 Found 250 hits with Last Name = 'mikulski' and Initial = 'm'
Found 250 hits with Last Name = 'mikulski' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
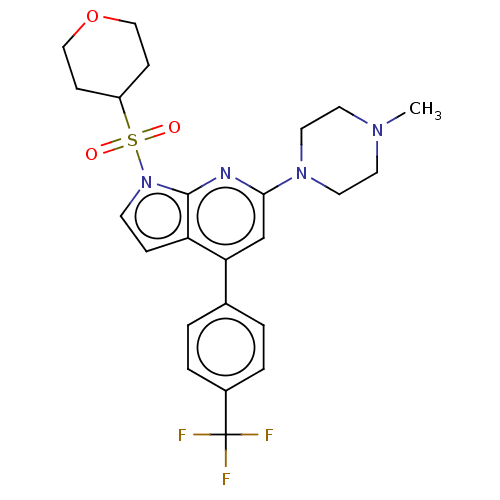
(Homo sapiens (Human)) | BDBM50166849
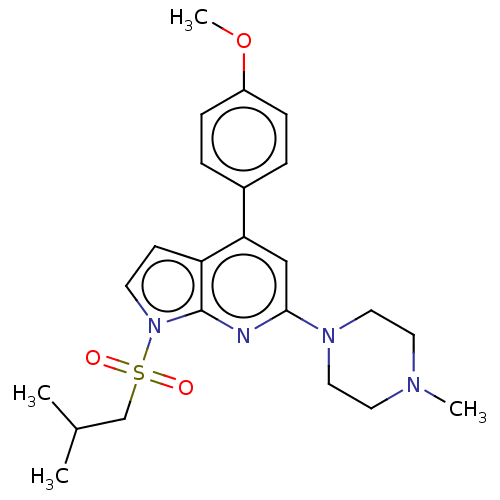
(CHEMBL3797651)Show SMILES COc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C23H30N4O3S/c1-17(2)16-31(28,29)27-10-9-20-21(18-5-7-19(30-4)8-6-18)15-22(24-23(20)27)26-13-11-25(3)12-14-26/h5-10,15,17H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166863

