

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085677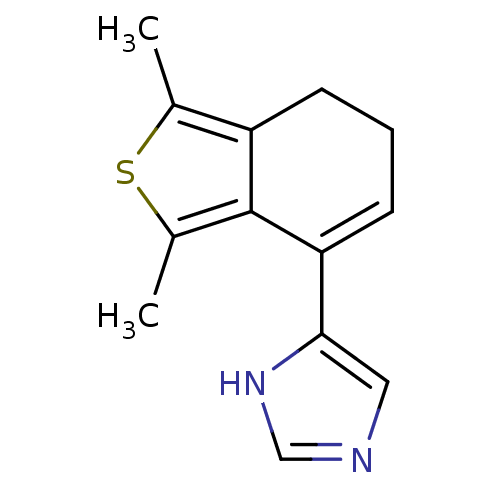 (4-(1,3-Dimethyl-6,7-dihydro-benzo[c]thiophen-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085677 (4-(1,3-Dimethyl-6,7-dihydro-benzo[c]thiophen-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085683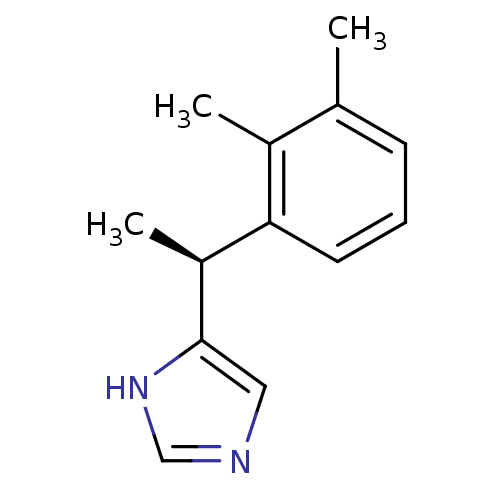 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085683 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029326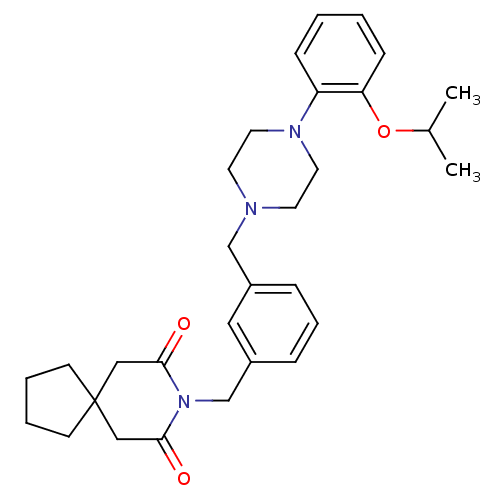 (8-{3-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064563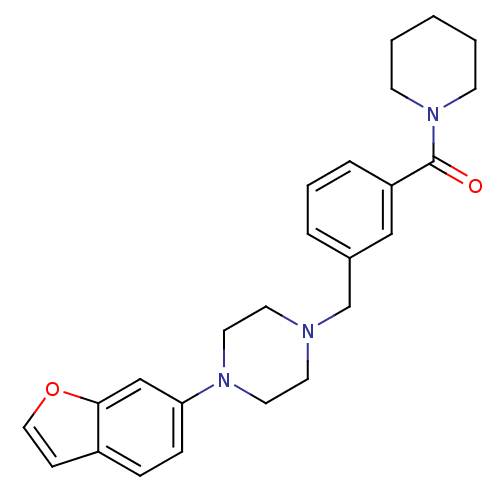 (CHEMBL61816 | [3-(4-Benzofuran-6-yl-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064537 (CHEMBL293658 | {3-[4-(2,3-Dihydro-benzo[1,4]dioxin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085679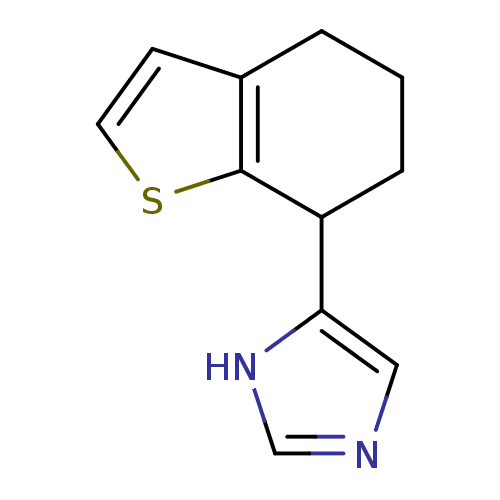 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-7-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085679 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-7-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity with Dopamine receptor D2 using membranes prepared from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50016897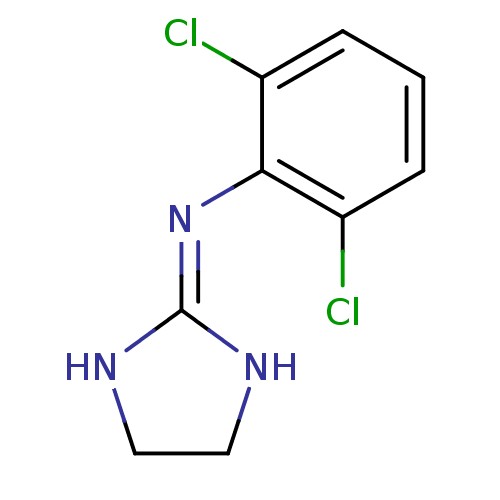 (2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50016897 (2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064529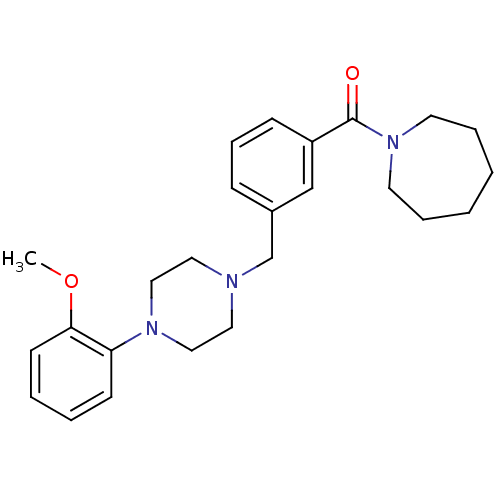 (Azepan-1-yl-{3-[4-(2-methoxy-phenyl)-piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]RTX binding to human Vanilloid receptor subtype 1 expressed in HEK293 cells | Bioorg Med Chem Lett 12: 1189-92 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085678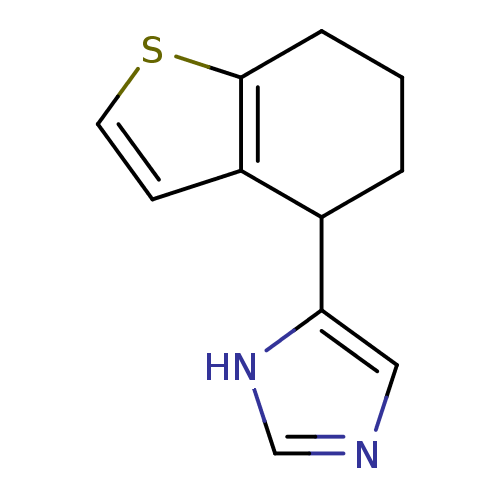 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-4-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085678 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-4-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064548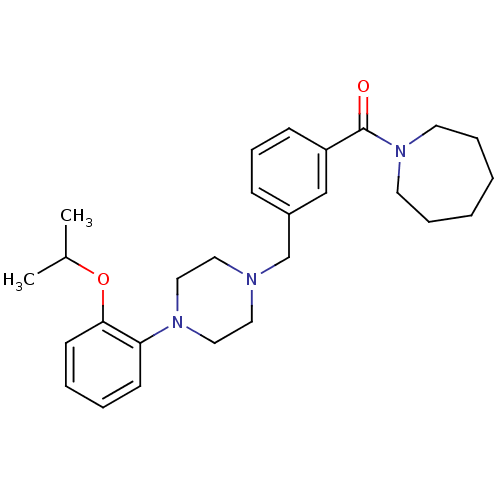 (Azepan-1-yl-{3-[4-(2-isopropoxy-phenyl)-piperazin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064536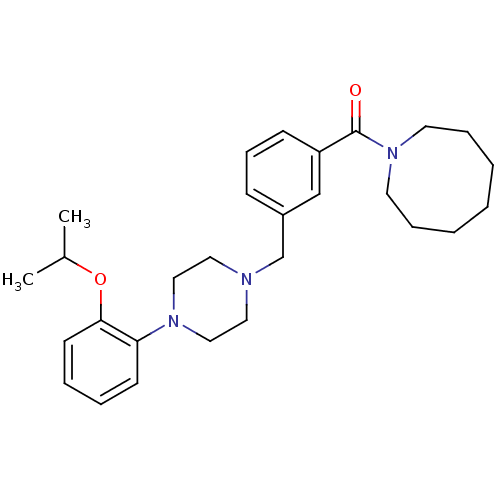 (Azocan-1-yl-{3-[4-(2-isopropoxy-phenyl)-piperazin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50111690 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]RTX binding to human Vanilloid receptor subtype 1 expressed in HEK293 cells | Bioorg Med Chem Lett 12: 1189-92 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064547 (Azepan-1-yl-{3-[4-(2-ethoxy-phenyl)-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064534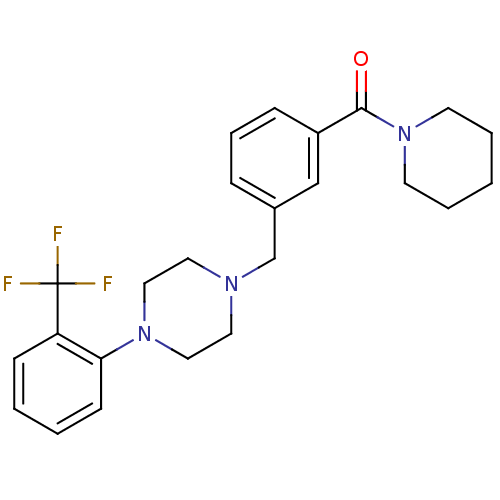 (CHEMBL413546 | Piperidin-1-yl-{3-[4-(2-trifluorome...