 Found 75 hits with Last Name = 'miller' and Initial = 'dp'
Found 75 hits with Last Name = 'miller' and Initial = 'dp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23222
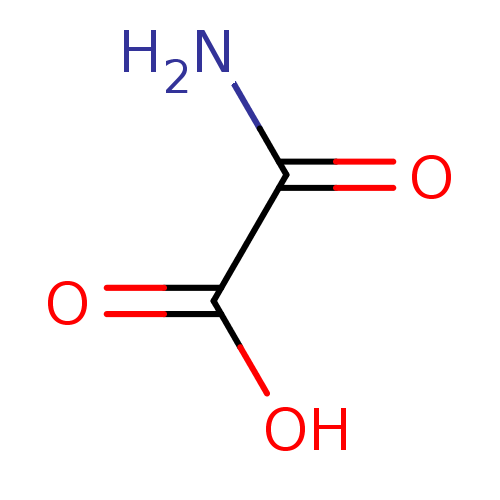
(Oxalamic acid | Oxamate | Oxamate, 3 | Oxamidic Ac...)Show InChI InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50146014
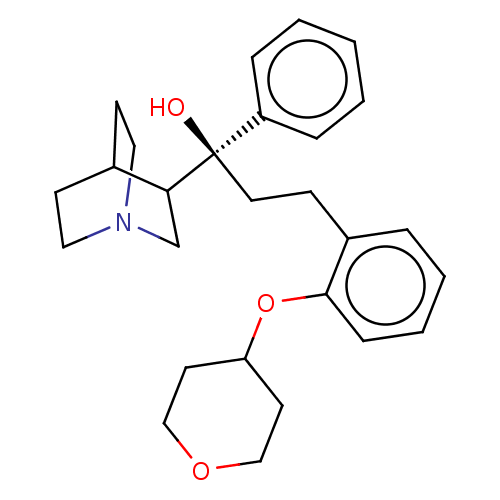
(CHEMBL3765554)Show SMILES O[C@@](CCc1ccccc1OC1CCOCC1)(C1CN2CCC1CC2)c1ccccc1 |r,wU:1.1,(.04,-1,;1.1,-1.63,;-.24,-2.39,;-.26,-3.93,;-1.6,-4.68,;-2.93,-3.9,;-4.27,-4.66,;-4.28,-6.2,;-2.96,-6.98,;-1.62,-6.22,;-.29,-7.01,;-.31,-8.55,;1.01,-9.34,;.99,-10.88,;-.36,-11.63,;-1.68,-10.84,;-1.66,-9.3,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;1.11,-.09,;2.45,.67,;2.47,2.21,;1.14,2.99,;-.2,2.24,;-.22,.7,)| Show InChI InChI=1S/C27H35NO3/c29-27(23-7-2-1-3-8-23,25-20-28-16-11-21(25)12-17-28)15-10-22-6-4-5-9-26(22)31-24-13-18-30-19-14-24/h1-9,21,24-25,29H,10-20H2/t25?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ChoKalpha using choline as substrate by ultraviolet spectroscopic assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145944
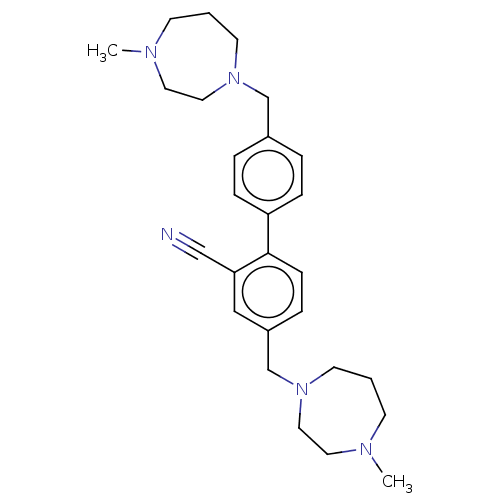
(CHEMBL3763540)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(CN3CCCN(C)CC3)cc2C#N)CC1 Show InChI InChI=1S/C27H37N5/c1-29-11-3-13-31(17-15-29)21-23-5-8-25(9-6-23)27-10-7-24(19-26(27)20-28)22-32-14-4-12-30(2)16-18-32/h5-10,19H,3-4,11-18,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145946
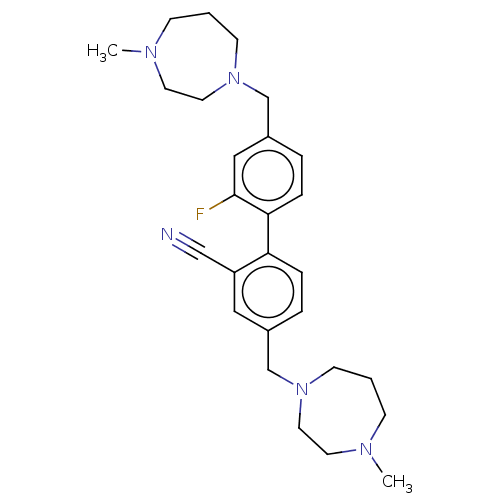
(CHEMBL3763931)Show SMILES CN1CCCN(Cc2ccc(c(F)c2)-c2ccc(CN3CCCN(C)CC3)cc2C#N)CC1 Show InChI InChI=1S/C27H36FN5/c1-30-9-3-11-32(15-13-30)20-22-5-7-25(24(17-22)19-29)26-8-6-23(18-27(26)28)21-33-12-4-10-31(2)14-16-33/h5-8,17-18H,3-4,9-16,20-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425797
