

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236571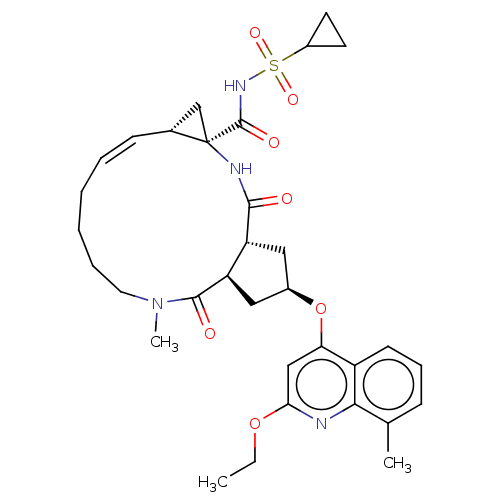 (US9365582, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236563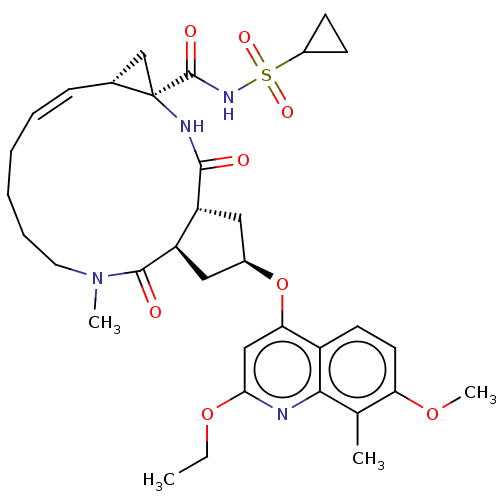 (US9365582, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236565 (US9365582, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236566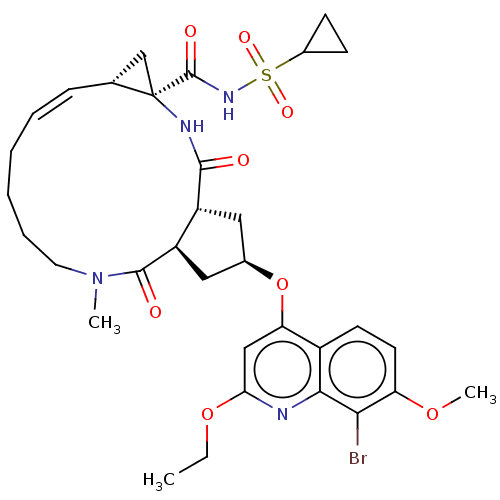 (US9365582, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236573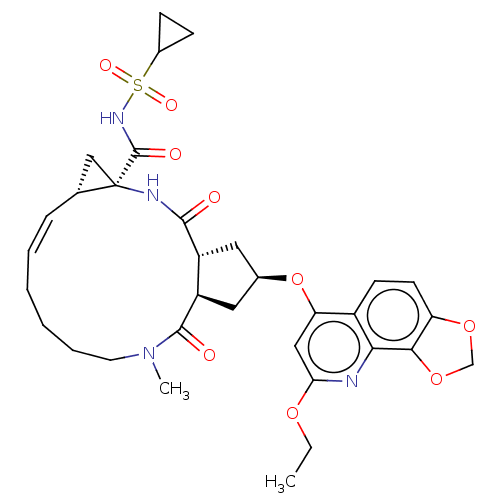 (US9365582, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236569 (US9365582, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236567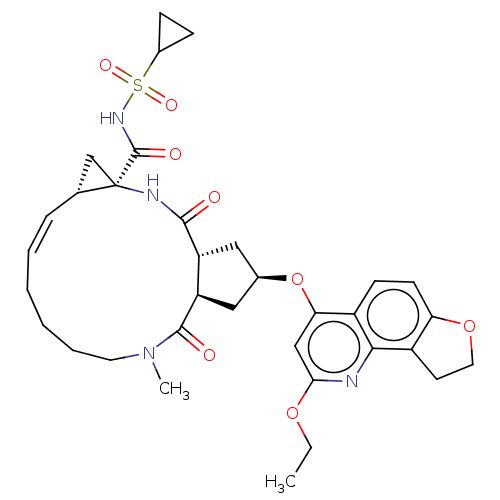 (US9365582, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236568 (US9365582, 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236570 (US9365582, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236562 (US9365582, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236564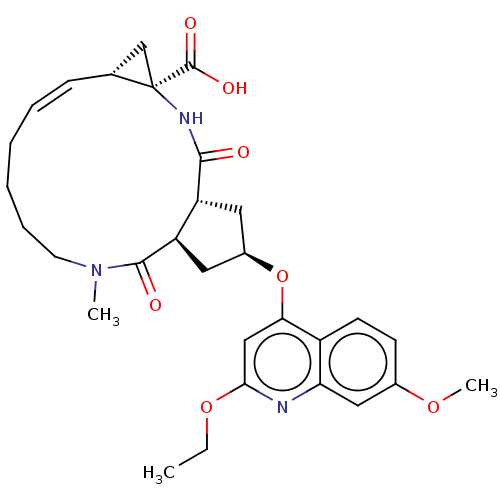 (US9365582, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 46 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236572 (US9365582, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194684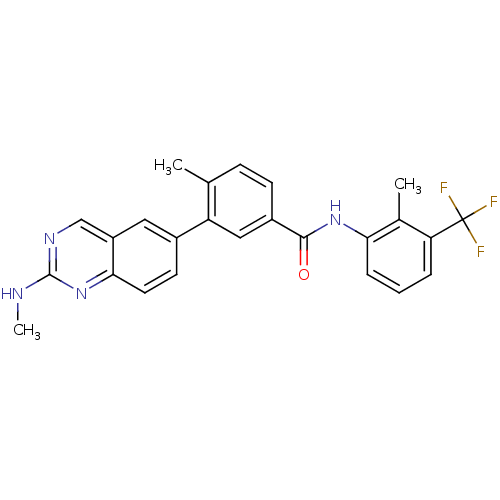 (4-methyl-N-(2-methyl-3-4-methyl-N-(2-methyl-3-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194688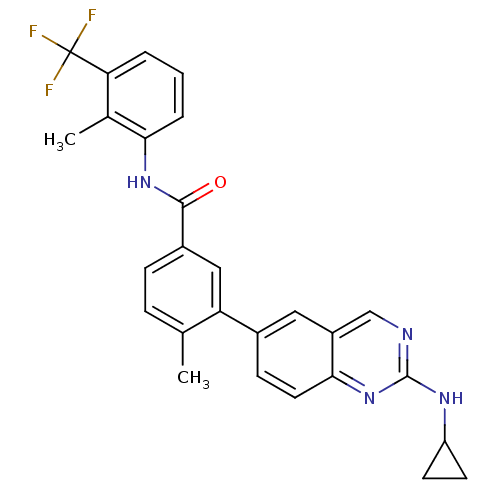 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM14949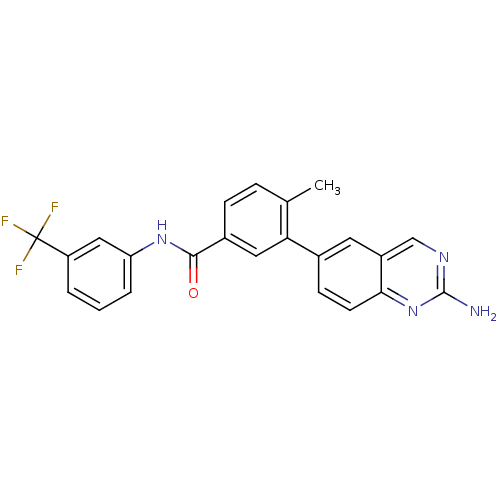 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hemagglutinin () | BDBM611021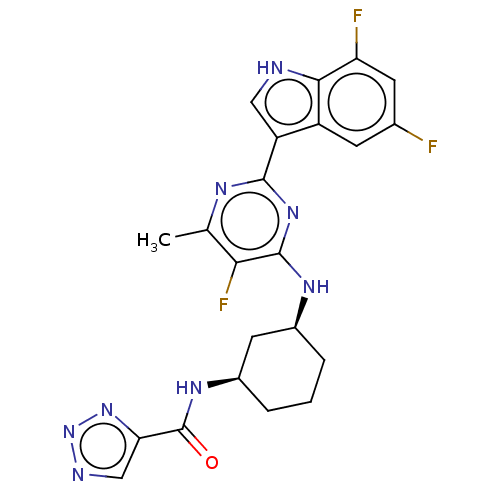 (US10626108, Compound 