(CHEMBL3799529)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCNCC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H19NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166852
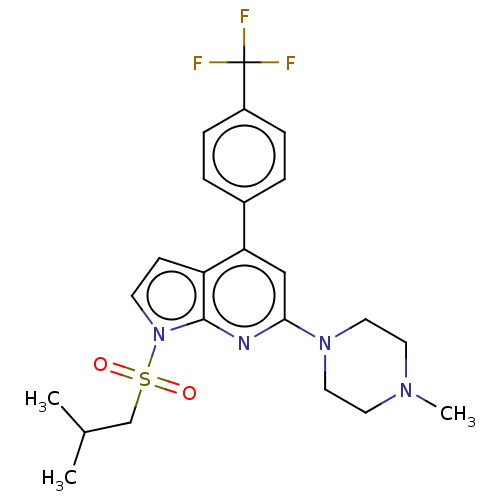
(CHEMBL3797717)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-19-20(17-4-6-18(7-5-17)23(24,25)26)14-21(27-22(19)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166844
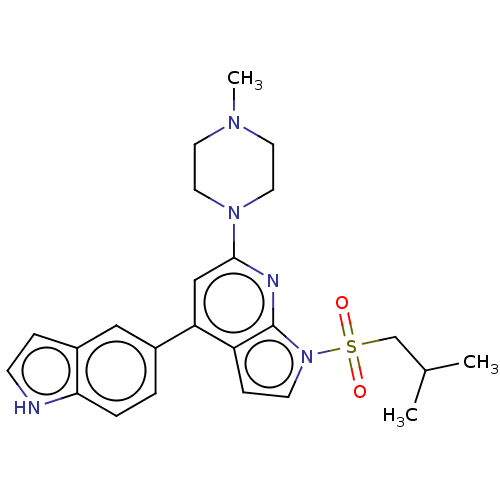
(CHEMBL3798478)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C24H29N5O2S/c1-17(2)16-32(30,31)29-9-7-20-21(18-4-5-22-19(14-18)6-8-25-22)15-23(26-24(20)29)28-12-10-27(3)11-13-28/h4-9,14-15,17,25H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166859
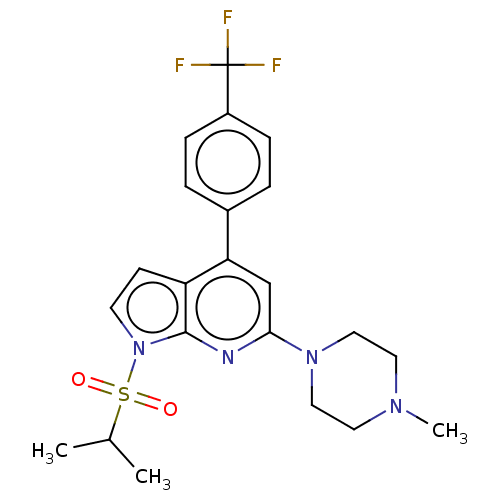
(CHEMBL3799120)Show SMILES CC(C)S(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O2S/c1-15(2)32(30,31)29-9-8-18-19(16-4-6-17(7-5-16)22(23,24)25)14-20(26-21(18)29)28-12-10-27(3)11-13-28/h4-9,14-15H,10-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166861
(CHEMBL3799448)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCOCC1 Show InChI InChI=1S/C26H23NO5/c1-14-3-4-17(11-15(14)2)21(28)13-32-26(31)16-7-9-20(10-8-16)27-24(29)22-18-5-6-19(12-18)23(22)25(27)30/h3-11,18-19,29-30H,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166846
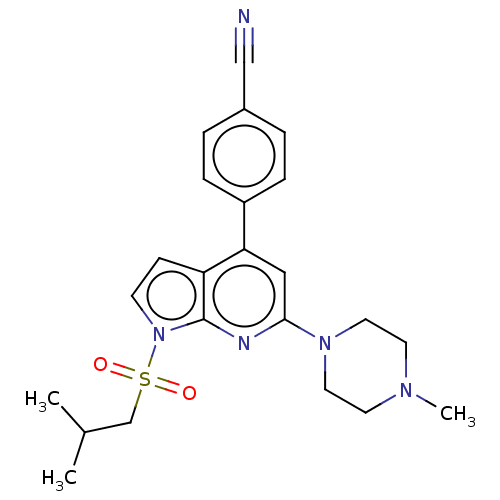
(CHEMBL3797435)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2S/c1-17(2)16-31(29,30)28-9-8-20-21(19-6-4-18(15-24)5-7-19)14-22(25-23(20)28)27-12-10-26(3)11-13-27/h4-9,14,17H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166862
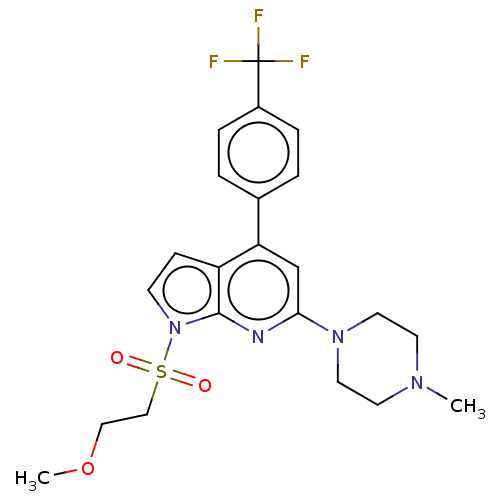
(CHEMBL3798490)Show SMILES COCCS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H21NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11,25-26H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610836

(MSC-4381)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccc(Cl)cc1C#Cc1cnc(cc1OC)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166853

