 Found 3610 hits with Last Name = 'mei' and Initial = 'j'
Found 3610 hits with Last Name = 'mei' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50240829
(CHEMBL3559801)Show InChI InChI=1S/C16H24O2/c1-16(2,3)12-8-10-13(11-9-12)18-15-7-5-4-6-14(15)17/h8-11,14-15,17H,4-7H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leipzig University
Curated by ChEMBL
| Assay Description
Effect on pancreatic polypeptide-mediated displacement of 125I-pancreatic polypeptide from human C-terminal eYFP-tagged NY4 receptor expressed in HEK... |
J Med Chem 60: 7605-7612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00976
BindingDB Entry DOI: 10.7270/Q22B915R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50451631

(CHEMBL4212454)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(nn1C)C(C)(C)C |r| Show InChI InChI=1S/C21H28N6O2/c1-21(2,3)18-13-17(26(5)24-18)19(28)23-16(12-15-10-8-7-9-11-15)20(29)27(6)25(4)14-22/h7-11,13,16H,12H2,1-6H3,(H,23,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549366

(CHEMBL4755248)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NS(=O)(=O)c1cccc(Br)c1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050511
((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050511

((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50451625

(CHEMBL4217414)Show SMILES CC(C)C[C@H](NC(=O)c1cc(nn1C)C(C)(C)C)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C18H30N6O2/c1-12(2)9-13(17(26)24(8)22(6)11-19)20-16(25)14-10-15(18(3,4)5)21-23(14)7/h10,12-13H,9H2,1-8H3,(H,20,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549370

(CHEMBL4746022)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Br)c1)NC(=O)c1cc(nn1C)C(C)(C)C)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549372
(CHEMBL4744431)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(Br)c1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50431227
(CHEMBL2333024)Show SMILES CC(C)[C@H](NC(=O)c1cccnc1)C(=O)N1CCC[C@H]1B(O)O |r| Show InChI InChI=1S/C15H22BN3O4/c1-10(2)13(18-14(20)11-5-3-7-17-9-11)15(21)19-8-4-6-12(19)16(22)23/h3,5,7,9-10,12-13,22-23H,4,6,8H2,1-2H3,(H,18,20)/t12-,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis |
J Med Chem 56: 3467-77 (2013)
Article DOI: 10.1021/jm400351a
BindingDB Entry DOI: 10.7270/Q2C53N7W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549374

(CHEMBL4746374)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(c1)-c1cccnc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271221
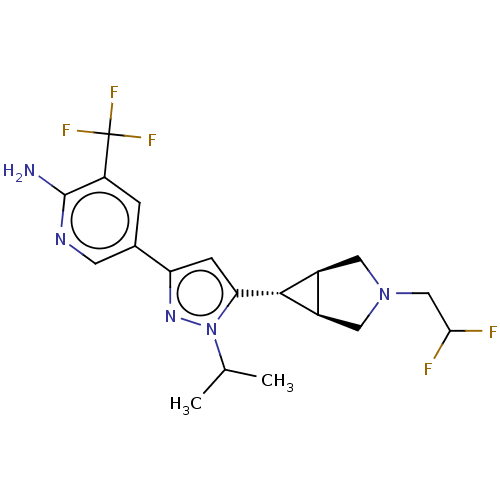
(CHEMBL3717769)Show SMILES [H][C@@]12CN(CC(F)F)C[C@]1([H])[C@H]2c1cc(nn1C(C)C)-c1cnc(N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H22F5N5/c1-9(2)29-15(17-11-6-28(7-12(11)17)8-16(20)21)4-14(27-29)10-3-13(19(22,23)24)18(25)26-5-10/h3-5,9,11-12,16-17H,6-8H2,1-2H3,(H2,25,26)/t11-,12+,17+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM31992
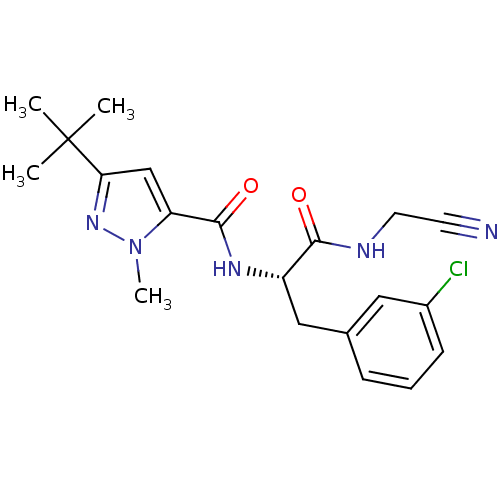
(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP8 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50451625

