 Found 203 hits with Last Name = 'miyauchi' and Initial = 's'
Found 203 hits with Last Name = 'miyauchi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50333455
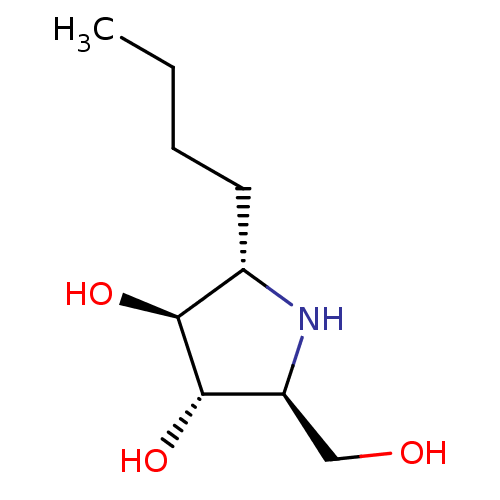
((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-6-8(12)9(13)7(5-11)10-6/h6-13H,2-5H2,1H3/t6-,7-,8-,9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50242271
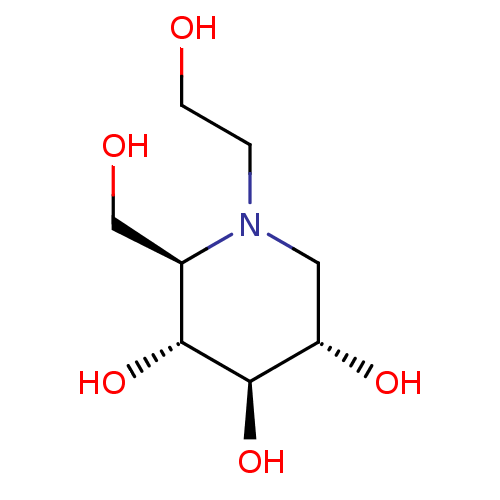
((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...)Show SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C8H17NO5/c10-2-1-9-3-6(12)8(14)7(13)5(9)4-11/h5-8,10-14H,1-4H2/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238110
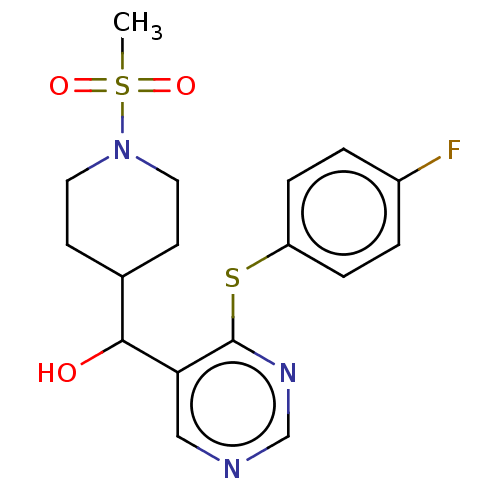
(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238110

(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115
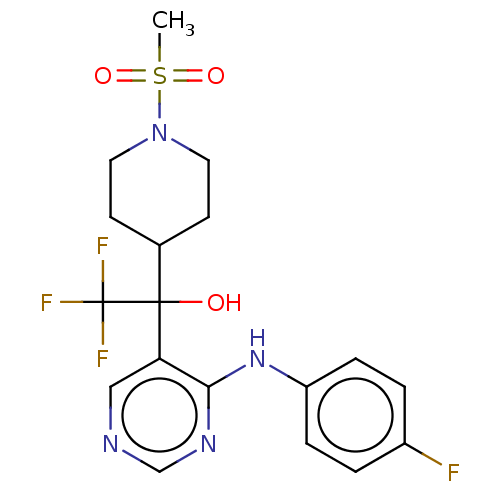
(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238108
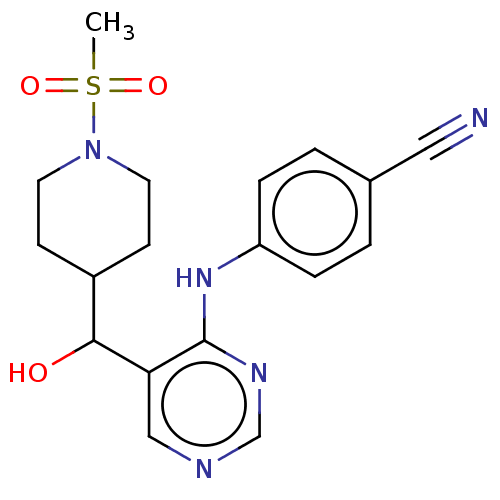