(CHEMBL3798953)Show SMILES CCc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C24H32N4O2S/c1-5-19-6-8-20(9-7-19)22-16-23(27-14-12-26(4)13-15-27)25-24-21(22)10-11-28(24)31(29,30)17-18(2)3/h6-11,16,18H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610833

(CHEMBL5276884)Show SMILES COc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(C)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166856
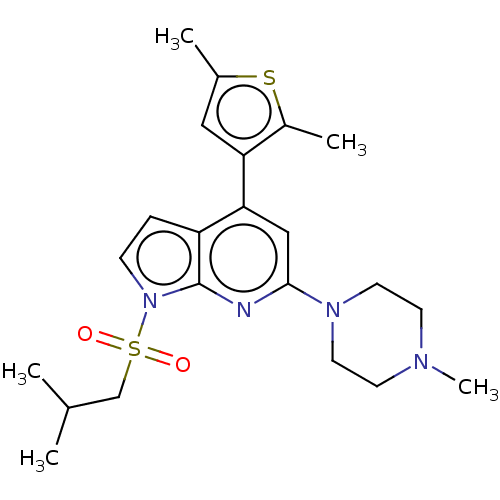
(CHEMBL3800251)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cc(C)sc1C Show InChI InChI=1S/C22H30N4O2S2/c1-15(2)14-30(27,28)26-7-6-18-20(19-12-16(3)29-17(19)4)13-21(23-22(18)26)25-10-8-24(5)9-11-25/h6-7,12-13,15H,8-11,14H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166860
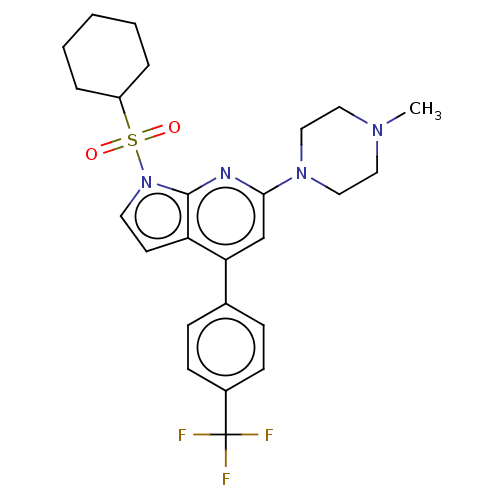
(CHEMBL3799330)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCCCC1 Show InChI InChI=1S/C25H29F3N4O2S/c1-30-13-15-31(16-14-30)23-17-22(18-7-9-19(10-8-18)25(26,27)28)21-11-12-32(24(21)29-23)35(33,34)20-5-3-2-4-6-20/h7-12,17,20H,2-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166854

