

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Uppsala Curated by ChEMBL | Assay Description Affinity of the compound for 5-hydroxytryptamine 1A receptor site | J Med Chem 32: 2311-8 (1989) BindingDB Entry DOI: 10.7270/Q2CJ8CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011261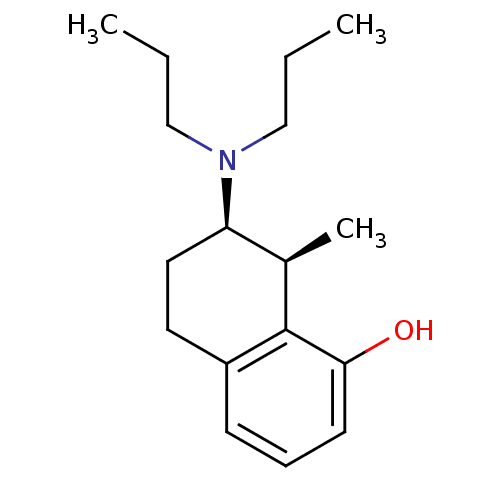 ((+)7-Dipropylamino-8-methyl-5,6,7,8-tetrahydro-nap...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011252 (7-Dipropylamino-8-methyl-5,6,7,8-tetrahydro-naphth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011252 (7-Dipropylamino-8-methyl-5,6,7,8-tetrahydro-naphth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011246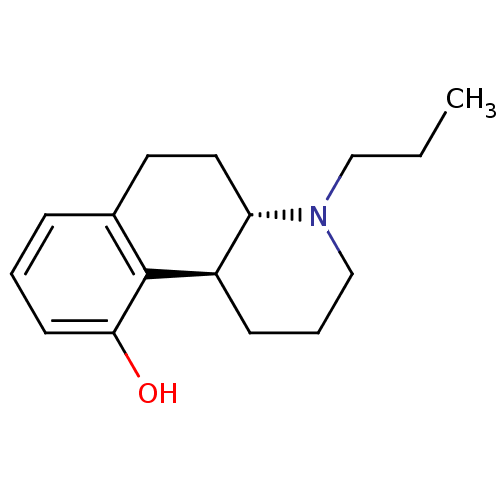 (4-Propyl-1,2,3,4,4a,5,6,10b-octahydro-benzo[f]quin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011247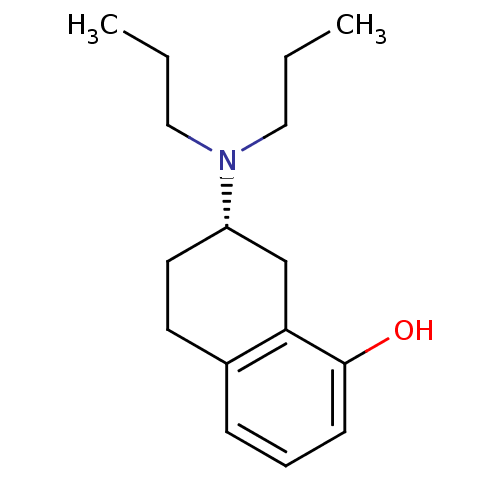 (7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen-1-ol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011247 (7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen-1-ol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50453032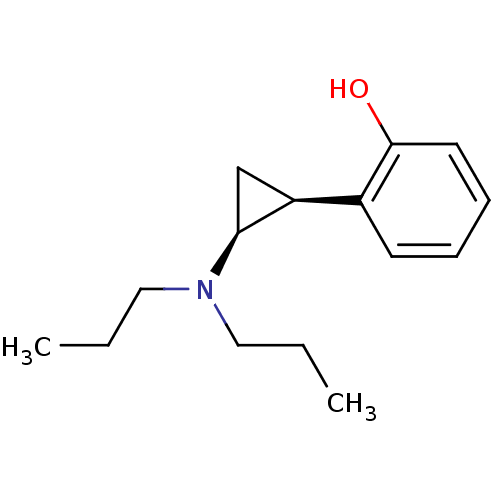 (CHEMBL2368632) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50453033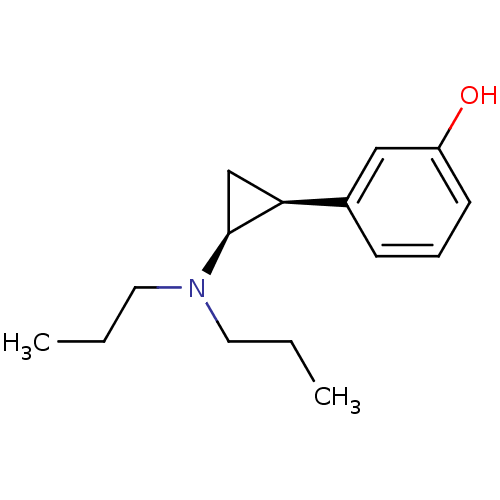 (CHEMBL2368621) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018586 (8-Dipropylamino-6,7,8,9-tetrahydro-5H-benzocyclohe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Uppsala Curated by ChEMBL | Assay Description Affinity of the compound for 5-hydroxytryptamine 1A receptor site | J Med Chem 32: 2311-8 (1989) BindingDB Entry DOI: 10.7270/Q2CJ8CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011251 (4-Propyl-1,2,3,4,4a,5,6,10b-octahydro-benzo[f]quin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011265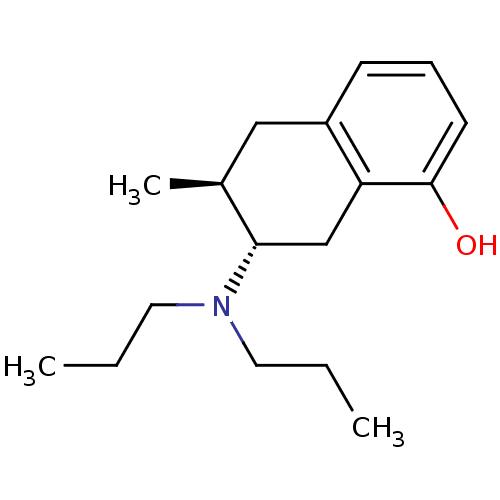 ((+)-7-Dipropylamino-6-methyl-5,6,7,8-tetrahydro-na...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011265 ((+)-7-Dipropylamino-6-methyl-5,6,7,8-tetrahydro-na...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011248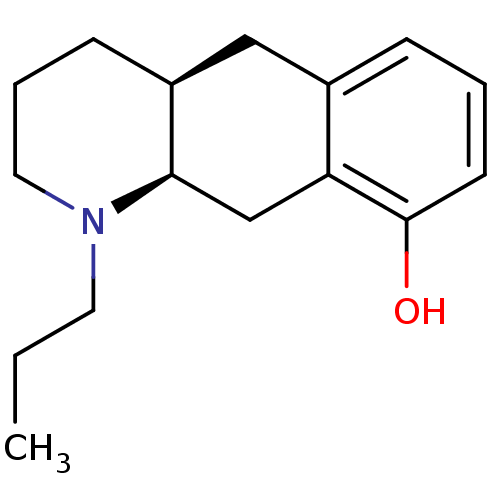 (1-Propyl-1,2,3,4,4a,5,10,10a-octahydro-benzo[g]qui...