (CHEMBL86223) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 2C receptor in rat cortex homogenates. | J Med Chem 42: 2504-26 (1999) Article DOI: 10.1021/jm9706325 BindingDB Entry DOI: 10.7270/Q2VD7257 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50472301 (CHEMBL86223) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1D receptor in calf caudate homogenates. | J Med Chem 42: 2504-26 (1999) Article DOI: 10.1021/jm9706325 BindingDB Entry DOI: 10.7270/Q2VD7257 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50471285 (CHEMBL76480) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for 5-hydroxytryptamine 2C receptor was measured in rat cortex homogenates. | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50471285 (CHEMBL76480) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for Alpha-2 adrenergic receptor was measured in rat cortex homogenates. | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50471285 (CHEMBL76480) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for 5-hydroxytryptamine 2A receptor was measured in rat cortex homogenates | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50471285 (CHEMBL76480) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for 5-hydroxytryptamine 1A receptor was measured in rat cortex homogenates. | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50471285 (CHEMBL76480) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for 5-hydroxytryptamine 1D receptor was measured in calf caudate homogenate | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50471285 (CHEMBL76480) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for Dopamine receptor D2 was measured in rat cortex homogenates | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50471285 (CHEMBL76480) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for Alpha-1 adrenergic receptor was measured in rat cortex homogenates | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50472301 (CHEMBL86223) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor in rat cortex homogenates. | J Med Chem 42: 2504-26 (1999) Article DOI: 10.1021/jm9706325 BindingDB Entry DOI: 10.7270/Q2VD7257 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50471285 (CHEMBL76480) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for Dopamine receptor D1 was measured in rat cortex homogenates. | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1/Beta-2/Beta-3 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50471285 (CHEMBL76480) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Affinity pKi for Beta adrenergic receptor was measured in rat cortex homogenates | J Med Chem 40: 2347-62 (1997) Article DOI: 10.1021/jm9605849 BindingDB Entry DOI: 10.7270/Q2QZ2DN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50472300 (CHEMBL84165) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor in rat cortex homogenates. | J Med Chem 42: 