(CHEMBL3799638)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1 Show InChI InChI=1S/C22H28N4O2S/c1-17(2)16-29(27,28)26-10-9-19-20(18-7-5-4-6-8-18)15-21(23-22(19)26)25-13-11-24(3)12-14-25/h4-10,15,17H,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610834
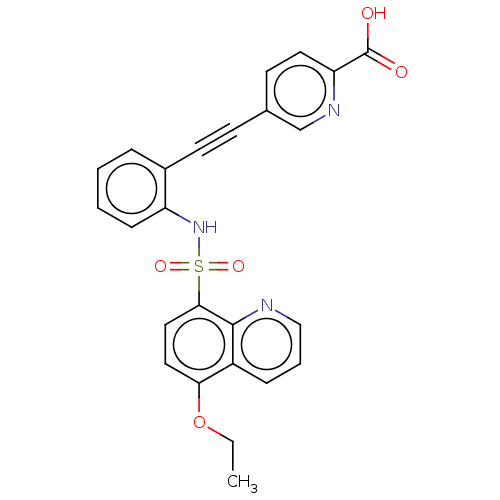
(CHEMBL5279064)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610837
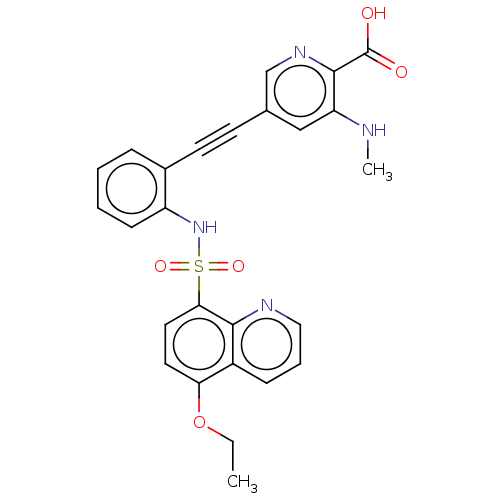
(CHEMBL5281492)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(NC)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610830
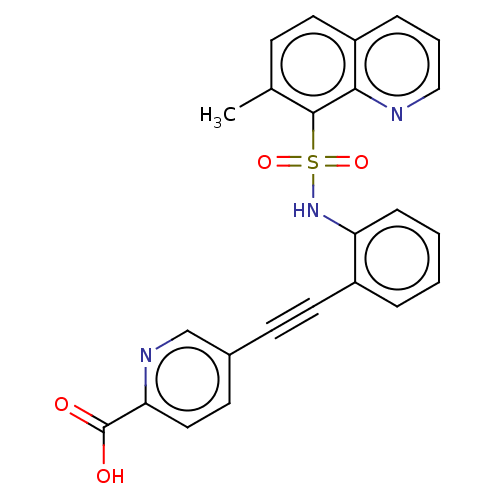
(CHEMBL5267752)Show SMILES Cc1ccc2cccnc2c1S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610835

(CHEMBL5267349)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(cc1OC)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166847
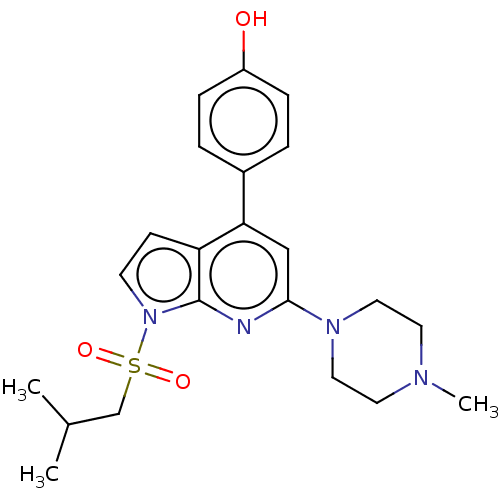
(CHEMBL3800539)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(O)cc1 Show InChI InChI=1S/C22H28N4O3S/c1-16(2)15-30(28,29)26-9-8-19-20(17-4-6-18(27)7-5-17)14-21(23-22(19)26)25-12-10-24(3)11-13-25/h4-9,14,16,27H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166849

(CHEMBL3797651)Show SMILES COc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C23H30N4O3S/c1-17(2)16-31(28,29)27-10-9-20-21(18-5-7-19(30-4)8-6-18)15-22(24-23(20)27)26-13-11-25(3)12-14-26/h5-10,15,17H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166850
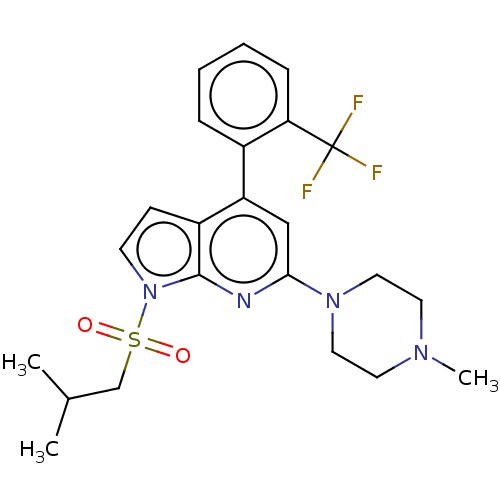
(CHEMBL3799547)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-18-19(17-6-4-5-7-20(17)23(24,25)26)14-21(27-22(18)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166859