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001869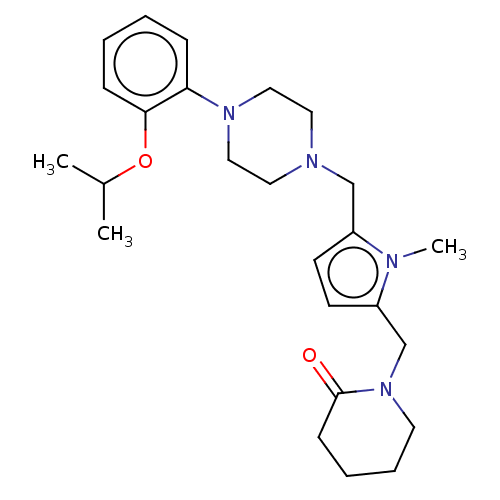 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the dopamine receptor D2 using [3H]spiperinone. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001869 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity with Dopamine receptor D2 using membranes prepared from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223336 (1-(1-(cyclopropylmethyl)-6-fluoro-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064565 (CHEMBL59167 | N,N-Dibutyl-3-[4-(2-isopropoxy-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50064564 (CHEMBL64528 | {3-[4-(2-Isopropoxy-phenyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D2 from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064564 (CHEMBL64528 | {3-[4-(2-Isopropoxy-phenyl)-piperidi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223314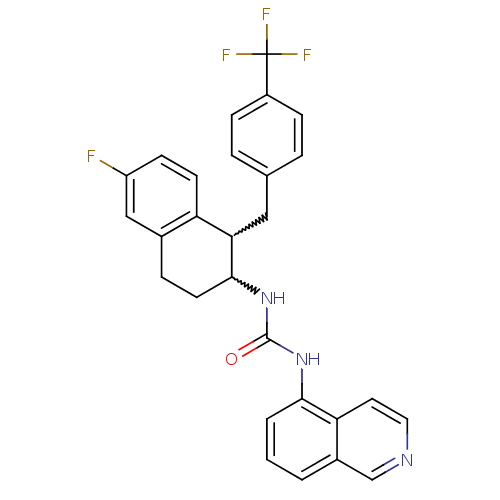 (1-(1-(4-(trifluoromethyl)benzyl)-6-fluoro-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50138518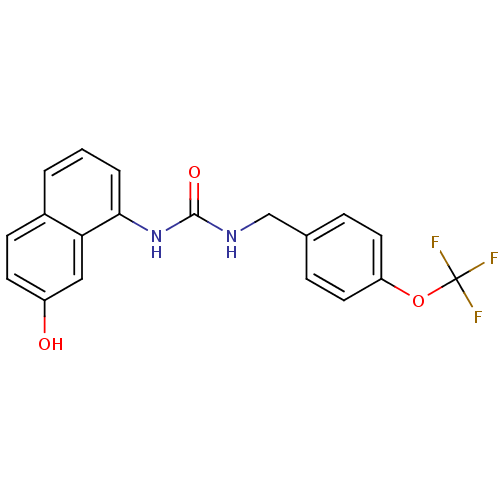 (1-(7-Hydroxy-naphthalen-1-yl)-3-(4-trifluoromethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Functional