2504-26 (1999) Article DOI: 10.1021/jm9706325 BindingDB Entry DOI: 10.7270/Q2VD7257 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297677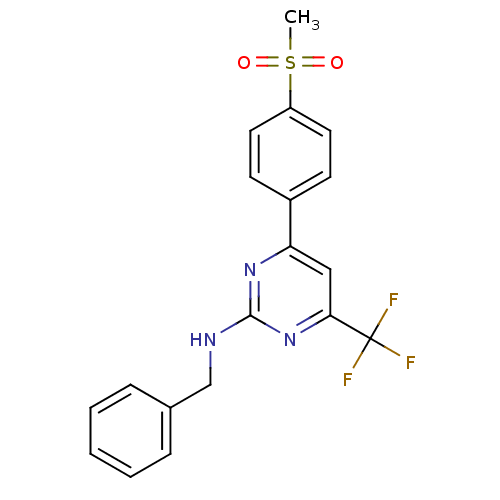 (CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297675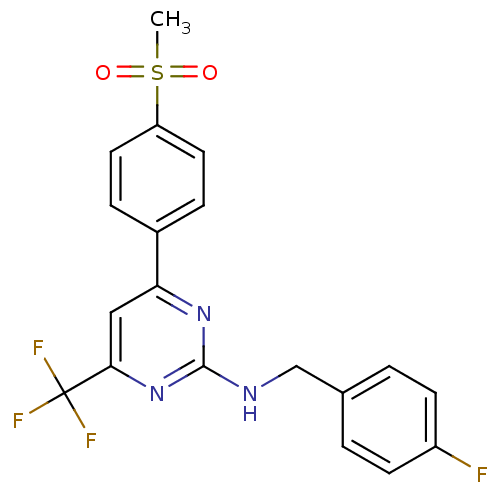 (CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572440 (CHEMBL4469438) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50153990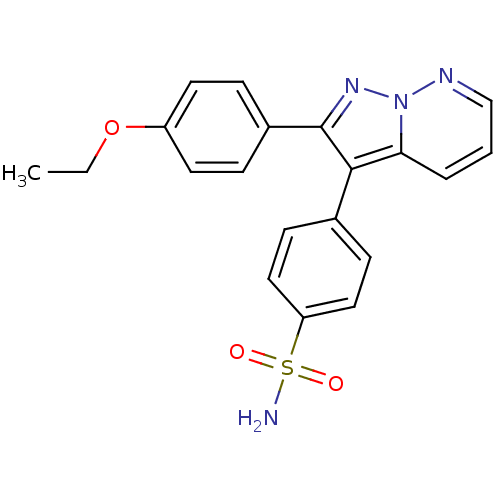 (4-[2-(4-Ethoxy-phenyl)-pyrazolo[1,5-b]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human cyclooxygenase-2 expressed in COS cells | Bioorg Med Chem Lett 14: 5445-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.089 BindingDB Entry DOI: 10.7270/Q2RJ4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297669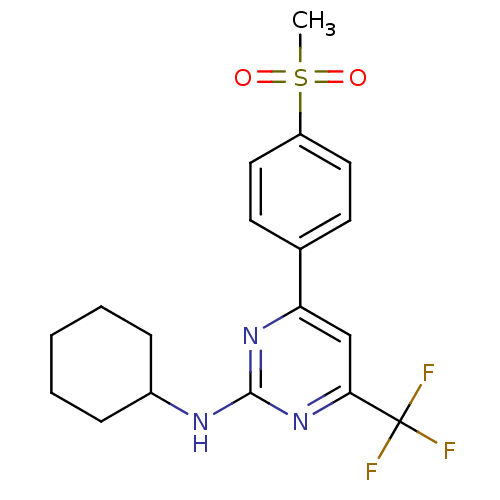 (CHEMBL561891 | N-cyclohexyl-4-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572444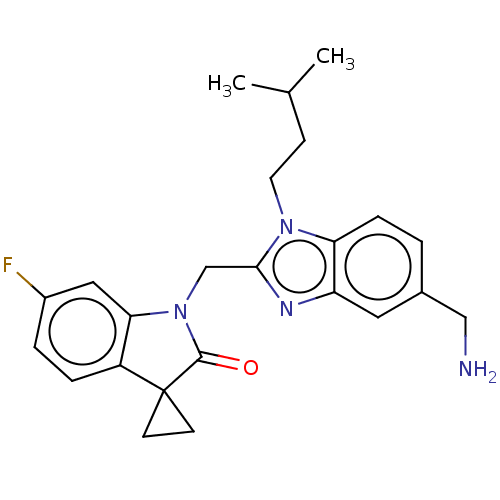 (CHEMBL4878302) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572466 (CHEMBL4876200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus fusion protein | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572442 (CHEMBL4872095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572463 (CHEMBL4871101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus fusion protein | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572450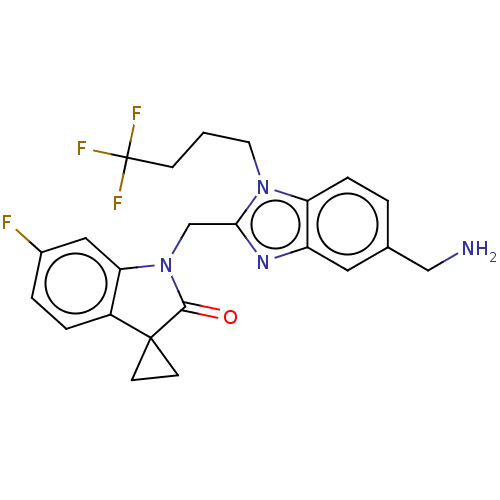 (Rv-521 | Rv521 | Sisunatovir) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572464 (CHEMBL4858040) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus fusion protein | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572451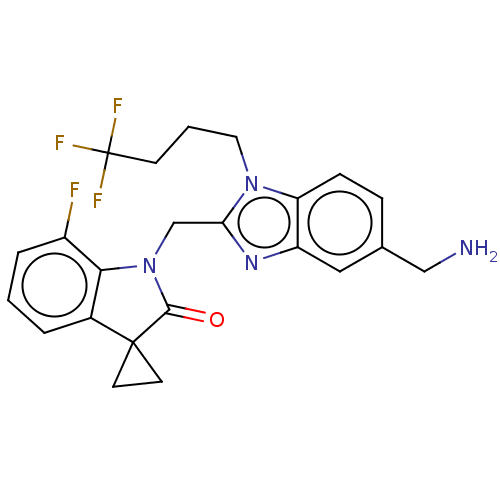 (CHEMBL4867108) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572465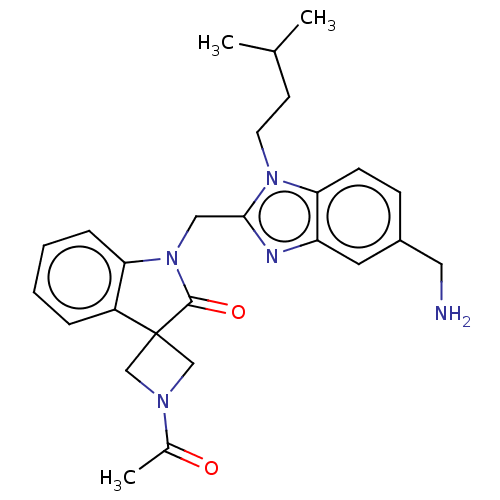 (CHEMBL4858949) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus fusion protein | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572445 (CHEMBL4865906) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572453 (CHEMBL4874709) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297672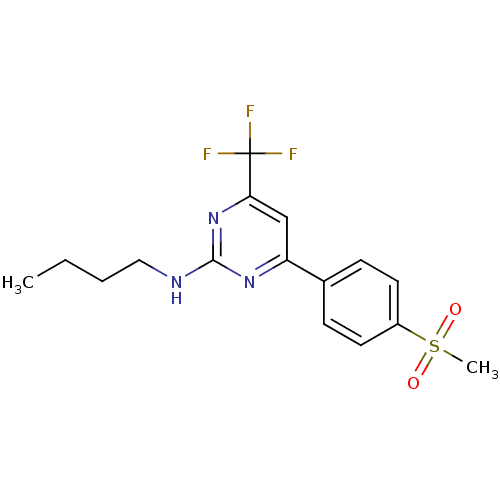 (CHEMBL549393 | N-butyl-4-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572447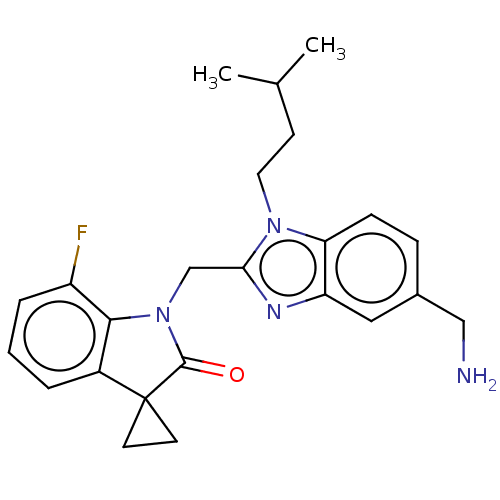 (CHEMBL4860271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297671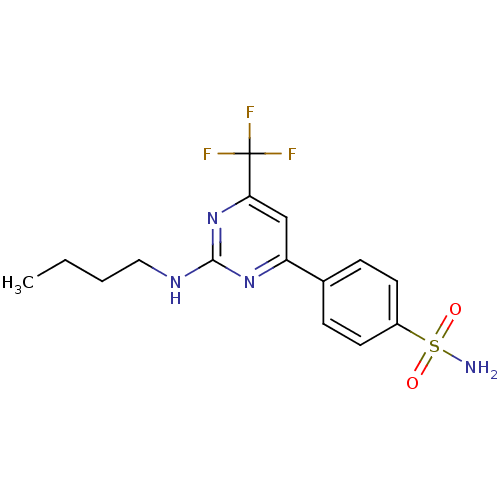 (4-(2-(butylamino)-6-(trifluoromethyl)pyrimidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572449 (CHEMBL4853602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297670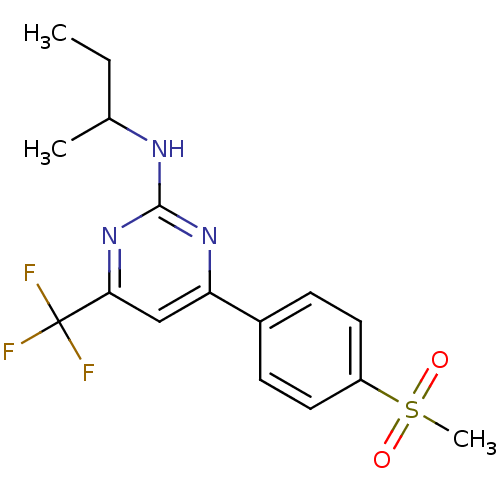 (CHEMBL539663 | GW-637185X | N-sec-butyl-4-(4-(meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50153994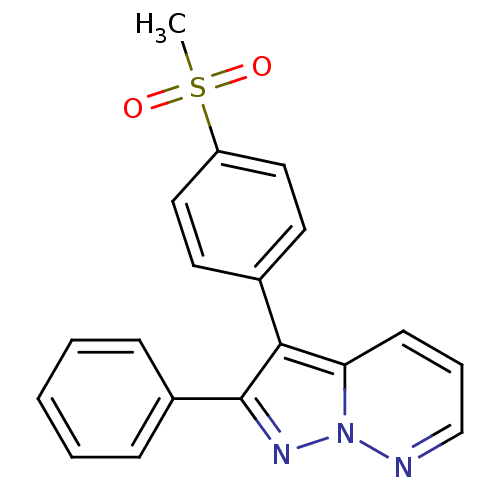 (3-(4-Methanesulfonyl-phenyl)-2-phenyl-pyrazolo[1,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human cyclooxygenase-2 