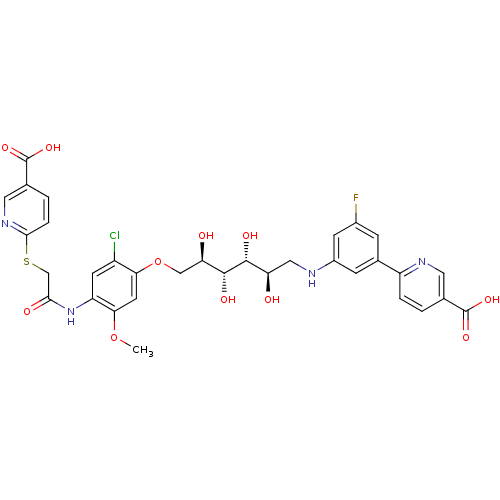
(CHEMBL2316885)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C33H32ClFN4O11S/c1-49-27-10-26(21(34)9-23(27)39-28(42)15-51-29-5-3-17(12-38-29)33(47)48)50-14-25(41)31(44)30(43)24(40)13-36-20-7-18(6-19(35)8-20)22-4-2-16(11-37-22)32(45)46/h2-12,24-25,30-31,36,40-41,43-44H,13-15H2,1H3,(H,39,42)(H,45,46)(H,47,48)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145989
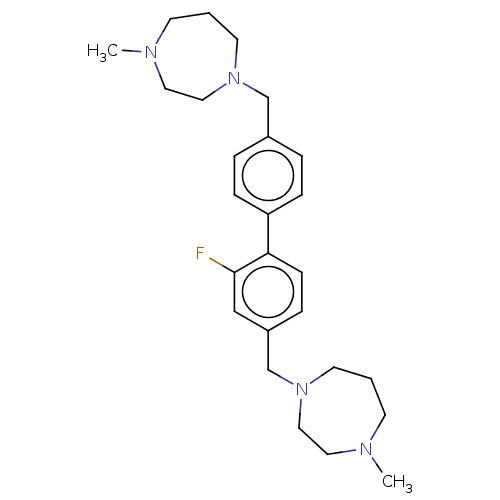
(CHEMBL3763982)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(CN3CCCN(C)CC3)cc2F)CC1 Show InChI InChI=1S/C26H37FN4/c1-28-11-3-13-30(17-15-28)20-22-5-8-24(9-6-22)25-10-7-23(19-26(25)27)21-31-14-4-12-29(2)16-18-31/h5-10,19H,3-4,11-18,20-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425798
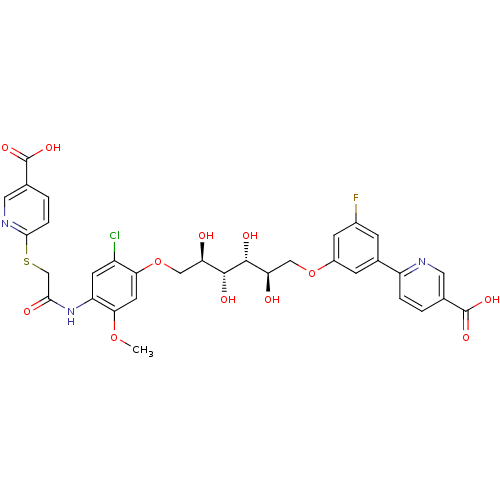
(CHEMBL2316886)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)COc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C33H31ClFN3O12S/c1-48-27-10-26(21(34)9-23(27)38-28(41)15-51-29-5-3-17(12-37-29)33(46)47)50-14-25(40)31(43)30(42)24(39)13-49-20-7-18(6-19(35)8-20)22-4-2-16(11-36-22)32(44)45/h2-12,24-25,30-31,39-40,42-43H,13-15H2,1H3,(H,38,41)(H,44,45)(H,46,47)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) in absence of 0.1% Triton |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145945
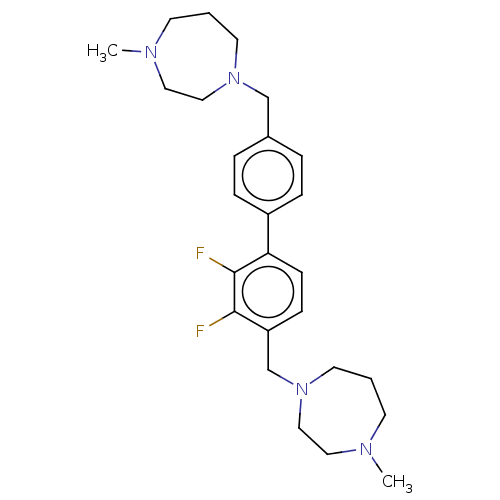
(CHEMBL3764278)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(CN3CCCN(C)CC3)c(F)c2F)CC1 Show InChI InChI=1S/C26H36F2N4/c1-29-11-3-13-31(17-15-29)19-21-5-7-22(8-6-21)24-10-9-23(25(27)26(24)28)20-32-14-4-12-30(2)16-18-32/h5-10H,3-4,11-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145942