(CHEMBL4217414)Show SMILES CC(C)C[C@H](NC(=O)c1cc(nn1C)C(C)(C)C)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C18H30N6O2/c1-12(2)9-13(17(26)24(8)22(6)11-19)20-16(25)14-10-15(18(3,4)5)21-23(14)7/h10,12-13H,9H2,1-8H3,(H,20,25)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Trypanosoma cruzi cruzain using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substra... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50451625

(CHEMBL4217414)Show SMILES CC(C)C[C@H](NC(=O)c1cc(nn1C)C(C)(C)C)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C18H30N6O2/c1-12(2)9-13(17(26)24(8)22(6)11-19)20-16(25)14-10-15(18(3,4)5)21-23(14)7/h10,12-13H,9H2,1-8H3,(H,20,25)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of Trypanosoma cruzi cruzipain using Z-Phe-Arg-7-amidomethylcoumarin as substrate measured after 30 mins by fluorometric analysis |
Citation and Details
Article DOI: 10.1039/d0md00097c
BindingDB Entry DOI: 10.7270/Q2DJ5K9T |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50451631

(CHEMBL4212454)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(nn1C)C(C)(C)C |r| Show InChI InChI=1S/C21H28N6O2/c1-21(2,3)18-13-17(26(5)24-18)19(28)23-16(12-15-10-8-7-9-11-15)20(29)27(6)25(4)14-22/h7-11,13,16H,12H2,1-6H3,(H,23,28)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Trypanosoma cruzi cruzain using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substra... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50253621
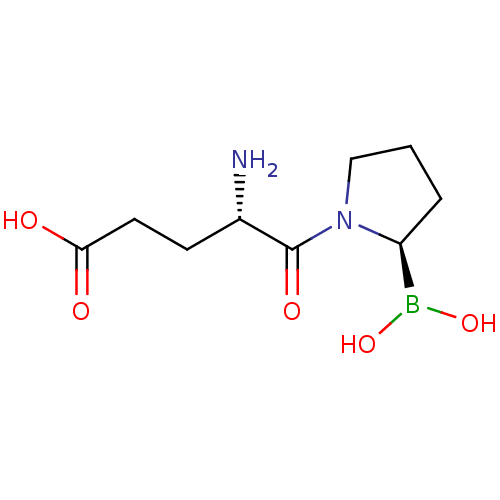
((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...)Show InChI InChI=1S/C9H17BN2O5/c11-6(3-4-8(13)14)9(15)12-5-1-2-7(12)10(16)17/h6-7,16-17H,1-5,11H2,(H,13,14)/t6-,7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP8 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50304793
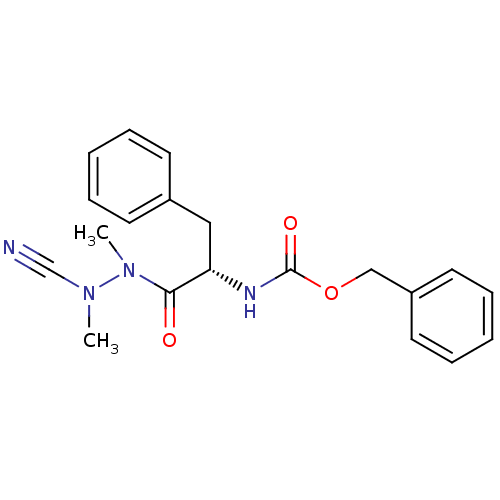
((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h3-12,18H,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of Trypanosoma cruzi cruzipain using Z-Phe-Arg-7-amidomethylcoumarin as substrate measured after 30 mins by fluorometric analysis |
Citation and Details
Article DOI: 10.1039/d0md00097c
BindingDB Entry DOI: 10.7270/Q2DJ5K9T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50476305
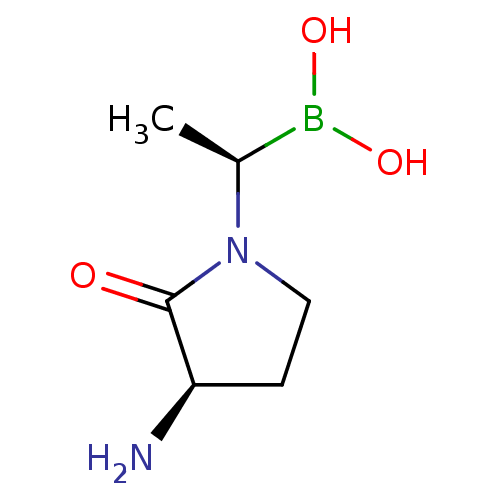
(CHEMBL2068511)Show InChI InChI=1S/C6H13BN2O3/c1-4(7(11)12)9-3-2-5(8)6(9)10/h4-5,11-12H,2-3,8H2,1H3/t4-,5+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271243
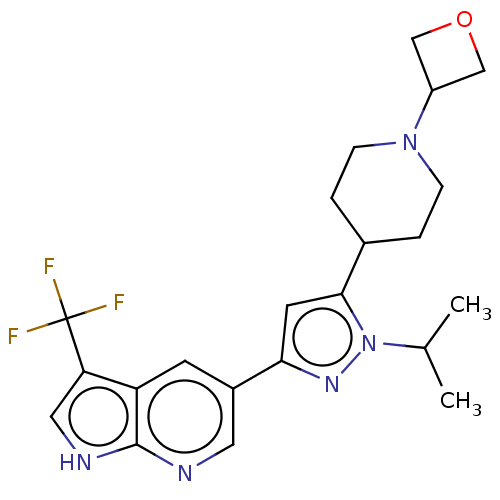
(CHEMBL3718344)Show SMILES CC(C)n1nc(cc1C1CCN(CC1)C1COC1)-c1cnc2[nH]cc(c2c1)C(F)(F)F Show InChI InChI=1S/C22H26F3N5O/c1-13(2)30-20(14-3-5-29(6-4-14)16-11-31-12-16)8-19(28-30)15-7-17-18(22(23,24)25)10-27-21(17)26-9-15/h7-10,13-14,16H,3-6,11-12H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50253621