expressed in COS cells | Bioorg Med Chem Lett 14: 5445-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.089 BindingDB Entry DOI: 10.7270/Q2RJ4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600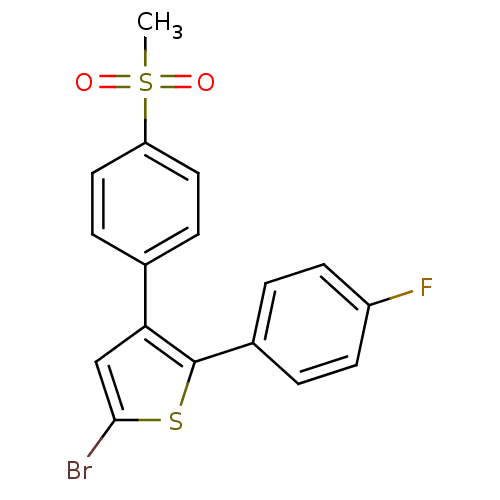 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297668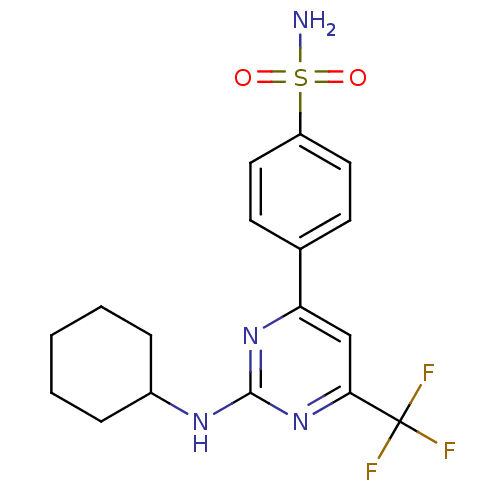 (4-(2-(cyclohexylamino)-6-(trifluoromethyl)pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572448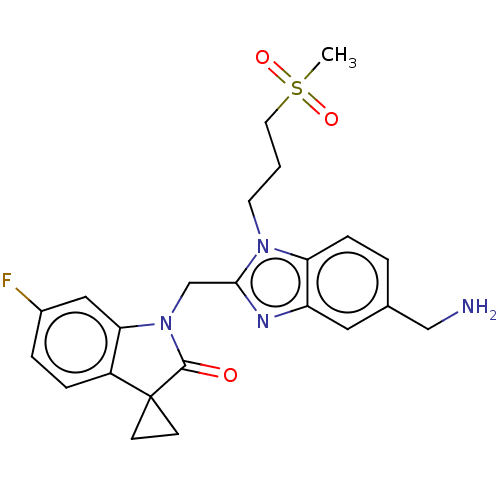 (CHEMBL4863172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572452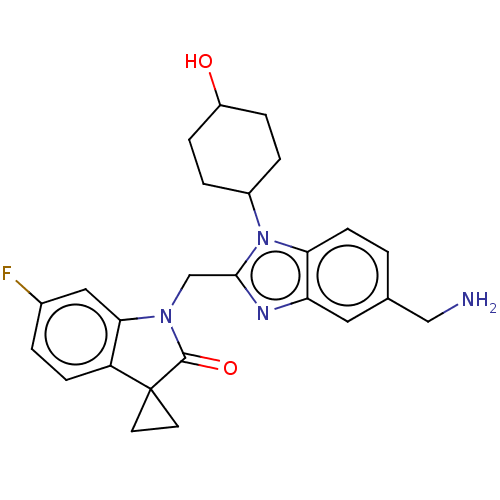 (CHEMBL4873528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50153982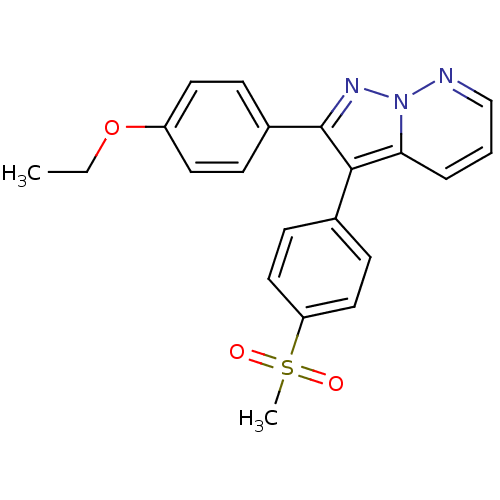 (2-(4-Ethoxy-phenyl)-3-(4-methanesulfonyl-phenyl)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human cyclooxygenase-2 expressed in COS cells | Bioorg Med Chem Lett 14: 5445-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.089 BindingDB Entry DOI: 10.7270/Q2RJ4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572454 (CHEMBL4855358) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297664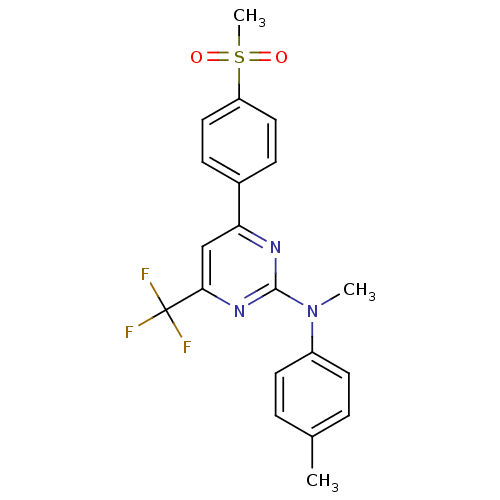 (CHEMBL559613 | N-methyl-4-(4-(methylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50151347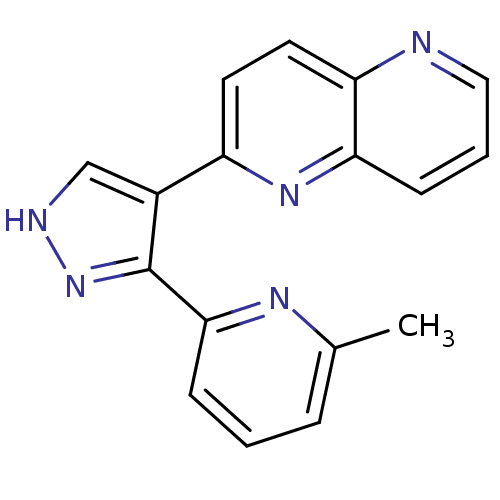 (2-(3-(6-methylpyridin-2-yl)-1H-pyrazol-4-yl)-1,5-n...