(CHEMBL3765239)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(cc2)C(=O)N2CCCN(C)CC2)CC1 Show InChI InChI=1S/C26H36N4O/c1-27-13-3-15-29(19-17-27)21-22-5-7-23(8-6-22)24-9-11-25(12-10-24)26(31)30-16-4-14-28(2)18-20-30/h5-12H,3-4,13-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50146003

(CHEMBL3763453)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2cc(F)c(CN3CCCN(C)CC3)c(F)c2)CC1 Show InChI InChI=1S/C26H36F2N4/c1-29-9-3-11-31(15-13-29)19-21-5-7-22(8-6-21)23-17-25(27)24(26(28)18-23)20-32-12-4-10-30(2)14-16-32/h5-8,17-18H,3-4,9-16,19-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145947

(CHEMBL3763792)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(CN3CCCN(C)CC3)c(Cl)c2)CC1 Show InChI InChI=1S/C26H37ClN4/c1-28-11-3-13-30(17-15-28)20-22-5-7-23(8-6-22)24-9-10-25(26(27)19-24)21-31-14-4-12-29(2)16-18-31/h5-10,19H,3-4,11-18,20-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425798

(CHEMBL2316886)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)COc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C33H31ClFN3O12S/c1-48-27-10-26(21(34)9-23(27)38-28(41)15-51-29-5-3-17(12-37-29)33(46)47)50-14-25(40)31(43)30(42)24(39)13-49-20-7-18(6-19(35)8-20)22-4-2-16(11-36-22)32(44)45/h2-12,24-25,30-31,39-40,42-43H,13-15H2,1H3,(H,38,41)(H,44,45)(H,46,47)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) in presence of 0.1% Triton |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425798

(CHEMBL2316886)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)COc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C33H31ClFN3O12S/c1-48-27-10-26(21(34)9-23(27)38-28(41)15-51-29-5-3-17(12-37-29)33(46)47)50-14-25(40)31(43)30(42)24(39)13-49-20-7-18(6-19(35)8-20)22-4-2-16(11-36-22)32(44)45/h2-12,24-25,30-31,39-40,42-43H,13-15H2,1H3,(H,38,41)(H,44,45)(H,46,47)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145943
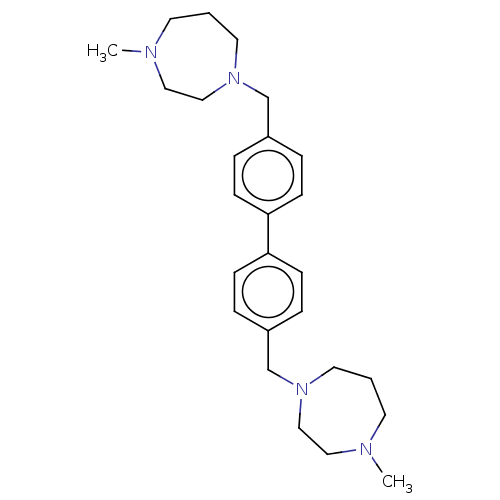
(CHEMBL3763801)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(CN3CCCN(C)CC3)cc2)CC1 Show InChI InChI=1S/C26H38N4/c1-27-13-3-15-29(19-17-27)21-23-5-9-25(10-6-23)26-11-7-24(8-12-26)22-30-16-4-14-28(2)18-20-30/h5-12H,3-4,13-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 457 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145946

(CHEMBL3763931)Show SMILES CN1CCCN(Cc2ccc(c(F)c2)-c2ccc(CN3CCCN(C)CC3)cc2C#N)CC1 Show InChI InChI=1S/C27H36FN5/c1-30-9-3-11-32(15-13-30)20-22-5-7-25(24(17-22)19-29)26-8-6-23(18-27(26)28)21-33-12-4-10-31(2)14-16-33/h5-8,17-18H,3-4,9-16,20-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ChoKalpha in human MDA-MB-415 cells assessed as reduction in phosphocholine level after 24 hrs by NMR analysis |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145931
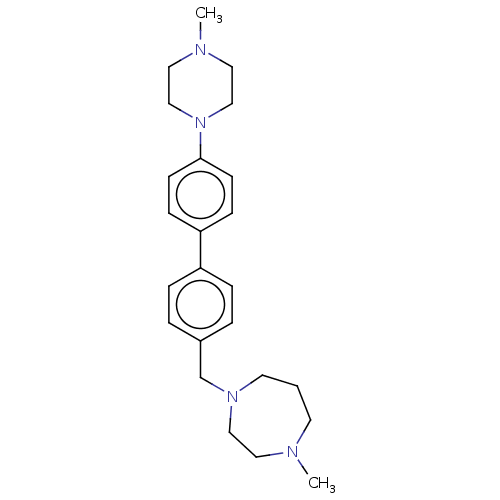
(CHEMBL3764898)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1ccc(CN2CCCN(C)CC2)cc1 Show InChI InChI=1S/C24H34N4/c1-25-12-3-13-27(17-14-25)20-21-4-6-22(7-5-21)23-8-10-24(11-9-23)28-18-15-26(2)16-19-28/h4-11H,3,12-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145939
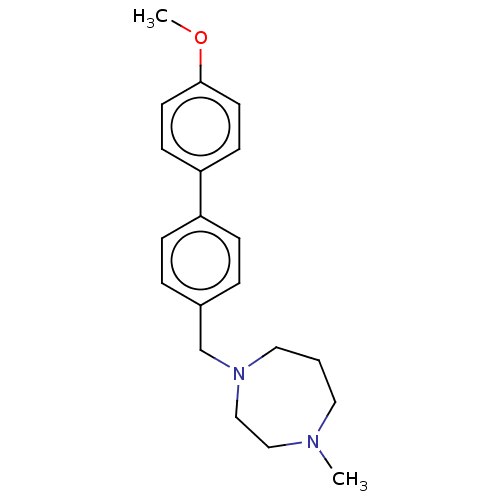
(CHEMBL3764767)Show InChI InChI=1S/C20H26N2O/c1-21-12-3-13-22(15-14-21)16-17-4-6-18(7-5-17)19-8-10-20(23-2)11-9-19/h4-11H,3,12-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145930