((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...)Show InChI InChI=1S/C9H17BN2O5/c11-6(3-4-8(13)14)9(15)12-5-1-2-7(12)10(16)17/h6-7,16-17H,1-5,11H2,(H,13,14)/t6-,7-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372007
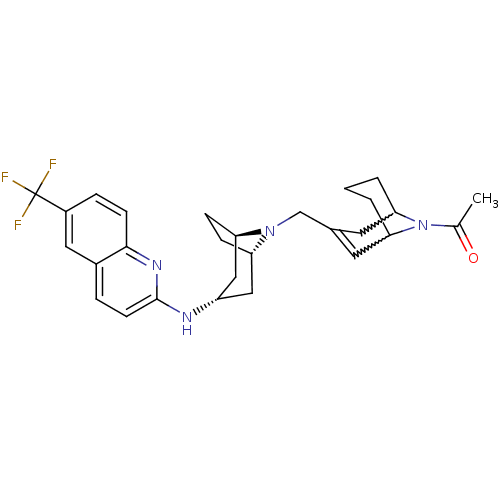
(CHEMBL257017)Show SMILES CC(=O)N1C2CCCC1C=C(CN1[C@H]3CC[C@@H]1C[C@H](C3)Nc1ccc3cc(ccc3n1)C(F)(F)F)C2 |w:4.40,8.9,t:10,TLB:1:3:10.35.9:7.5.6,THB:11:12:19.18.17:14.15| Show InChI InChI=1S/C28H33F3N4O/c1-17(36)35-24-3-2-4-25(35)12-18(11-24)16-34-22-7-8-23(34)15-21(14-22)32-27-10-5-19-13-20(28(29,30)31)6-9-26(19)33-27/h5-6,9-11,13,21-25H,2-4,7-8,12,14-16H2,1H3,(H,32,33)/t21-,22-,23+,24?,25? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227864
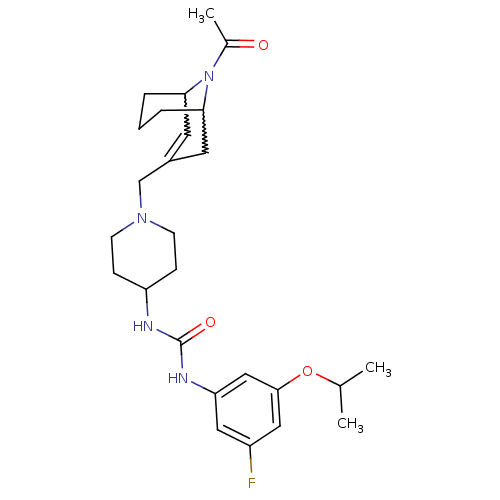
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cc(F)cc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:25.25,21.20,t:19,TLB:28:27:19.26.20:22.24.23| Show InChI InChI=1S/C26H37FN4O3/c1-17(2)34-25-14-20(27)13-22(15-25)29-26(33)28-21-7-9-30(10-8-21)16-19-11-23-5-4-6-24(12-19)31(23)18(3)32/h11,13-15,17,21,23-24H,4-10,12,16H2,1-3H3,(H2,28,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50087676
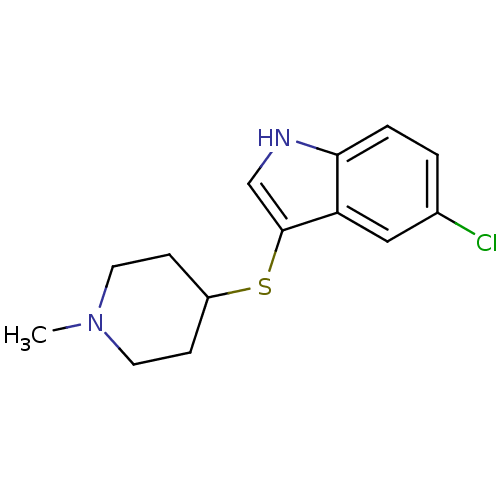
(5-Chloro-3-(1-methyl-piperidin-4-ylsulfanyl)-1H-in...)Show InChI InChI=1S/C14H17ClN2S/c1-17-6-4-11(5-7-17)18-14-9-16-13-3-2-10(15)8-12(13)14/h2-3,8-9,11,16H,4-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires UPSA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound on Serotonin transporter was determined in rat |
Bioorg Med Chem Lett 10: 805-9 (2000)
BindingDB Entry DOI: 10.7270/Q20V8C0T |
More data for this
Ligand-Target Pair | |
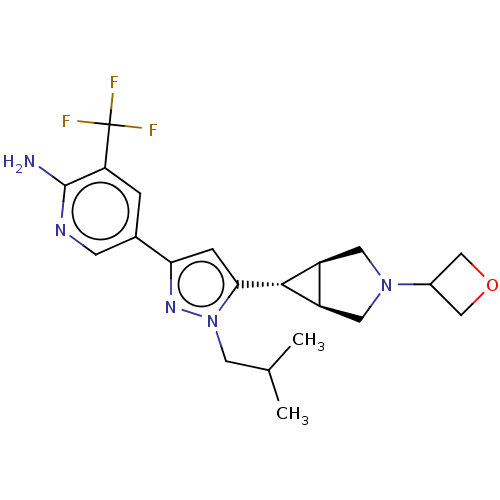