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant Activin A receptor type II-like kinase (ALK5) expressed in baculovirus/Sf9 cells | J Med Chem 47: 4494-506 (2004) Article DOI: 10.1021/jm0400247 BindingDB Entry DOI: 10.7270/Q2VQ325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572443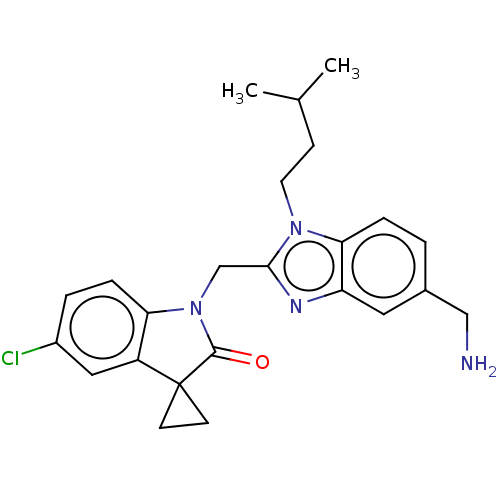 (CHEMBL4872263) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297665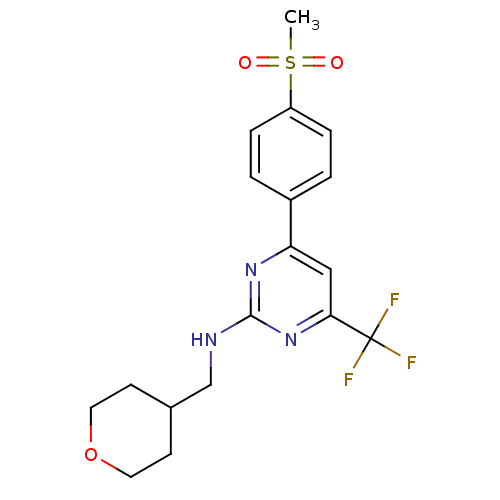 (4-(4-(methylsulfonyl)phenyl)-N-((tetrahydro-2H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297673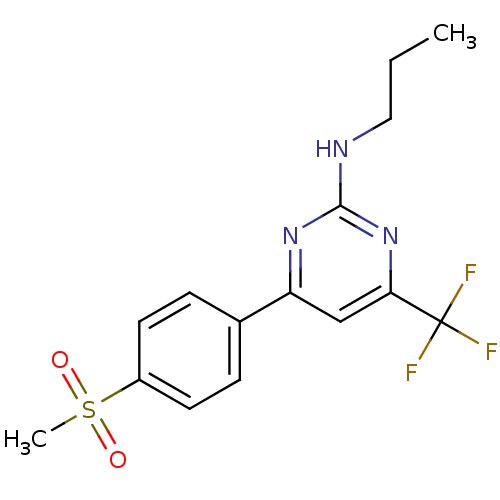 (4-(4-(methylsulfonyl)phenyl)-N-propyl-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297680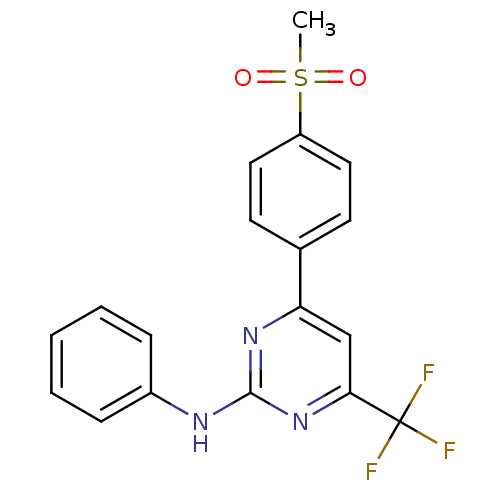 (4-(4-(methylsulfonyl)phenyl)-N-phenyl-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50151362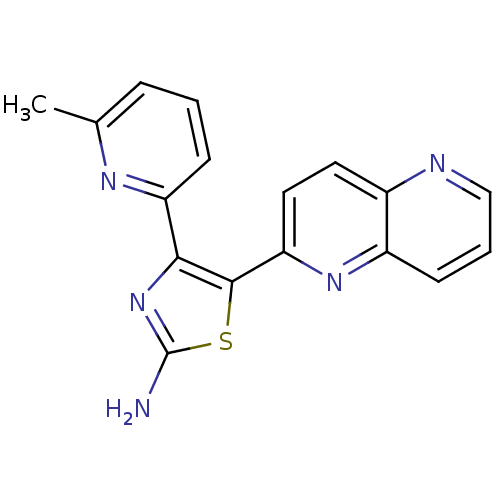 (4-(6-Methyl-pyridin-2-yl)-5-[1,5]naphthyridin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant Activin A receptor type II-like kinase (ALK5) expressed in baculovirus/Sf9 cells | J Med Chem 47: 4494-506 (2004) Article DOI: 10.1021/jm0400247 BindingDB Entry DOI: 10.7270/Q2VQ325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fusion glycoprotein F0 (Human respiratory syncytial virus A (strain A2)) | BDBM50572462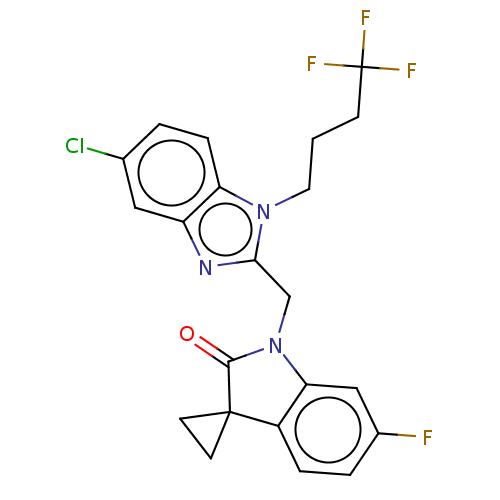 (CHEMBL4876843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01882 BindingDB Entry DOI: 10.7270/Q2QF8XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 316 total ) | Next | Last >> |