(CHEMBL3763340)Show InChI InChI=1S/C19H24N2/c1-20-12-5-13-21(15-14-20)16-17-8-10-19(11-9-17)18-6-3-2-4-7-18/h2-4,6-11H,5,12-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145943

(CHEMBL3763801)Show SMILES CN1CCCN(Cc2ccc(cc2)-c2ccc(CN3CCCN(C)CC3)cc2)CC1 Show InChI InChI=1S/C26H38N4/c1-27-13-3-15-29(19-17-27)21-23-5-9-25(10-6-23)26-11-7-24(8-12-26)22-30-16-4-14-28(2)18-20-30/h5-12H,3-4,13-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ChoKalpha in human MDA-MB-468 cells assessed as reduction in phosphocholine level after 24 hrs by NMR analysis |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425794

(CHEMBL2316883)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccccn1 |r| Show InChI InChI=1S/C32H32ClFN4O9S/c1-46-27-12-26(21(33)11-23(27)38-28(41)16-48-29-4-2-3-7-35-29)47-15-25(40)31(43)30(42)24(39)14-36-20-9-18(8-19(34)10-20)22-6-5-17(13-37-22)32(44)45/h2-13,24-25,30-31,36,39-40,42-43H,14-16H2,1H3,(H,38,41)(H,44,45)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425796
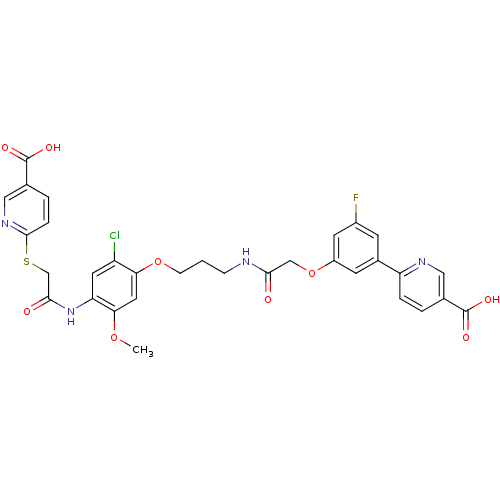
(CHEMBL2316884)Show SMILES COc1cc(OCCCNC(=O)COc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O Show InChI InChI=1S/C32H28ClFN4O9S/c1-45-27-13-26(23(33)12-25(27)38-29(40)17-48-30-6-4-19(15-37-30)32(43)44)46-8-2-7-35-28(39)16-47-22-10-20(9-21(34)11-22)24-5-3-18(14-36-24)31(41)42/h3-6,9-15H,2,7-8,16-17H2,1H3,(H,35,39)(H,38,40)(H,41,42)(H,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145940
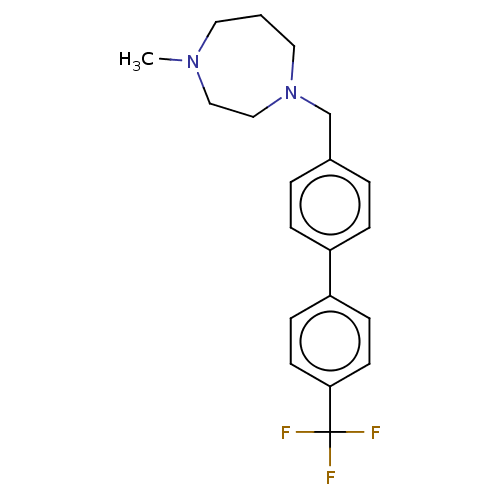
(CHEMBL3765567)Show InChI InChI=1S/C20H23F3N2/c1-24-11-2-12-25(14-13-24)15-16-3-5-17(6-4-16)18-7-9-19(10-8-18)20(21,22)23/h3-10H,2,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145929
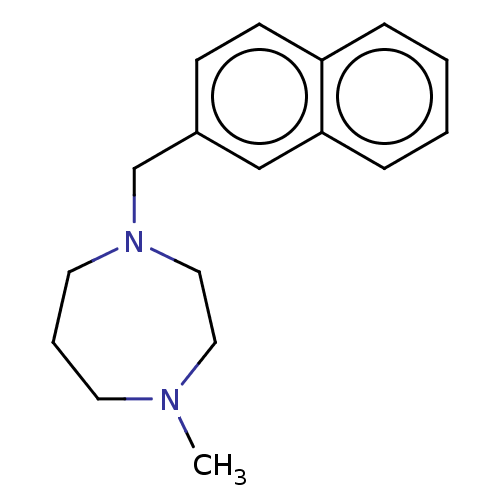
(CHEMBL3765650)Show InChI InChI=1S/C17H22N2/c1-18-9-4-10-19(12-11-18)14-15-7-8-16-5-2-3-6-17(16)13-15/h2-3,5-8,13H,4,9-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145928
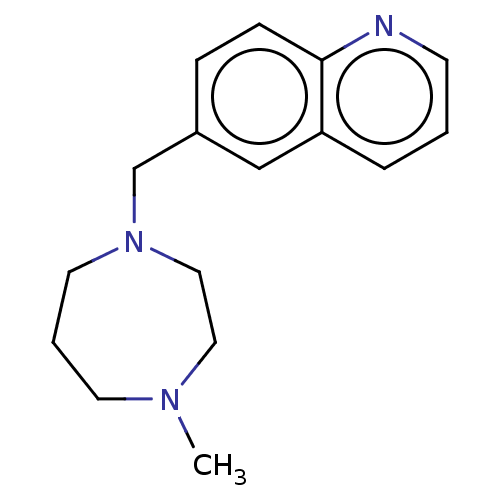
(CHEMBL3765077)Show InChI InChI=1S/C16H21N3/c1-18-8-3-9-19(11-10-18)13-14-5-6-16-15(12-14)4-2-7-17-16/h2,4-7,12H,3,8-11,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145934
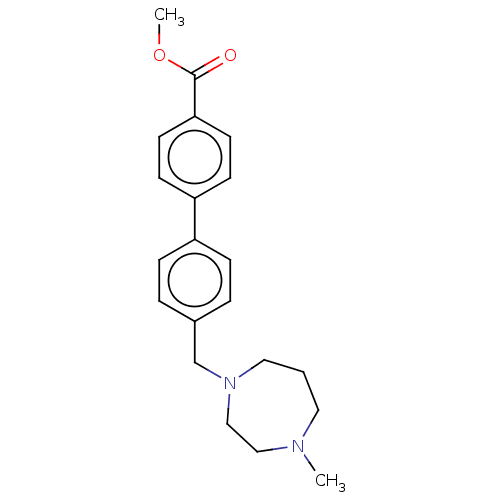
(CHEMBL3764904)Show InChI InChI=1S/C21H26N2O2/c1-22-12-3-13-23(15-14-22)16-17-4-6-18(7-5-17)19-8-10-20(11-9-19)21(24)25-2/h4-11H,3,12-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425793
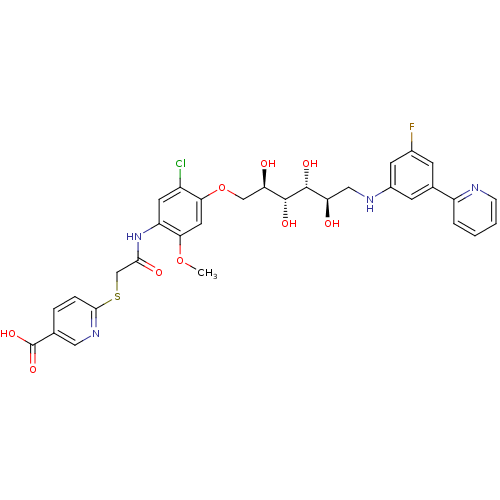
(CHEMBL2316881)Show SMILES COc1cc(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNc2cc(F)cc(c2)-c2ccccn2)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C32H32ClFN4O9S/c1-46-27-12-26(21(33)11-23(27)38-28(41)16-48-29-6-5-17(13-37-29)32(44)45)47-15-25(40)31(43)30(42)24(39)14-36-20-9-18(8-19(34)10-20)22-4-2-3-7-35-22/h2-13,24-25,30-31,36,39-40,42-43H,14-16H2,1H3,(H,38,41)(H,44,45)/t24-,25-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425795