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271208
(CHEMBL4128619)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1cc(nn1CC(C)C)-c1cnc(N)c(c1)C(F)(F)F)C1COC1 |r| Show InChI InChI=1S/C21H26F3N5O/c1-11(2)6-29-18(19-14-7-28(8-15(14)19)13-9-30-10-13)4-17(27-29)12-3-16(21(22,23)24)20(25)26-5-12/h3-5,11,13-15,19H,6-10H2,1-2H3,(H2,25,26)/t14-,15+,19+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
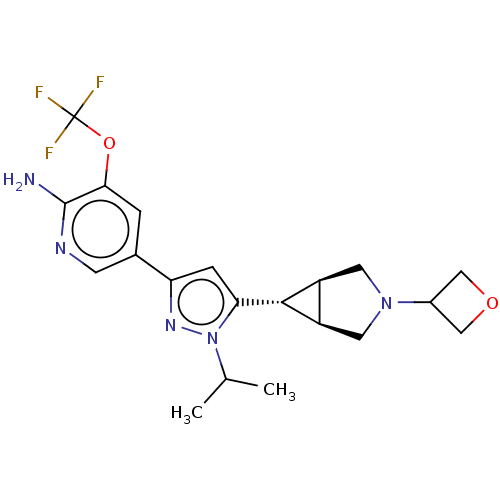
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271207
(CHEMBL3719135)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1cc(nn1C(C)C)-c1cnc(N)c(OC(F)(F)F)c1)C1COC1 |r| Show InChI InChI=1S/C20H24F3N5O2/c1-10(2)28-16(18-13-6-27(7-14(13)18)12-8-29-9-12)4-15(26-28)11-3-17(19(24)25-5-11)30-20(21,22)23/h3-5,10,12-14,18H,6-9H2,1-2H3,(H2,24,25)/t13-,14+,18+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
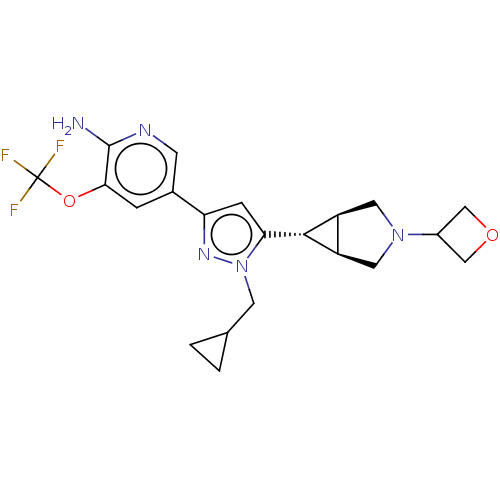
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271242
(CHEMBL4125721)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1cc(nn1CC1CC1)-c1cnc(N)c(OC(F)(F)F)c1)C1COC1 |r| Show InChI InChI=1S/C21H24F3N5O2/c22-21(23,24)31-18-3-12(5-26-20(18)25)16-4-17(29(27-16)6-11-1-2-11)19-14-7-28(8-15(14)19)13-9-30-10-13/h3-5,11,13-15,19H,1-2,6-10H2,(H2,25,26)/t14-,15+,19+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271233
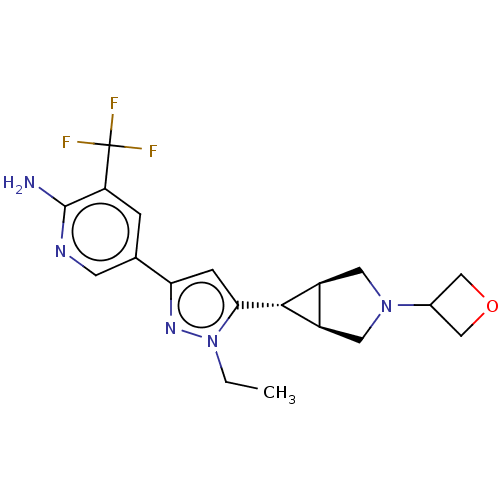
(CHEMBL4129958)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1cc(nn1CC)-c1cnc(N)c(c1)C(F)(F)F)C1COC1 |r| Show InChI InChI=1S/C19H22F3N5O/c1-2-27-16(17-12-6-26(7-13(12)17)11-8-28-9-11)4-15(25-27)10-3-14(19(20,21)22)18(23)24-5-10/h3-5,11-13,17H,2,6-9H2,1H3,(H2,23,24)/t12-,13+,17+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50050511