(CHEMBL3799120)Show SMILES CC(C)S(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O2S/c1-15(2)32(30,31)29-9-8-18-19(16-4-6-17(7-5-16)22(23,24)25)14-20(26-21(18)29)28-12-10-27(3)11-13-28/h4-9,14-15H,10-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610826

(CHEMBL5287351)Show SMILES Cc1cnc2c(cccc2c1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610827
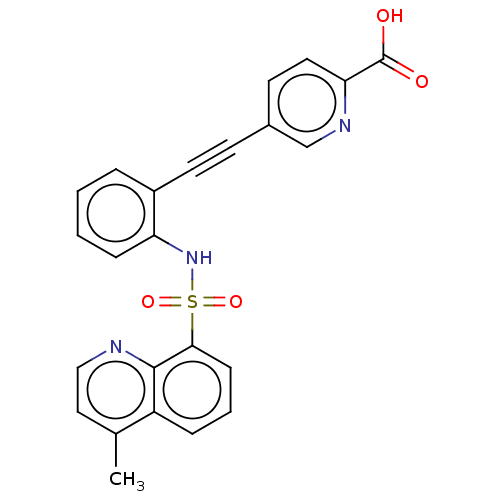
(CHEMBL5287780)Show SMILES Cc1ccnc2c(cccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166843
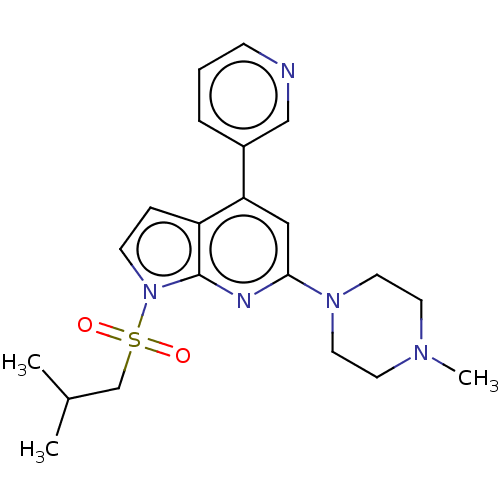
(CHEMBL3797293)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cccnc1 Show InChI InChI=1S/C21H27N5O2S/c1-16(2)15-29(27,28)26-8-6-18-19(17-5-4-7-22-14-17)13-20(23-21(18)26)25-11-9-24(3)10-12-25/h4-8,13-14,16H,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610824

(CHEMBL5268966)Show SMILES OC(=O)c1ccc(cn1)C#Cc1ccccc1NS(=O)(=O)c1cccc2cccnc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166851
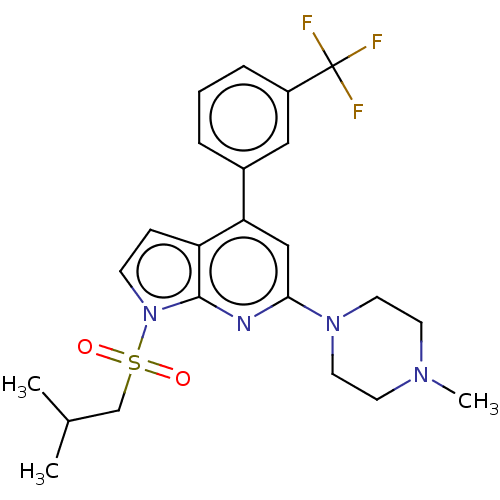
(CHEMBL3798840)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-8-7-19-20(17-5-4-6-18(13-17)23(24,25)26)14-21(27-22(19)30)29-11-9-28(3)10-12-29/h4-8,13-14,16H,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610829
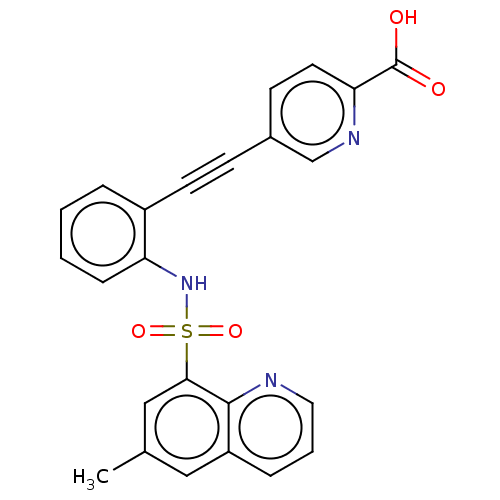
(CHEMBL5288904)Show SMILES Cc1cc(c2ncccc2c1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166862