(CHEMBL2316882)Show SMILES COc1cc(OC[C@H](O)[C@H](O)[C@@H](O)[C@@H](O)COc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C33H31ClFN3O12S/c1-48-27-10-26(21(34)9-23(27)38-28(41)15-51-29-5-3-17(12-37-29)33(46)47)50-14-25(40)31(43)30(42)24(39)13-49-20-7-18(6-19(35)8-20)22-4-2-16(11-36-22)32(44)45/h2-12,24-25,30-31,39-40,42-43H,13-15H2,1H3,(H,38,41)(H,44,45)(H,46,47)/t24-,25-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145927
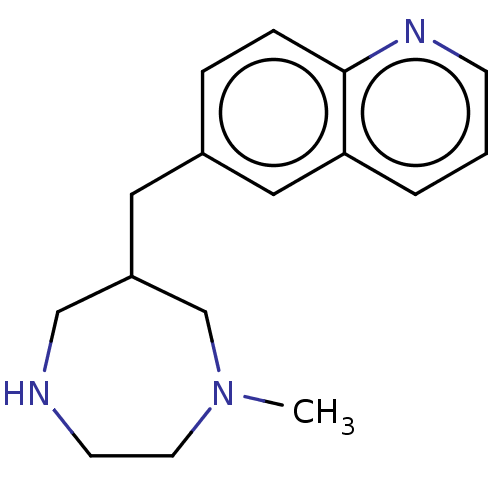
(CHEMBL3764395)Show InChI InChI=1S/C16H21N3/c1-19-8-7-17-11-14(12-19)9-13-4-5-16-15(10-13)3-2-6-18-16/h2-6,10,14,17H,7-9,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) in absence of 0.1% Triton |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425799
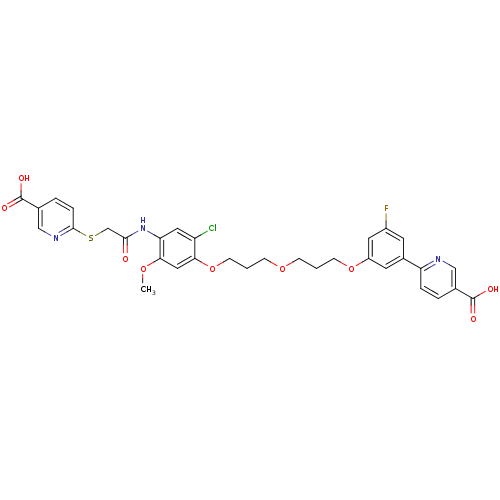
(CHEMBL2316888)Show SMILES COc1cc(OCCCOCCCOc2cc(F)cc(c2)-c2ccc(cn2)C(O)=O)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O Show InChI InChI=1S/C33H31ClFN3O9S/c1-44-29-16-28(25(34)15-27(29)38-30(39)19-48-31-7-5-21(18-37-31)33(42)43)47-11-3-9-45-8-2-10-46-24-13-22(12-23(35)14-24)26-6-4-20(17-36-26)32(40)41/h4-7,12-18H,2-3,8-11,19H2,1H3,(H,38,39)(H,40,41)(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145918
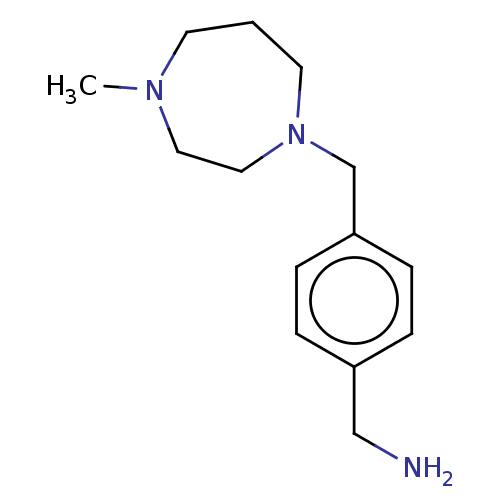