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM14949 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194668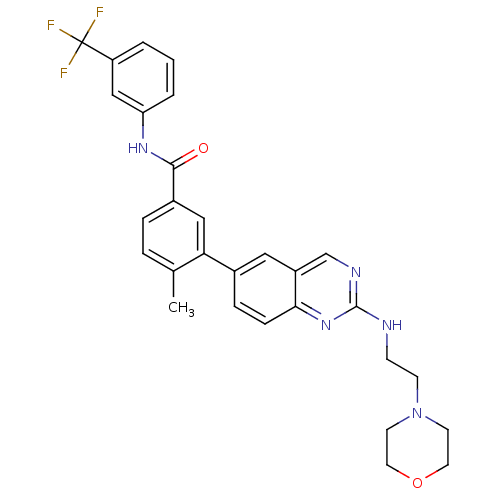 (4-methyl-3-(2-(2-morpholinoethylamino)quinazolin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194691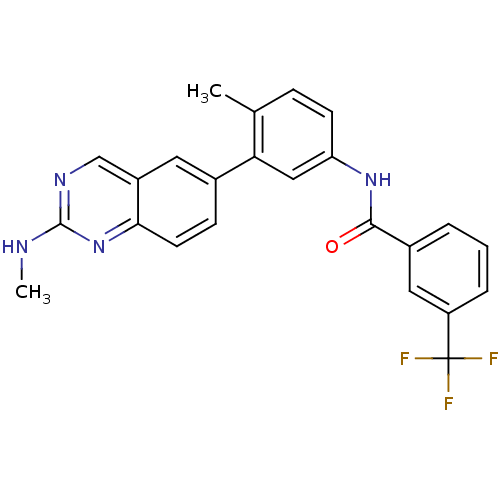 (CHEMBL212128 | N-(4-methyl-3-(2-(methylamino)quina...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194694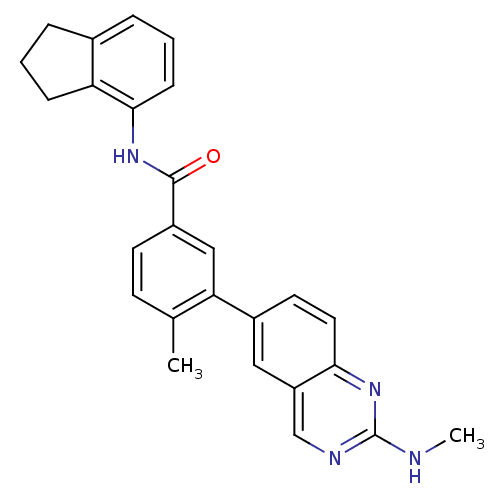 (CHEMBL427233 | N-(2,3-dihydro-1H-inden-4-yl)-4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM35317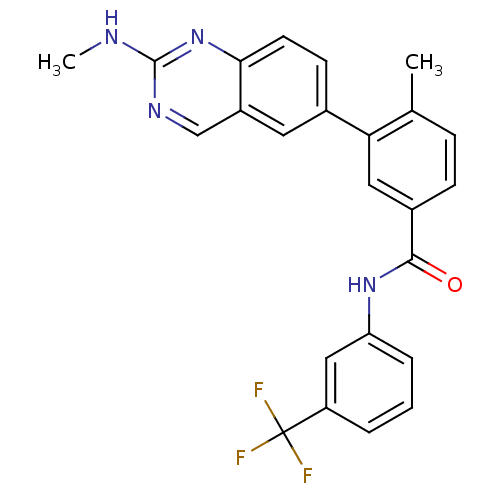 (4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26381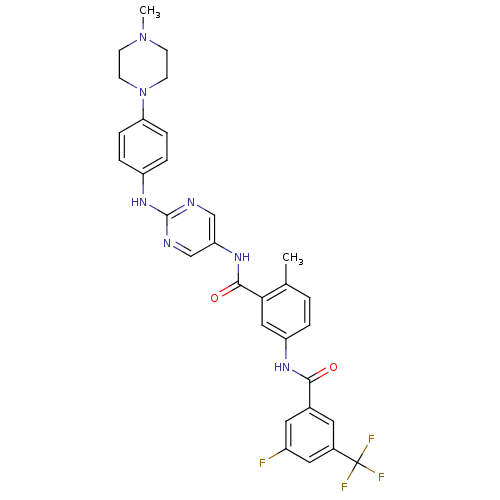 (1-N-[3-fluoro-5-(trifluoromethyl)benzene]-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194678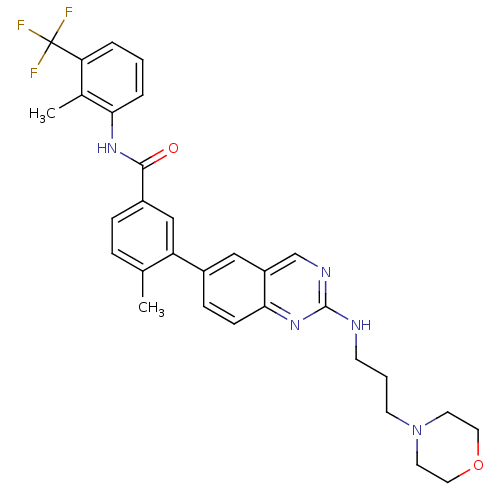 