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018585 (8-Dipropylamino-6,7,8,9-tetrahydro-5H-benzocyclohe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Uppsala Curated by ChEMBL | Assay Description Affinity of the compound for 5-hydroxytryptamine 1A receptor site | J Med Chem 32: 2311-8 (1989) BindingDB Entry DOI: 10.7270/Q2CJ8CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011262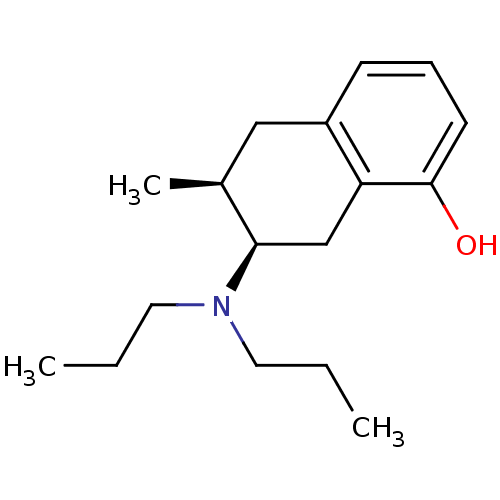 (7-Dipropylamino-6-methyl-5,6,7,8-tetrahydro-naphth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 394 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368163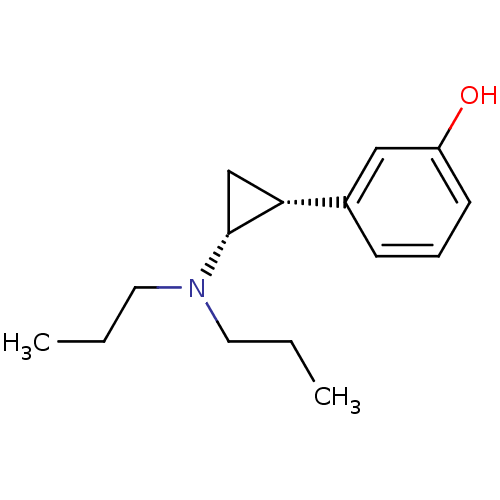 (CHEMBL545098) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011256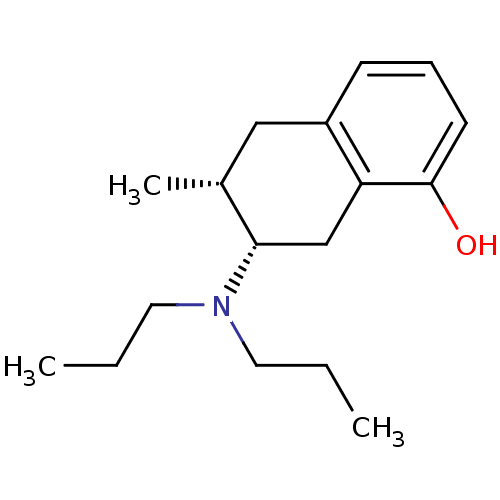 ((+)-7-Dipropylamino-6-methyl-5,6,7,8-tetrahydro-na...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 656 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011256 ((+)-7-Dipropylamino-6-methyl-5,6,7,8-tetrahydro-na...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 656 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368165 (CHEMBL540354) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 923 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011263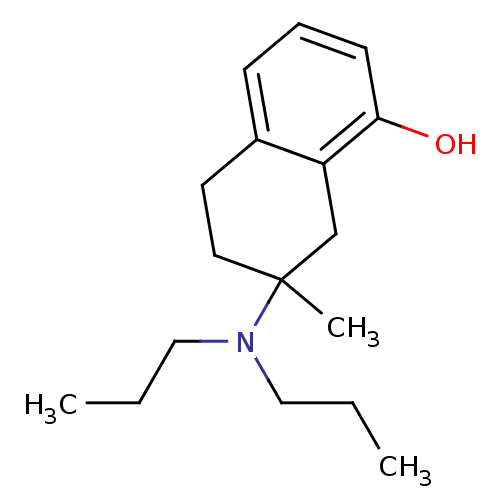 (7-Dipropylamino-7-methyl-5,6,7,8-tetrahydro-naphth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011257 (7-Dipropylamino-6-methyl-5,6,7,8-tetrahydro-naphth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011249 (1-Propyl-1,2,3,4,4a,5,10,10a-octahydro-benzo[g]qui...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011252 (7-Dipropylamino-8-methyl-5,6,7,8-tetrahydro-naphth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011253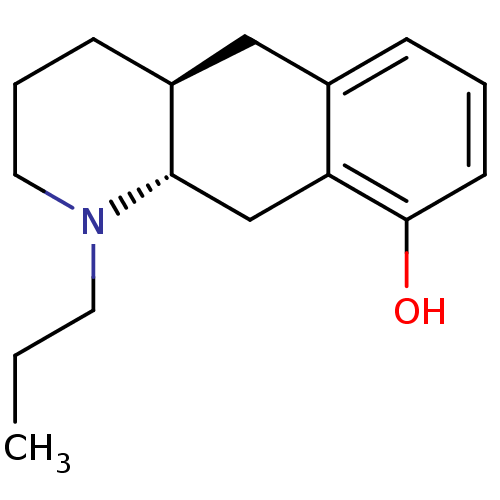 (1-Propyl-1,2,3,4,4a,5,10,10a-octahydro-benzo[g]qui...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011258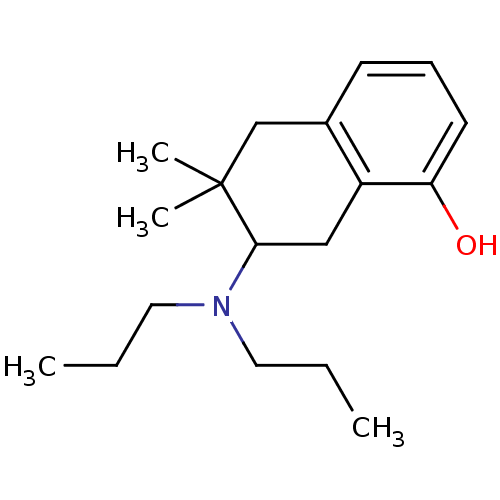 (7-Dipropylamino-6,6-dimethyl-5,6,7,8-tetrahydro-na...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011255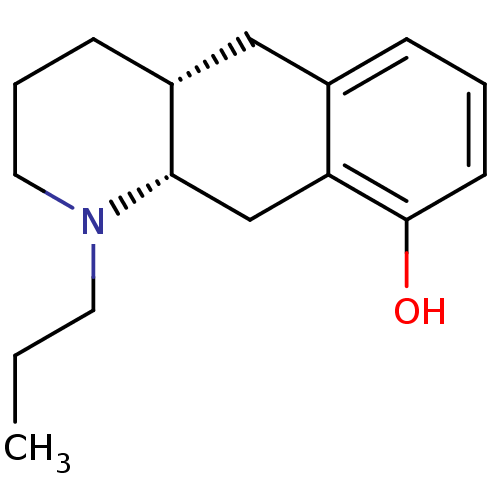 (1-Propyl-1,2,3,4,4a,5,10,10a-octahydro-benzo[g]qui...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. | J Med Chem 34: 497-510 (1991) BindingDB Entry DOI: 10.7270/Q2PK0GRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18864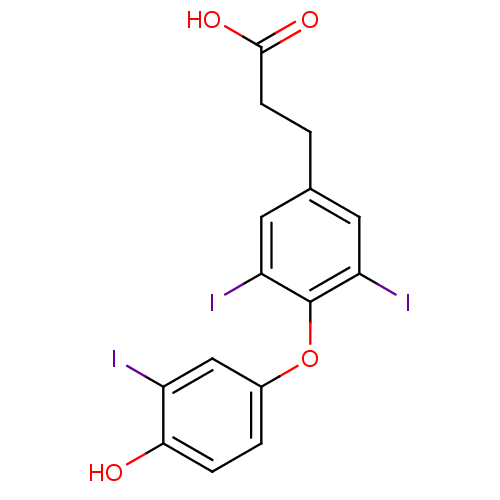 (3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18865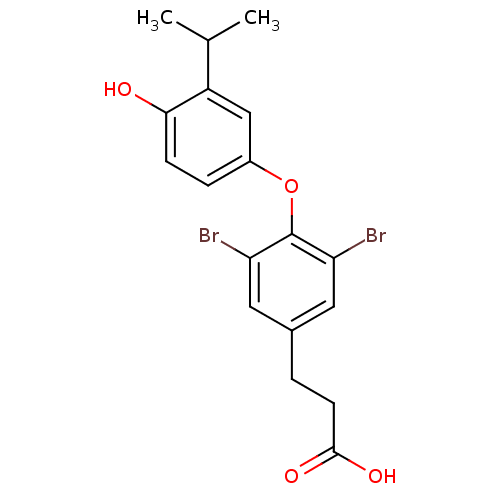 (3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18864 (3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18862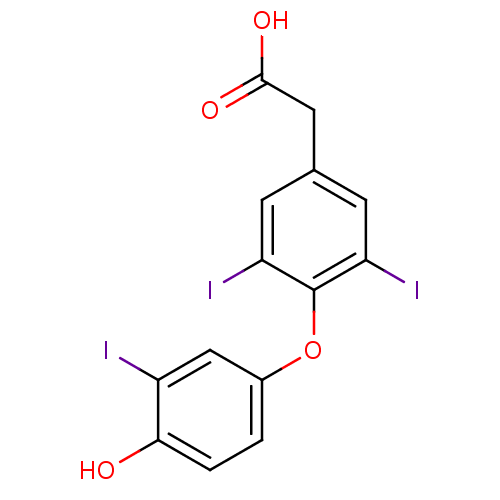 (2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18867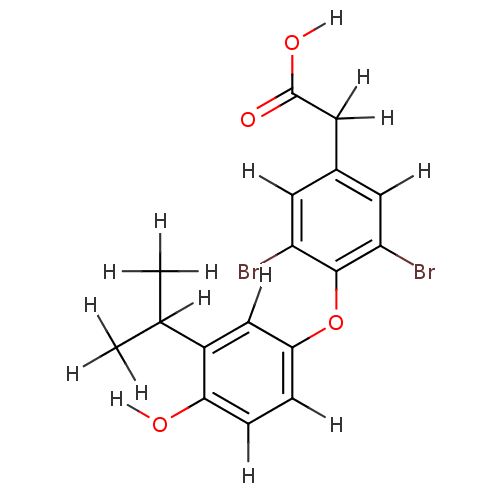 (2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.0950 | n/a | 0.200 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18865 (3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18863 ((2R)-2-amino-3-{4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18862 (2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18863 ((2R)-2-amino-3-{4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040674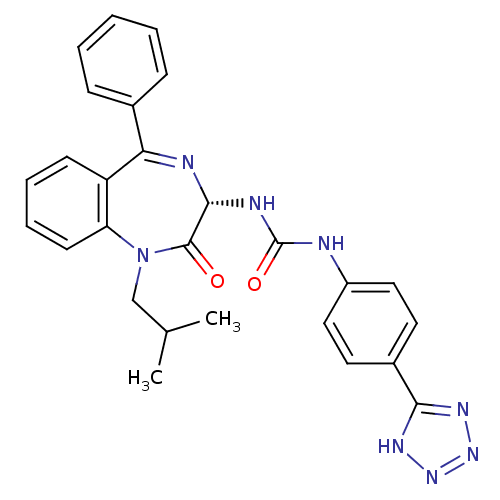 (1-((S)-1-Isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.142 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18870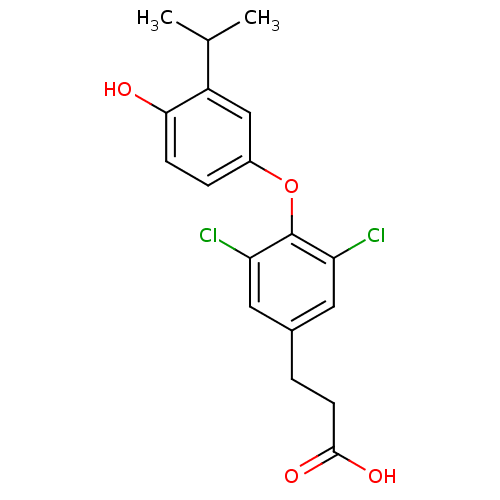 (3-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.150 | n/a | 0.280 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043556 (CHEMBL137516 | N,N-Diethyl-2-{3-[3-(3-methoxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043546 (1-(3-Methoxy-phenyl)-3-[2-oxo-1-(2-oxo-2-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50456295 (CHEMBL2111920) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040678 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.266 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043493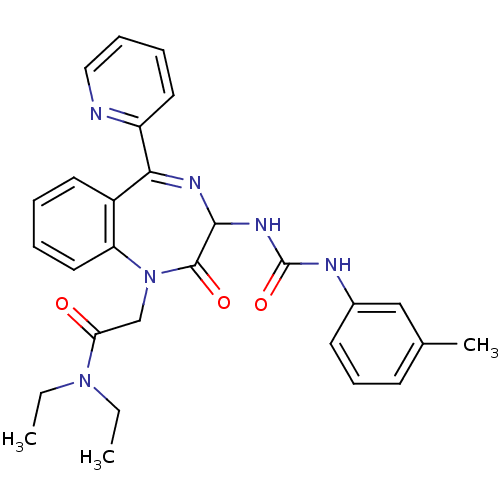 (CHEMBL313813 | N,N-Diethyl-2-[2-oxo-5-pyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043575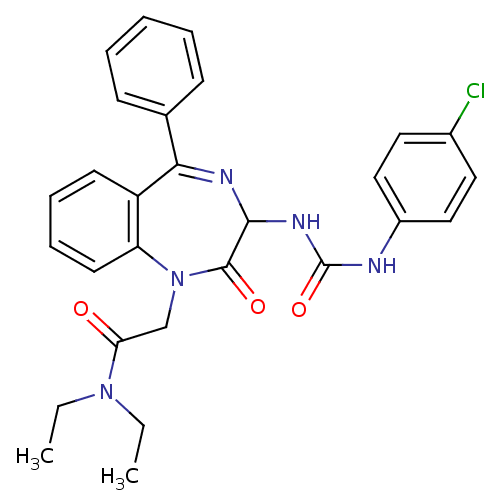 (2-{3-[3-(4-Chloro-phenyl)-ureido]-2-oxo-5-phenyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18870 (3-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.760 | n/a | 0.300 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... | J Med Chem 46: 1580-8 (2003) Article DOI: 10.1021/jm021080f BindingDB Entry DOI: 10.7270/Q20K26TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043548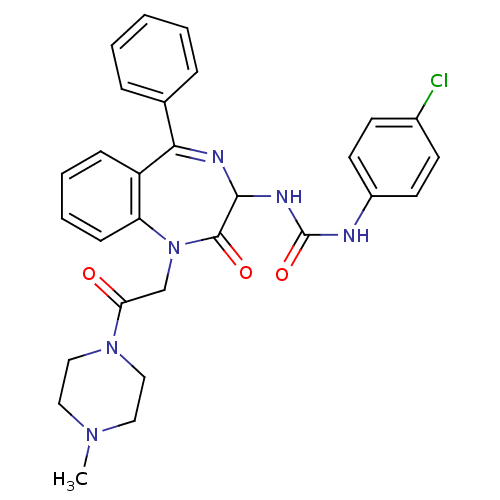 (1-(4-Chloro-phenyl)-3-{1-[2-(4-methyl-piperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020269 ((RS)-{3-[3-(4-Chloro-phenyl)-ureido]-2-oxo-5-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 318 total ) | Next | Last >> |