antagonistic activity against human vanilloid receptor subtype 1 in HEK293 cell membranes was determined as inhibition of agonist-induced ... | Bioorg Med Chem Lett 14: 531-4 (2003) BindingDB Entry DOI: 10.7270/Q2VQ323X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50138515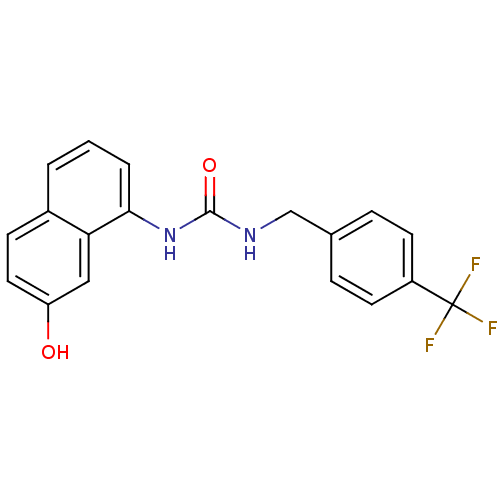 (1-(7-Hydroxy-naphthalen-1-yl)-3-(4-trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards cloned human vanilloid receptor subtype 1 in HEK293 cell membranes using [3H]-RTX as radioligand | Bioorg Med Chem Lett 14: 531-4 (2003) BindingDB Entry DOI: 10.7270/Q2VQ323X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223313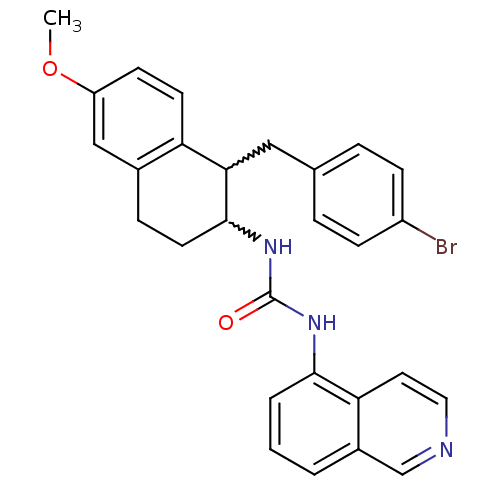 (1-(1-(4-bromobenzyl)-6-methoxy-1,2,3,4-tetrahydron...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50138503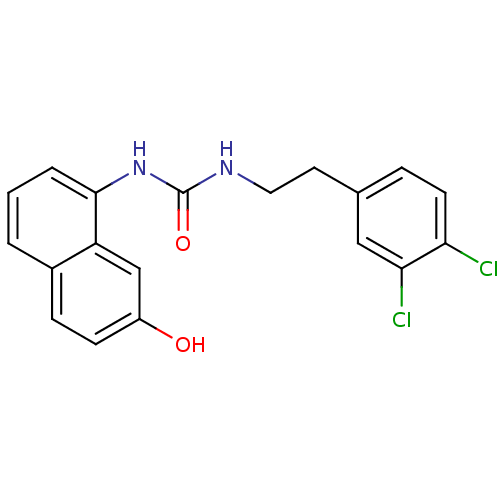 (1-[2-(3,4-Dichloro-phenyl)-ethyl]-3-(7-hydroxy-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Functional antagonistic activity against human vanilloid receptor subtype 1 in HEK293 cell membranes was determined as inhibition of agonist-induced ... | Bioorg Med Chem Lett 14: 531-4 (2003) BindingDB Entry DOI: 10.7270/Q2VQ323X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064556 (1-(2-Isopropoxy-phenyl)-4-[3-(piperidine-1-sulfony...