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26367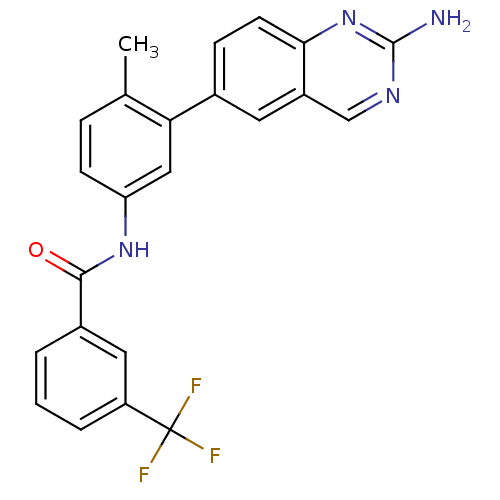 (Aminoquinazoline amide, 35 | N-[3-(2-aminoquinazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194686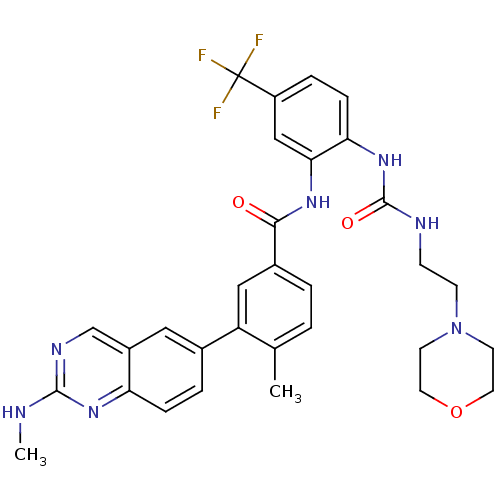 (1-(2-(4-methyl-3-(2-(methylamino)quinazolin-6-yl)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194670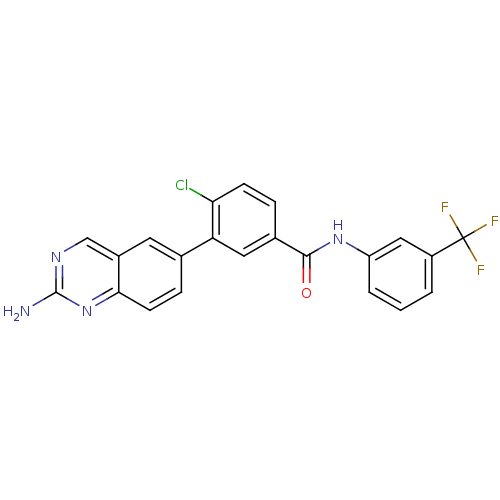 (3-(2-aminoquinazolin-6-yl)-4-chloro-N-(3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194690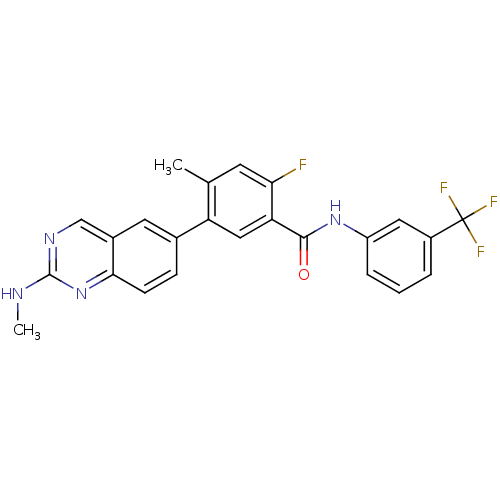 (2-fluoro-4-methyl-5-(2-(methylamino)quinazolin-6-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194671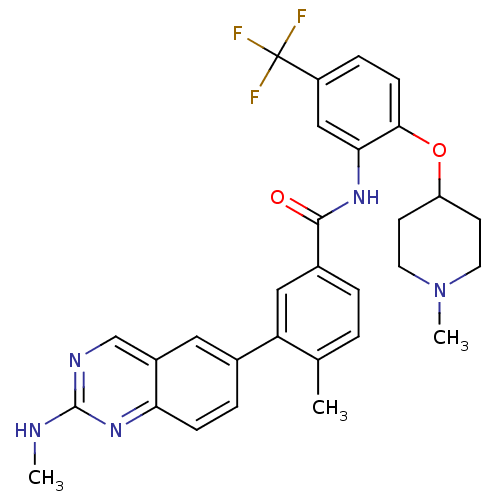 (4-methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194683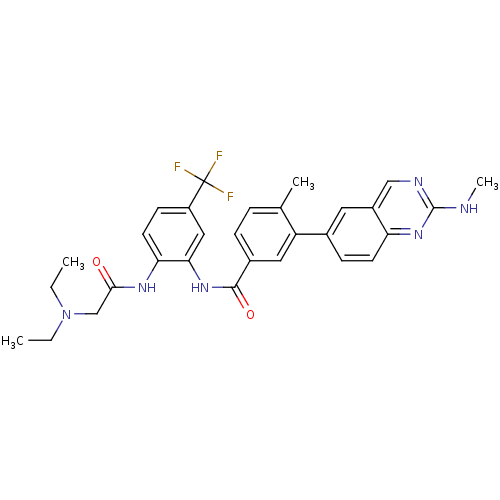 (CHEMBL212953 | N-(2-(2-(diethylamino)acetamido)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194681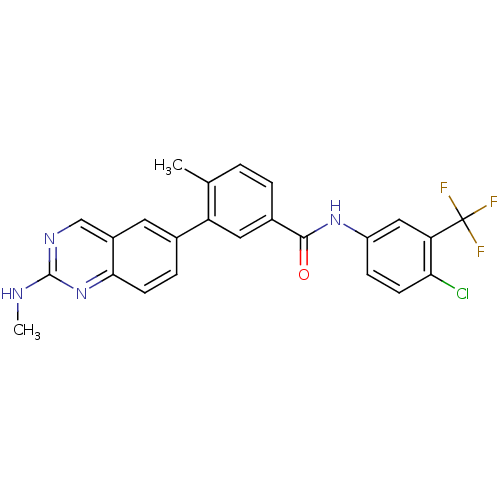 (CHEMBL215019 | N-(4-chloro-3-(trifluoromethyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26366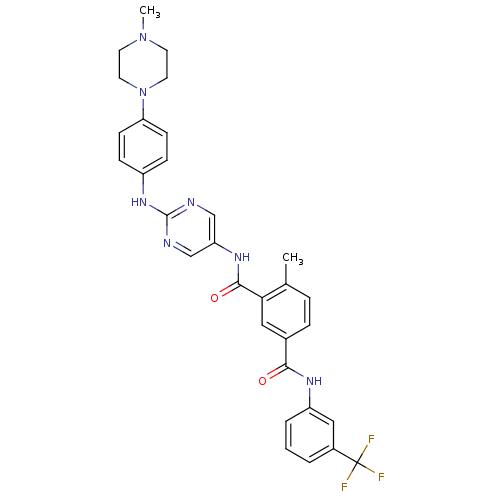 (4-methyl-3-N-(2-{[4-(4-methylpiperazin-1-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26372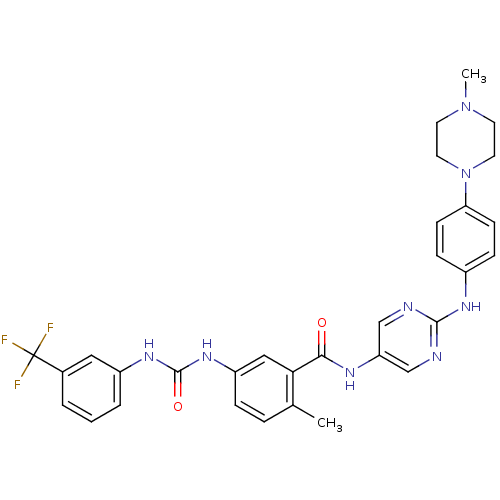 (2-methyl-N-(2-{[4-(4-methylpiperazin-1-yl)phenyl]a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26369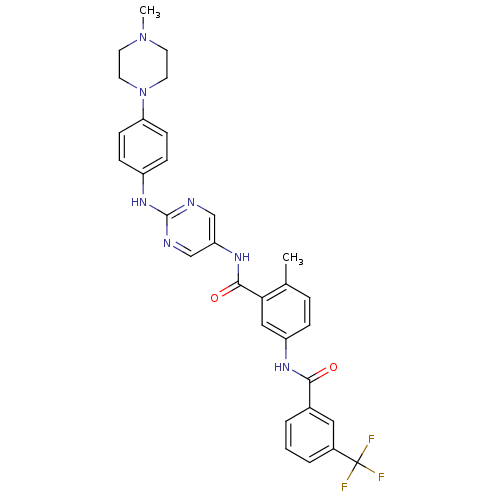 (4-methyl-3-N-(2-{[4-(4-methylpiperazin-1-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194679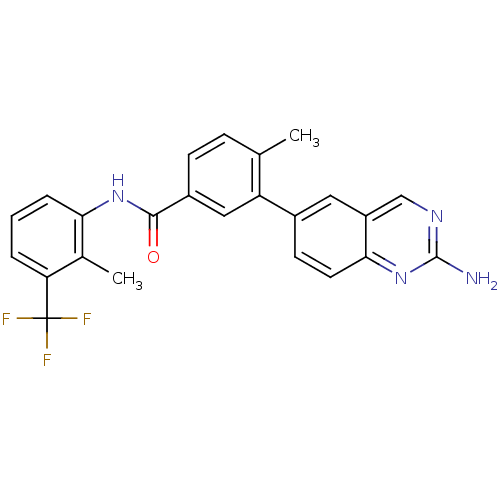 (3-(2-aminoquinazolin-6-yl)-4-methyl-N-(2-methyl-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194675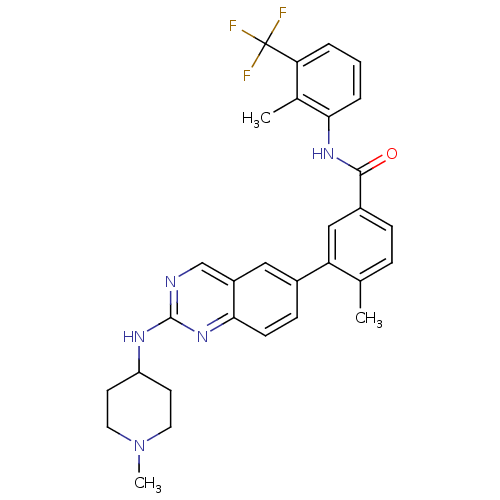 