(CHEMBL3763993)Show InChI InChI=1S/C14H23N3/c1-16-7-2-8-17(10-9-16)12-14-5-3-13(11-15)4-6-14/h3-6H,2,7-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145925
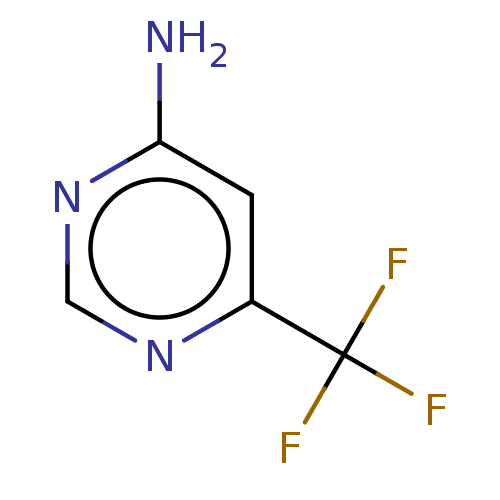
(CHEMBL3764475)Show InChI InChI=1S/C5H4F3N3/c6-5(7,8)3-1-4(9)11-2-10-3/h1-2H,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145921
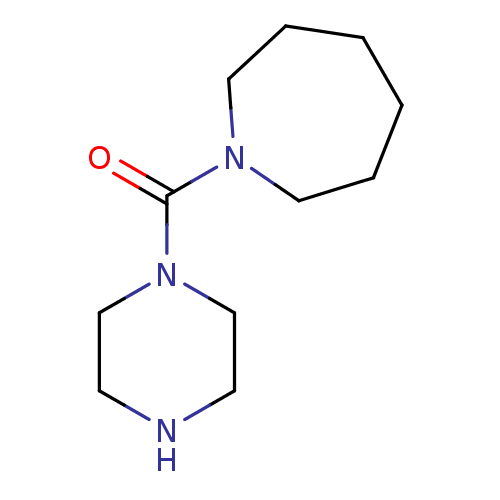
(CHEMBL3763196)Show InChI InChI=1S/C11H21N3O/c15-11(14-9-5-12-6-10-14)13-7-3-1-2-4-8-13/h12H,1-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425800
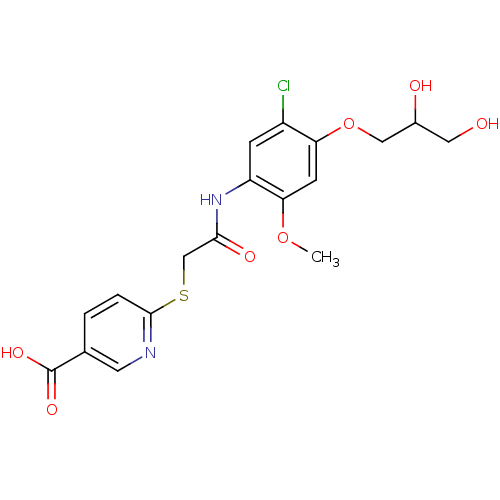
(CHEMBL2316887)Show SMILES COc1cc(OCC(O)CO)c(Cl)cc1NC(=O)CSc1ccc(cn1)C(O)=O Show InChI InChI=1S/C18H19ClN2O7S/c1-27-15-5-14(28-8-11(23)7-22)12(19)4-13(15)21-16(24)9-29-17-3-2-10(6-20-17)18(25)26/h2-6,11,22-23H,7-9H2,1H3,(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425801
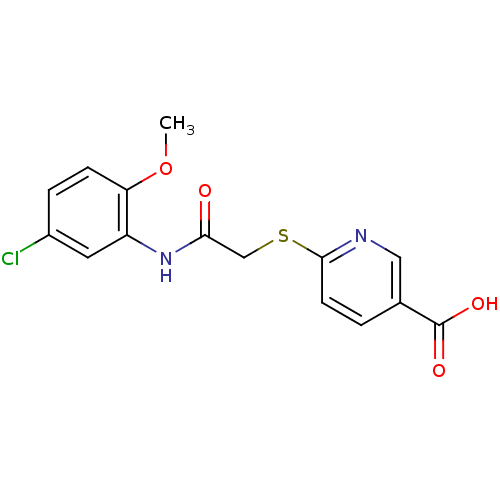
(CHEMBL2316891)Show InChI InChI=1S/C15H13ClN2O4S/c1-22-12-4-3-10(16)6-11(12)18-13(19)8-23-14-5-2-9(7-17-14)15(20)21/h2-7H,8H2,1H3,(H,18,19)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425802
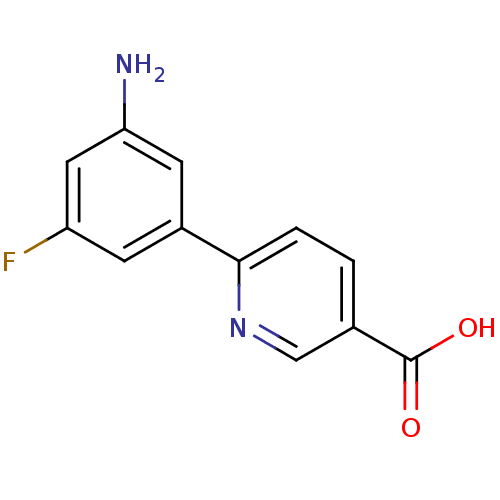
(CHEMBL2316889)Show InChI InChI=1S/C12H9FN2O2/c13-9-3-8(4-10(14)5-9)11-2-1-7(6-15-11)12(16)17/h1-6H,14H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145919
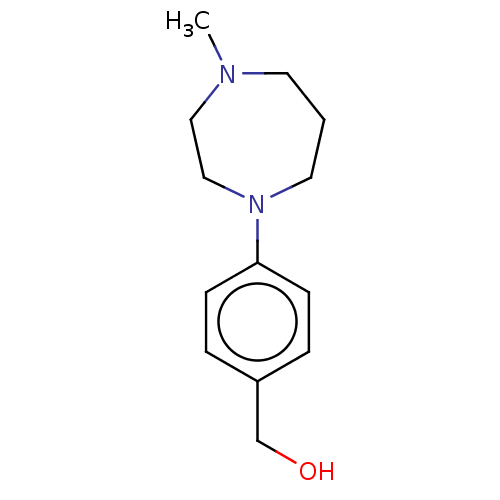