((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP8 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50171556

((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...)Show InChI InChI=1S/C7H17BN2O3/c1-4(2)6(9)7(11)10-5(3)8(12)13/h4-6,12-13H,9H2,1-3H3,(H,10,11)/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372000
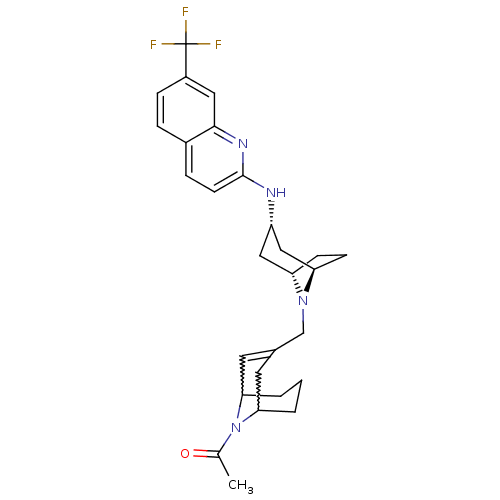
(CHEMBL256851)Show SMILES CC(=O)N1C2CCCC1C=C(CN1[C@H]3CC[C@@H]1C[C@H](C3)Nc1ccc3ccc(cc3n1)C(F)(F)F)C2 |w:4.40,8.9,t:10,TLB:1:3:10.35.9:7.5.6,THB:11:12:19.18.17:14.15| Show InChI InChI=1S/C28H33F3N4O/c1-17(36)35-24-3-2-4-25(35)12-18(11-24)16-34-22-8-9-23(34)15-21(14-22)32-27-10-6-19-5-7-20(28(29,30)31)13-26(19)33-27/h5-7,10-11,13,21-25H,2-4,8-9,12,14-16H2,1H3,(H,32,33)/t21-,22-,23+,24?,25? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271228
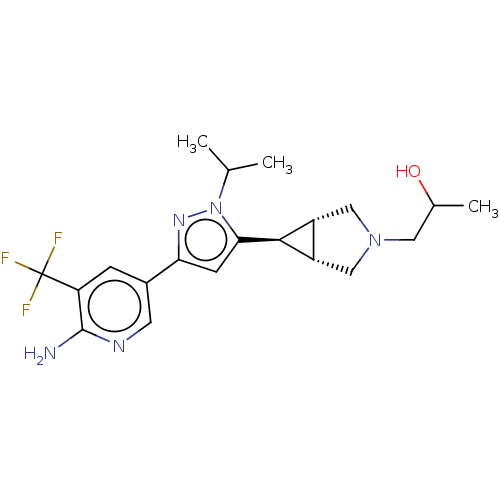
(CHEMBL4128889)Show SMILES [H][C@@]12CN(CC(C)O)C[C@]1([H])[C@H]2c1cc(nn1C(C)C)-c1cnc(N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H26F3N5O/c1-10(2)28-17(18-13-8-27(7-11(3)29)9-14(13)18)5-16(26-28)12-4-15(20(21,22)23)19(24)25-6-12/h4-6,10-11,13-14,18,29H,7-9H2,1-3H3,(H2,24,25)/t11?,13-,14+,18+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271212
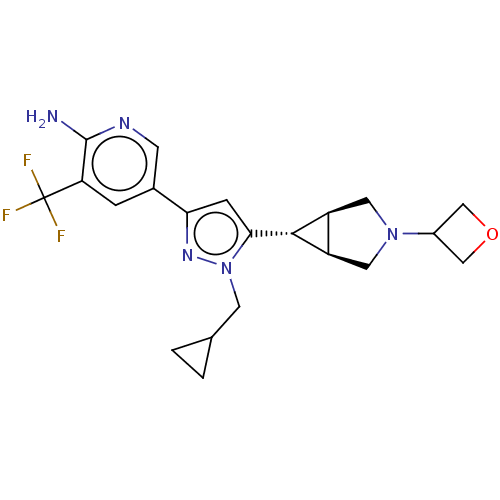
(CHEMBL3715238)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1cc(nn1CC1CC1)-c1cnc(N)c(c1)C(F)(F)F)C1COC1 |r| Show InChI InChI=1S/C21H24F3N5O/c22-21(23,24)16-3-12(5-26-20(16)25)17-4-18(29(27-17)6-11-1-2-11)19-14-7-28(8-15(14)19)13-9-30-10-13/h3-5,11,13-15,19H,1-2,6-10H2,(H2,25,26)/t14-,15+,19+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50554304