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17744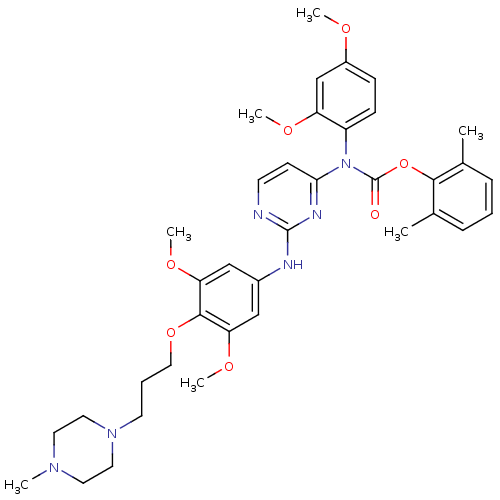 (2,6-dimethylphenyl N-[2-({3,5-dimethoxy-4-[3-(4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26382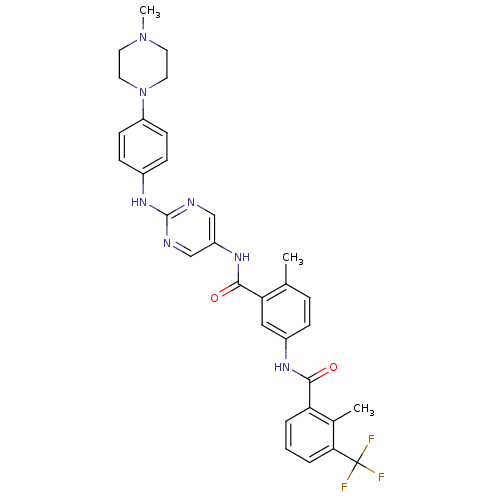 (4-methyl-1-N-[2-methyl-3-(trifluoromethyl)benzene]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50194691 (CHEMBL212128 | N-(4-methyl-3-(2-(methylamino)quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of p38-alpha by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194700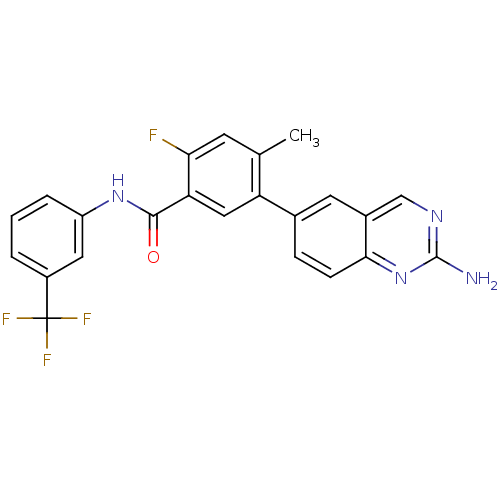 (5-(2-aminoquinazolin-6-yl)-2-fluoro-4-methyl-N-(3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM26379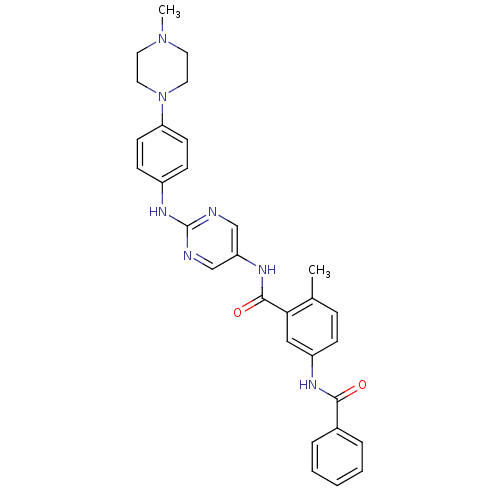 (1-N-benzene-4-methyl-3-N-(2-{[4-(4-methylpiperazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529403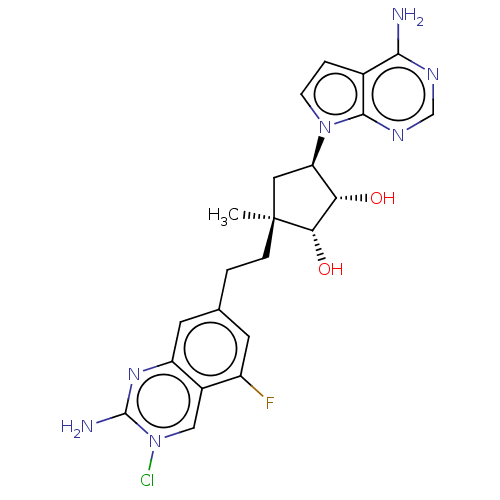 (US11198699, Compound 30) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529405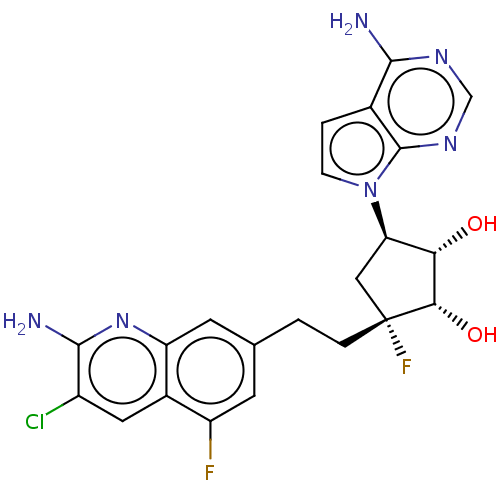 (US11198699, Compound 36) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529406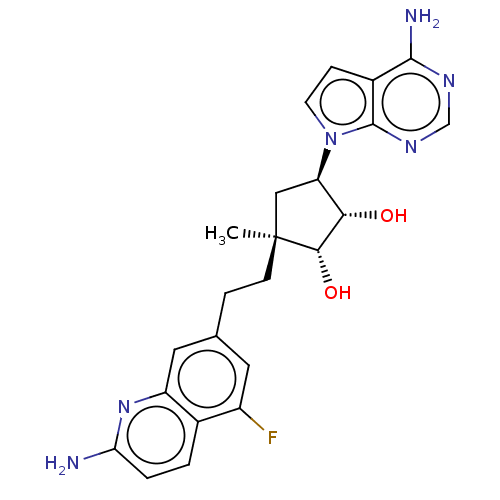 (US11198699, Compound 37) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529408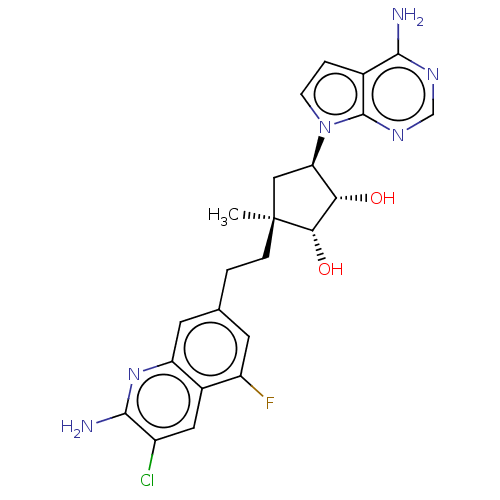 (US11198699, Compound 39) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529409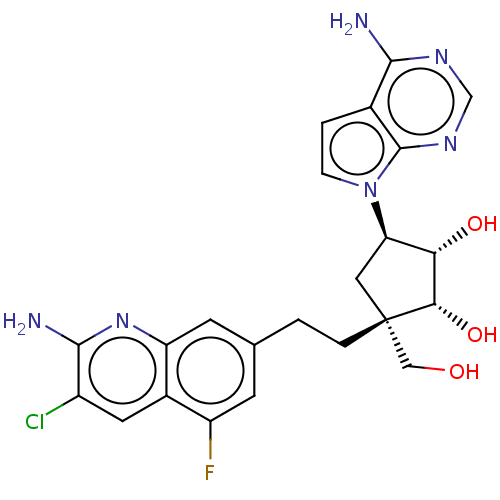 (US11198699, Compound 40) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529374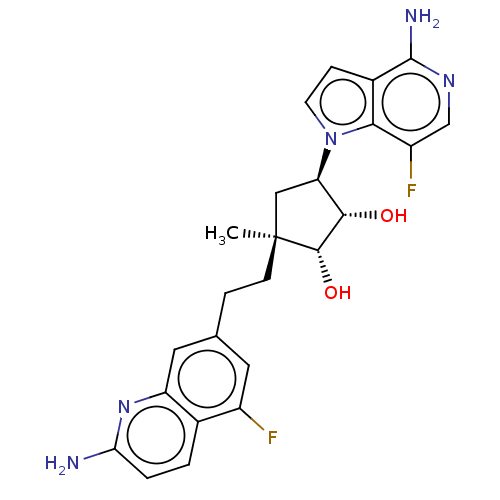 (US11198699, Compound 4 | US11198699, Compound 42) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529412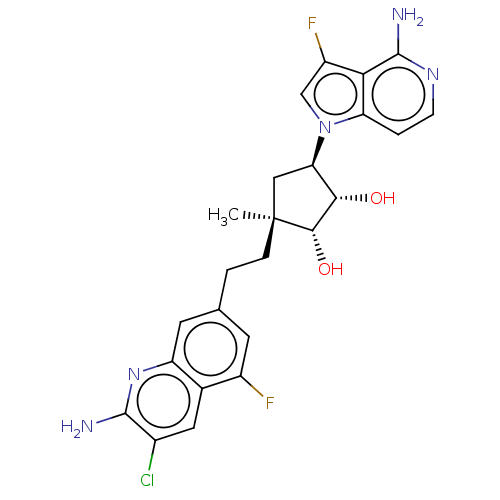 (US11198699, Compound 43) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529414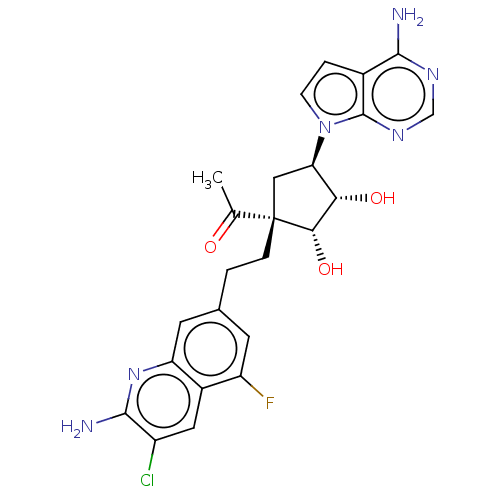 (US11198699, Compound 45) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529415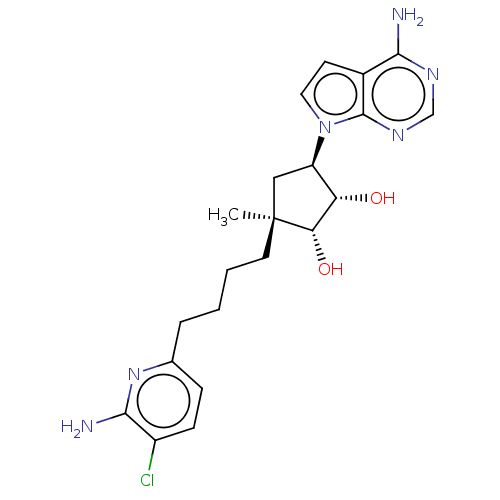 (US11198699, Compound 46) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM529416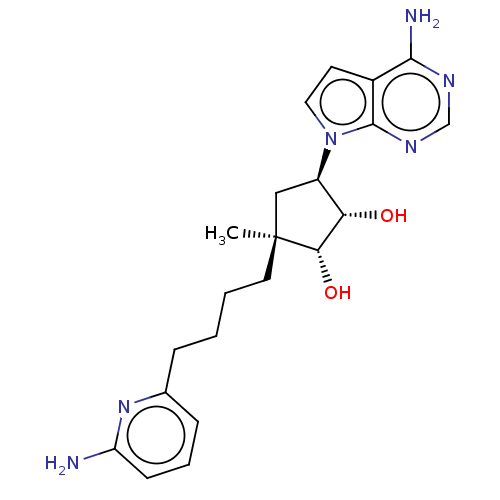 (US11198699, Compound 47) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 2: Compounds were tested for inhibition of methyltransferase activity in 384-well plate assay format using mass spectrometry technology. In thi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WS8XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 859 total ) | Next | Last >> |