(CHEMBL3765610)Show InChI InChI=1S/C13H20N2O/c1-14-7-2-8-15(10-9-14)13-5-3-12(11-16)4-6-13/h3-6,16H,2,7-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal truncated ChoKalpha1 (75 to 457 residues) using choline chloride as substrate measured over 10 to 30 mins by coupled A... |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425804
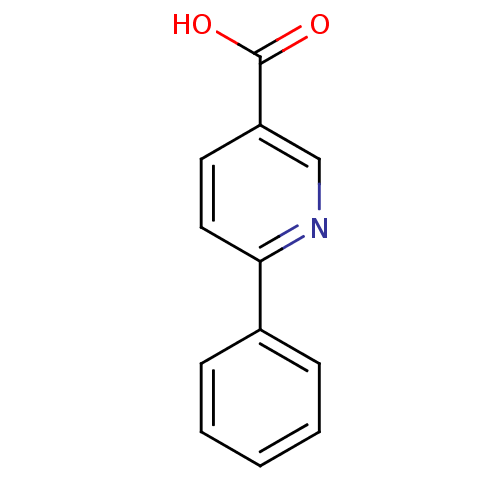
(6-Phenylnicotinic Acid | CHEMBL2316892)Show InChI InChI=1S/C12H9NO2/c14-12(15)10-6-7-11(13-8-10)9-4-2-1-3-5-9/h1-8H,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425803
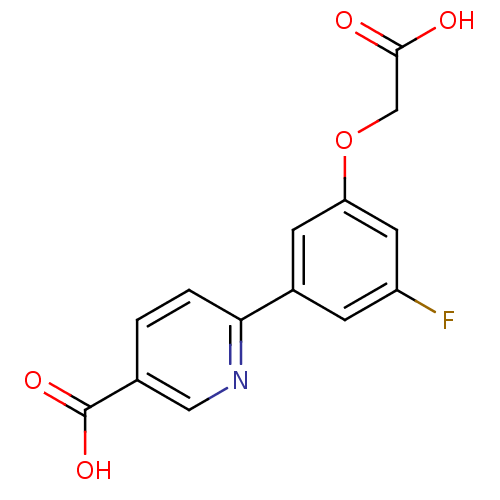
(CHEMBL2316893)Show InChI InChI=1S/C14H10FNO5/c15-10-3-9(4-11(5-10)21-7-13(17)18)12-2-1-8(6-16-12)14(19)20/h1-6H,7H2,(H,17,18)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50425792
(CHEMBL2316880)Show SMILES COC(=O)c1ccc(SCC(=O)Nc2cc(Cl)c(OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNc3cc(F)cc(c3)-c3ccc(cn3)C(=O)OC)cc2OC)nc1 |r| Show InChI InChI=1S/C35H36ClFN4O11S/c1-49-29-12-28(23(36)11-25(29)41-30(44)17-53-31-7-5-19(14-40-31)35(48)51-3)52-16-27(43)33(46)32(45)26(42)15-38-22-9-20(8-21(37)10-22)24-6-4-18(13-39-24)34(47)50-2/h4-14,26-27,32-33,38,42-43,45-46H,15-17H2,1-3H3,(H,41,44)/t26-,27-,32-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LDH-A (unknown origin) by cell based assay |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145918