(CHEMBL4752430)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271241
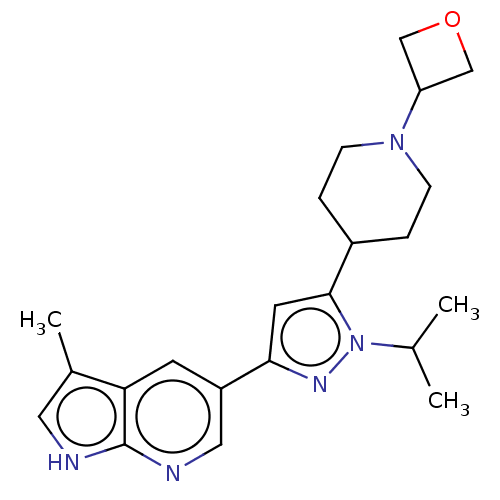
(CHEMBL3718912)Show SMILES CC(C)n1nc(cc1C1CCN(CC1)C1COC1)-c1cnc2[nH]cc(C)c2c1 Show InChI InChI=1S/C22H29N5O/c1-14(2)27-21(16-4-6-26(7-5-16)18-12-28-13-18)9-20(25-27)17-8-19-15(3)10-23-22(19)24-11-17/h8-11,14,16,18H,4-7,12-13H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372006