(CHEMBL3798490)Show SMILES COCCS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H21NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11,25-26H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166858
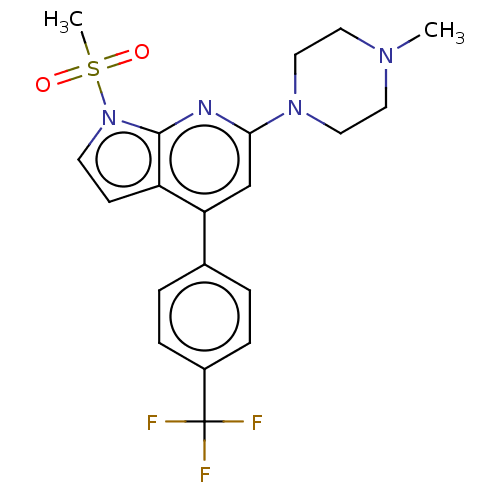
(CHEMBL3800153)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(C)(=O)=O Show InChI InChI=1S/C20H21F3N4O2S/c1-25-9-11-26(12-10-25)18-13-17(14-3-5-15(6-4-14)20(21,22)23)16-7-8-27(19(16)24-18)30(2,28)29/h3-8,13H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610831
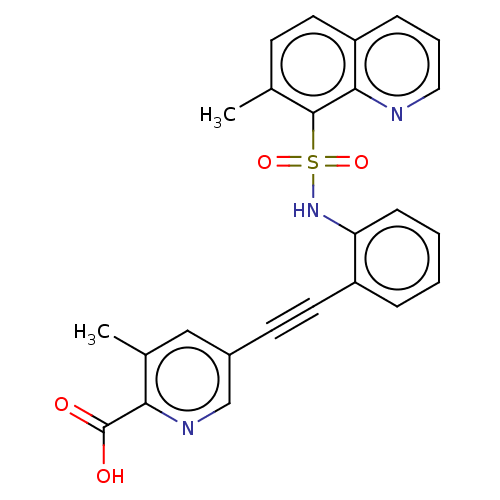
(CHEMBL5265956)Show SMILES Cc1ccc2cccnc2c1S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(C)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166848
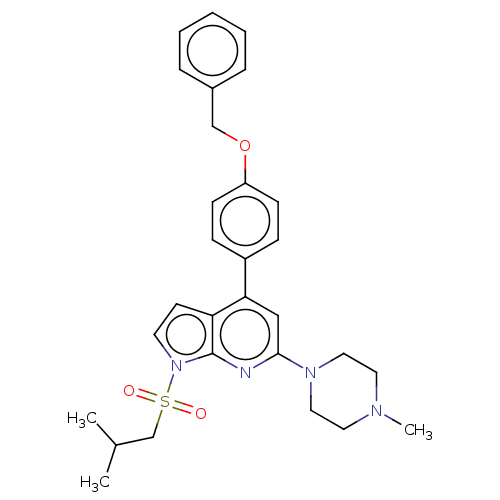
(CHEMBL3800150)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C29H34N4O3S/c1-22(2)21-37(34,35)33-14-13-26-27(19-28(30-29(26)33)32-17-15-31(3)16-18-32)24-9-11-25(12-10-24)36-20-23-7-5-4-6-8-23/h4-14,19,22H,15-18,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166855