(CHEMBL3763993)Show InChI InChI=1S/C14H23N3/c1-16-7-2-8-17(10-9-16)12-14-5-3-13(11-15)4-6-14/h3-6H,2,7-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal truncated ChoKalpha1 (75 to 457 residues) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145919

(CHEMBL3765610)Show InChI InChI=1S/C13H20N2O/c1-14-7-2-8-15(10-9-14)13-5-3-12(11-16)4-6-13/h3-6,16H,2,7-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.94E+5 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal truncated ChoKalpha1 (75 to 457 residues) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50145918

(CHEMBL3763993)Show InChI InChI=1S/C14H23N3/c1-16-7-2-8-17(10-9-16)12-14-5-3-13(11-15)4-6-14/h3-6H,2,7-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.90E+5 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to carbonic anhydrase2 (unknown origin) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145921

(CHEMBL3763196)Show InChI InChI=1S/C11H21N3O/c15-11(14-9-5-12-6-10-14)13-7-3-1-2-4-8-13/h12H,1-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.40E+5 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal truncated ChoKalpha1 (75 to 457 residues) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50145921

(CHEMBL3763196)Show InChI InChI=1S/C11H21N3O/c15-11(14-9-5-12-6-10-14)13-7-3-1-2-4-8-13/h12H,1-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+6 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to carbonic anhydrase2 (unknown origin) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145925

(CHEMBL3764475)Show InChI InChI=1S/C5H4F3N3/c6-5(7,8)3-1-4(9)11-2-10-3/h1-2H,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.30E+5 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal truncated ChoKalpha1 (75 to 457 residues) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50145925

(CHEMBL3764475)Show InChI InChI=1S/C5H4F3N3/c6-5(7,8)3-1-4(9)11-2-10-3/h1-2H,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.70E+5 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to carbonic anhydrase2 (unknown origin) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145927

(CHEMBL3764395)Show InChI InChI=1S/C16H21N3/c1-19-8-7-17-11-14(12-19)9-13-4-5-16-15(10-13)3-2-6-18-16/h2-6,10,14,17H,7-9,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal truncated ChoKalpha1 (75 to 457 residues) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145928

(CHEMBL3765077)Show InChI InChI=1S/C16H21N3/c1-18-8-3-9-19(11-10-18)13-14-5-6-16-15(12-14)4-2-7-17-16/h2,4-7,12H,3,8-11,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal truncated ChoKalpha1 (75 to 457 residues) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50145929

(CHEMBL3765650)Show InChI InChI=1S/C17H22N2/c1-18-9-4-10-19(12-11-18)14-15-7-8-16-5-2-3-6-17(16)13-15/h2-3,5-8,13H,4,9-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human N-terminal truncated ChoKalpha1 (75 to 457 residues) by surface plasmon resonance assay |
J Med Chem 59: 671-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01552
BindingDB Entry DOI: 10.7270/Q23X88HX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data