(CHEMBL256850)Show SMILES CC(C)Oc1ccc2ccc(N[C@H]3C[C@@H]4CC[C@H](C3)N4CC3=CC4CCCC(C3)N4C(C)=O)nc2c1 |w:27.29,23.24,t:23,TLB:30:29:21.28.22:24.26.25,THB:20:19:13.12.18:15.16| Show InChI InChI=1S/C30H40N4O2/c1-19(2)36-28-11-7-22-8-12-30(32-29(22)17-28)31-23-15-24-9-10-25(16-23)33(24)18-21-13-26-5-4-6-27(14-21)34(26)20(3)35/h7-8,11-13,17,19,23-27H,4-6,9-10,14-16,18H2,1-3H3,(H,31,32)/t23-,24-,25+,26?,27? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 12
(Homo sapiens (Human)) | BDBM50271219
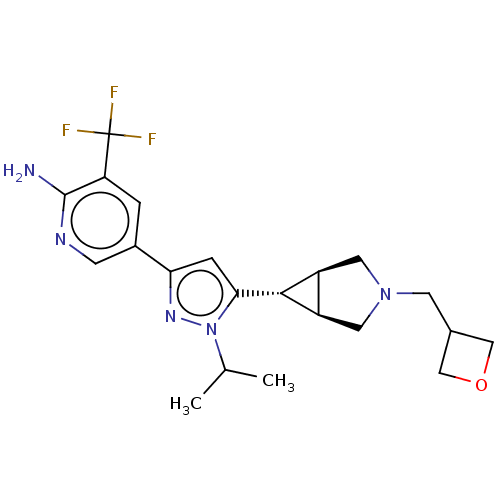
(CHEMBL4125824)Show SMILES [H][C@@]12CN(CC3COC3)C[C@]1([H])[C@H]2c1cc(nn1C(C)C)-c1cnc(N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H26F3N5O/c1-11(2)29-18(19-14-7-28(8-15(14)19)6-12-9-30-10-12)4-17(27-29)13-3-16(21(22,23)24)20(25)26-5-13/h3-5,11-12,14-15,19H,6-10H2,1-2H3,(H2,25,26)/t14-,15+,19+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... |
J Med Chem 60: 8083-8102 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00843
BindingDB Entry DOI: 10.7270/Q2639S7K |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50554312