(CHEMBL3800576)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cnn(C)c1 Show InChI InChI=1S/C20H28N6O2S/c1-15(2)14-29(27,28)26-6-5-17-18(16-12-21-24(4)13-16)11-19(22-20(17)26)25-9-7-23(3)8-10-25/h5-6,11-13,15H,7-10,14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610825

(CHEMBL5266104)Show SMILES Cc1ccc2cccc(c2n1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166847

(CHEMBL3800539)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(O)cc1 Show InChI InChI=1S/C22H28N4O3S/c1-16(2)15-30(28,29)26-9-8-19-20(17-4-6-18(27)7-5-17)14-21(23-22(19)26)25-12-10-24(3)11-13-25/h4-9,14,16,27H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166850

(CHEMBL3799547)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-18-19(17-6-4-5-7-20(17)23(24,25)26)14-21(27-22(18)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166857

(CHEMBL3800055)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1c(C)noc1C |(.88,-6.25,;1.7,-5.33,;2.91,-5.58,;1.22,-3.86,;2.25,-2.71,;3.46,-2.96,;2.64,-3.88,;1.77,-1.24,;2.67,.02,;1.77,1.24,;.3,.77,;-1.03,1.56,;-2.39,.77,;-2.39,-.77,;-1.03,-1.56,;.3,-.77,;-3.73,-1.54,;-3.73,-3.09,;-5.07,-3.85,;-6.41,-3.08,;-7.48,-3.69,;-6.4,-1.53,;-5.06,-.76,;-1.03,3.1,;.22,4,;1.39,3.61,;-.27,5.46,;-1.81,5.46,;-2.28,3.99,;-3.45,3.59,)| Show InChI InChI=1S/C21H29N5O3S/c1-14(2)13-30(27,28)26-7-6-17-18(20-15(3)23-29-16(20)4)12-19(22-21(17)26)25-10-8-24(5)9-11-25/h6-7,12,14H,8-11,13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166846

(CHEMBL3797435)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2S/c1-17(2)16-31(29,30)28-9-8-20-21(19-6-4-18(15-24)5-7-19)14-22(25-23(20)28)27-12-10-26(3)11-13-27/h4-9,14,17H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610828
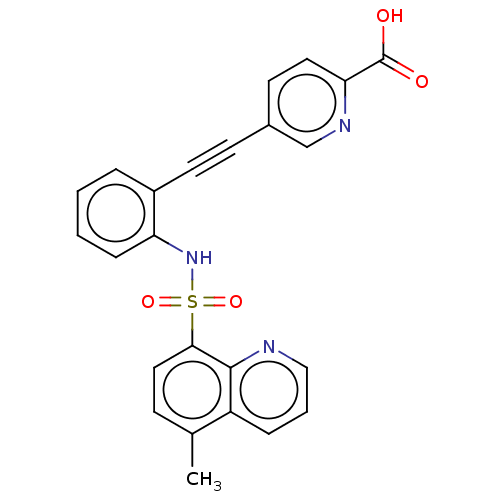
(CHEMBL5271816)Show SMILES Cc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166842
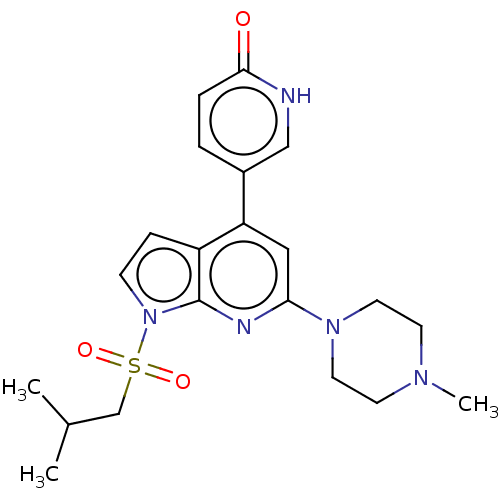
(CHEMBL3800442)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(=O)[nH]c1 Show InChI InChI=1S/C21H27N5O3S/c1-15(2)14-30(28,29)26-7-6-17-18(16-4-5-20(27)22-13-16)12-19(23-21(17)26)25-10-8-24(3)9-11-25/h4-7,12-13,15H,8-11,14H2,1-3H3,(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166848