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50064556 (1-(2-Isopropoxy-phenyl)-4-[3-(piperidine-1-sulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D2 from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064561 (CHEMBL60122 | {3-[4-(2-Isopropoxy-phenyl)-piperazi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064569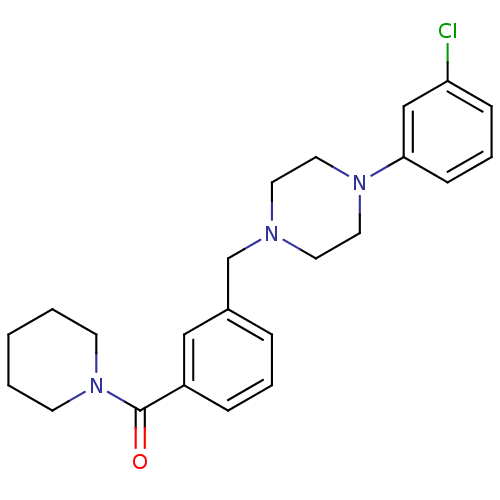 (CHEMBL61117 | {3-[4-(3-Chloro-phenyl)-piperazin-1-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064568 (CHEMBL292107 | N-Cyclohexyl-3-[4-(2-isopropoxy-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029301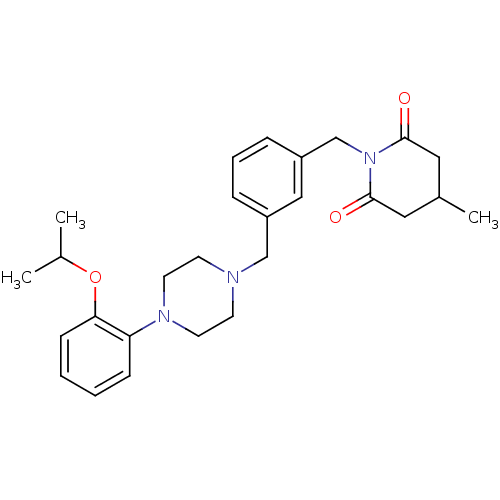 (1-{3-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50111692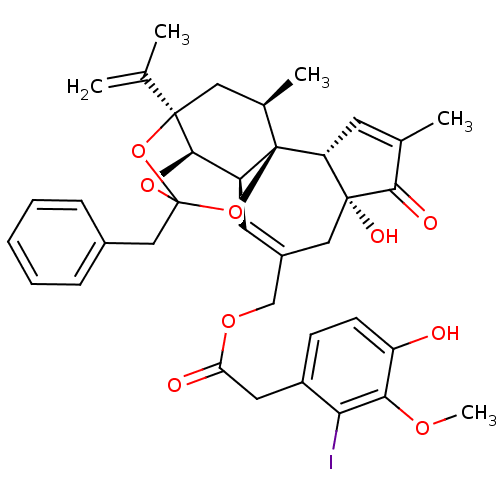 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]RTX binding to human Vanilloid receptor subtype 1 expressed in HEK293 cells | Bioorg Med Chem Lett 12: 1189-92 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the dopamine receptor D2 using [3H]spiperinone. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085680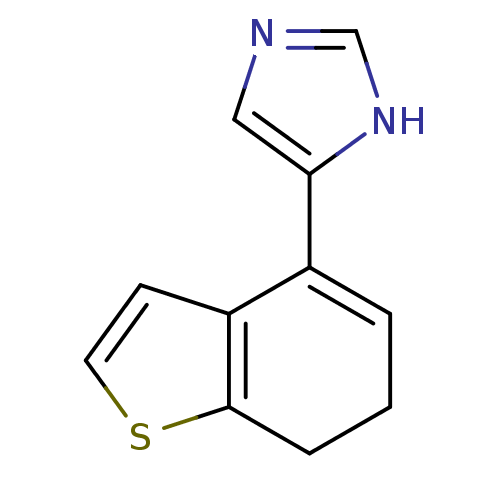 (4-(6,7-Dihydro-benzo[b]thiophen-4-yl)-1H-imidazole...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085680 (4-(6,7-Dihydro-benzo[b]thiophen-4-yl)-1H-imidazole...