(CHEMBL4792408)Show SMILES CC(C)C[C@H](NC(=O)C1(CC1)c1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50554305
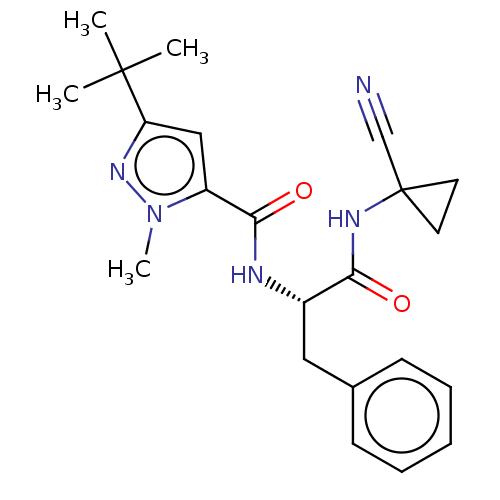
(CHEMBL4776795)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1ccccc1)C(=O)NC1(CC1)C#N)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50554310
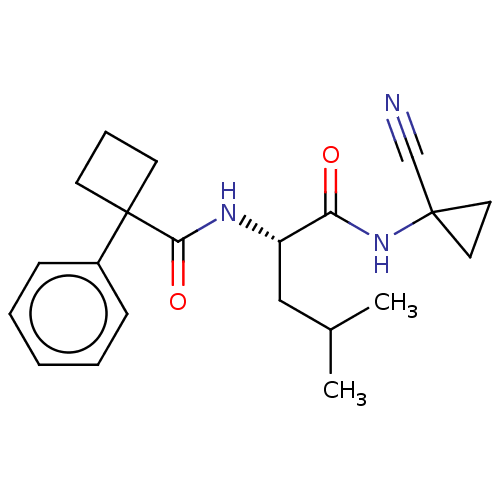
(CHEMBL4760625)Show SMILES CC(C)C[C@H](NC(=O)C1(CCC1)c1ccccc1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50549374

(CHEMBL4746374)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(c1)-c1cccnc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin B using Z-Phe-Arg-AMC fluorogenic peptide as substrate preincubated for 2 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372005

(CHEMBL429598)Show SMILES CC(C)Oc1cc(F)c2ccc(N[C@H]3C[C@@H]4CC[C@H](C3)N4CC3=CC4CCCC(C3)N4C(C)=O)nc2c1 |w:28.30,24.25,t:24,TLB:31:30:22.29.23:25.27.26,THB:21:20:14.13.19:16.17| Show InChI InChI=1S/C30H39FN4O2/c1-18(2)37-26-15-28(31)27-9-10-30(33-29(27)16-26)32-21-13-22-7-8-23(14-21)34(22)17-20-11-24-5-4-6-25(12-20)35(24)19(3)36/h9-11,15-16,18,21-25H,4-8,12-14,17H2,1-3H3,(H,32,33)/t21-,22-,23+,24?,25? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372008
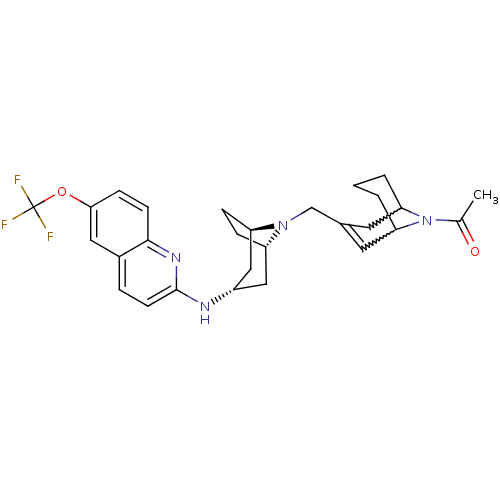
(CHEMBL444542)Show SMILES CC(=O)N1C2CCCC1C=C(CN1[C@H]3CC[C@@H]1C[C@H](C3)Nc1ccc3cc(OC(F)(F)F)ccc3n1)C2 |w:4.41,8.9,t:10,TLB:1:3:10.36.9:7.5.6,THB:11:12:19.18.17:14.15| Show InChI InChI=1S/C28H33F3N4O2/c1-17(36)35-23-3-2-4-24(35)12-18(11-23)16-34-21-6-7-22(34)15-20(14-21)32-27-10-5-19-13-25(37-28(29,30)31)8-9-26(19)33-27/h5,8-11,13,20-24H,2-4,6-7,12,14-16H2,1H3,(H,32,33)/t20-,21-,22+,23?,24? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data