(CHEMBL3800150)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C29H34N4O3S/c1-22(2)21-37(34,35)33-14-13-26-27(19-28(30-29(26)33)32-17-15-31(3)16-18-32)24-9-11-25(12-10-24)36-20-23-7-5-4-6-8-23/h4-14,19,22H,15-18,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Mus musculus) | BDBM50610868
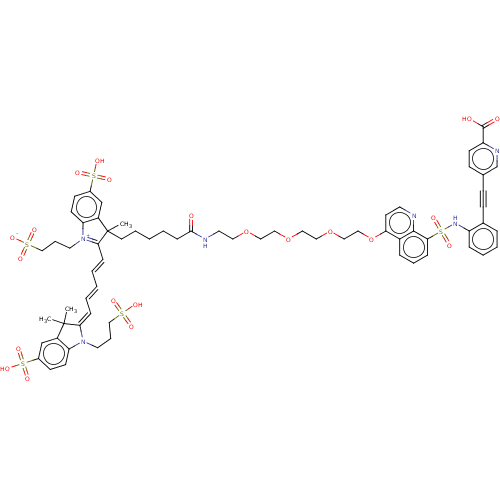
(CHEMBL5282978)Show SMILES CC1(C)\C(=C\C=C\C=C\C2=[N+](CCCS([O-])(=O)=O)c3ccc(cc3C2(C)CCCCCC(=O)NCCOCCOCCOCCOc2ccnc3c(cccc23)S(=O)(=O)Nc2ccccc2C#Cc2ccc(nc2)C(O)=O)S(O)(=O)=O)N(CCCS(O)(=O)=O)c2ccc(cc12)S(O)(=O)=O |c:9| | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166852

(CHEMBL3797717)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-19-20(17-4-6-18(7-5-17)23(24,25)26)14-21(27-22(19)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166844

(CHEMBL3798478)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C24H29N5O2S/c1-17(2)16-32(30,31)29-9-7-20-21(18-4-5-22-19(14-18)6-8-25-22)15-23(26-24(20)29)28-12-10-27(3)11-13-28/h4-9,14-15,17,25H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166854

(CHEMBL3799638)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1 Show InChI InChI=1S/C22H28N4O2S/c1-17(2)16-29(27,28)26-10-9-19-20(18-7-5-4-6-8-18)15-21(23-22(19)26)25-13-11-24(3)12-14-25/h4-10,15,17H,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 547 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166853

(CHEMBL3798953)Show SMILES CCc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C24H32N4O2S/c1-5-19-6-8-20(9-7-19)22-16-23(27-14-12-26(4)13-15-27)25-24-21(22)10-11-28(24)31(29,30)17-18(2)3/h6-11,16,18H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 614 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166851

(CHEMBL3798840)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-8-7-19-20(17-5-4-6-18(13-17)23(24,25)26)14-21(27-22(19)30)29-11-9-28(3)10-12-29/h4-8,13-14,16H,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166856

(CHEMBL3800251)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cc(C)sc1C Show InChI InChI=1S/C22H30N4O2S2/c1-15(2)14-30(27,28)26-7-6-18-20(19-12-16(3)29-17(19)4)13-21(23-22(18)26)25-10-8-24(5)9-11-25/h6-7,12-13,15H,8-11,14H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166858

(CHEMBL3800153)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(C)(=O)=O Show InChI InChI=1S/C20H21F3N4O2S/c1-25-9-11-26(12-10-25)18-13-17(14-3-5-15(6-4-14)20(21,22)23)16-7-8-27(19(16)24-18)30(2,28)29/h3-8,13H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166863

(CHEMBL3799529)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCNCC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H19NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data