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50111691 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]RTX binding to human Vanilloid receptor subtype 1 expressed in HEK293 cells | Bioorg Med Chem Lett 12: 1189-92 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029319 (1-{2-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029257 ((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029257 ((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029257 ((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability to displace serotonin 5-HT1A receptor binding to rat cerebral cortex in competition experiments against [3H]-8-(hydroxy)dipropyl ami... | Bioorg Med Chem Lett 7: 1927-1930 (1997) Article DOI: 10.1016/S0960-894X(97)00336-3 BindingDB Entry DOI: 10.7270/Q2SQ90CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064558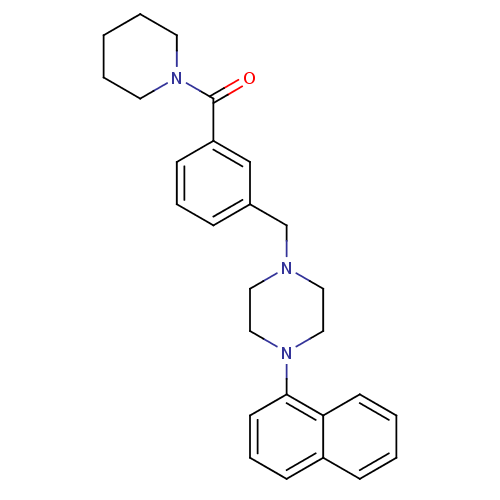 (CHEMBL59853 | [3-(4-Naphthalen-1-yl-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223321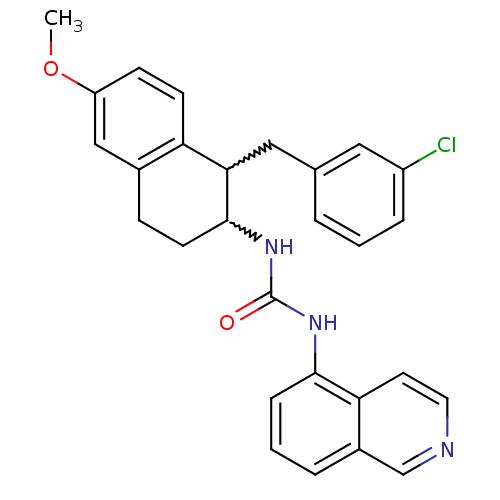 (1-(1-(3-chlorobenzyl)-6-methoxy-1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223338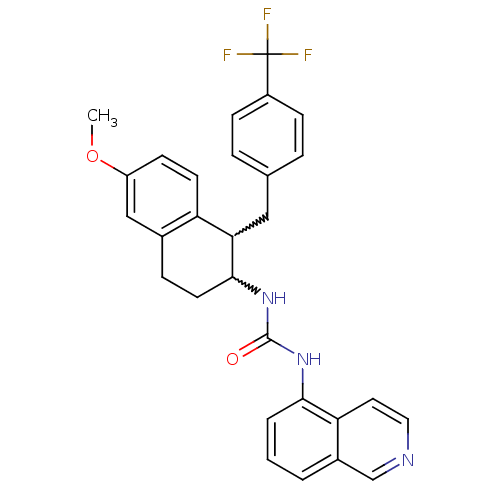 (1-(1-(4-(trifluoromethyl)benzyl)-6-methoxy-1,2,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 577 total ) | Next | Last >> |