(CHEMBL4070230)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C#N Show InChI InChI=1S/C18H21N5O3S/c1-27(25,26)23-8-6-14(7-9-23)17(24)16-11-20-12-21-18(16)22-15-4-2-13(10-19)3-5-15/h2-5,11-12,14,17,24H,6-9H2,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238109
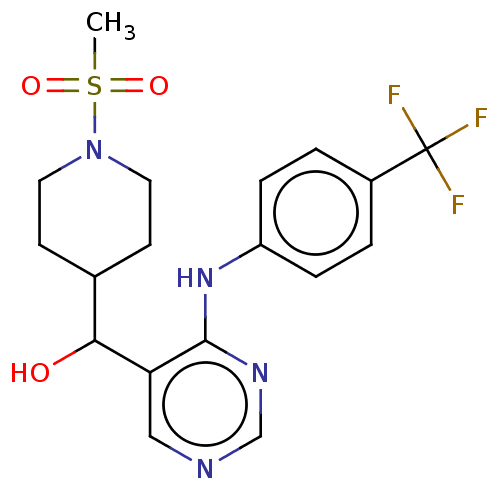
(CHEMBL4091167)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3S/c1-29(27,28)25-8-6-12(7-9-25)16(26)15-10-22-11-23-17(15)24-14-4-2-13(3-5-14)18(19,20)21/h2-5,10-12,16,26H,6-9H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50047262
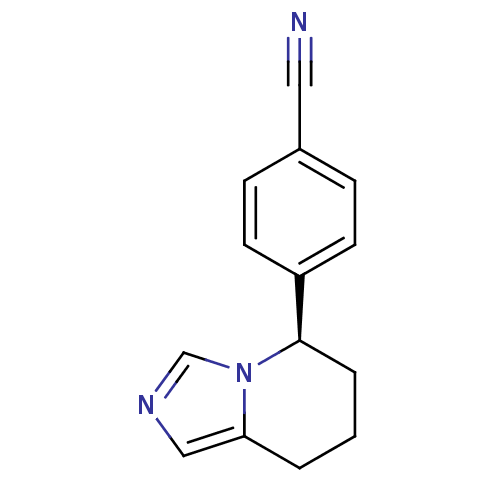
((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238106
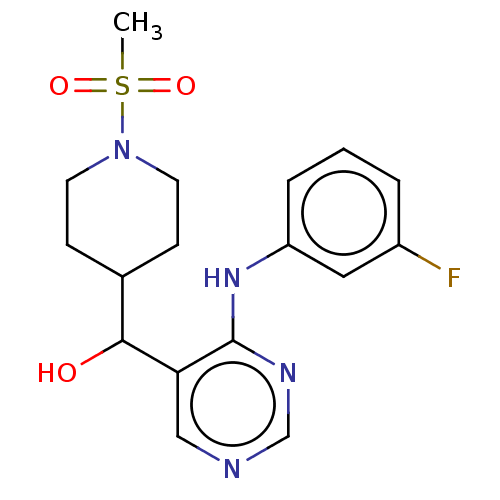
(CHEMBL4072943)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc(F)c1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-7-5-12(6-8-22)16(23)15-10-19-11-20-17(15)21-14-4-2-3-13(18)9-14/h2-4,9-12,16,23H,5-8H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238120
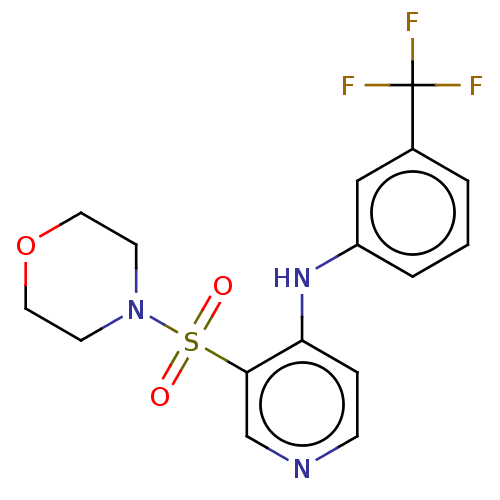
(CHEMBL4097020)Show SMILES FC(F)(F)c1cccc(Nc2ccncc2S(=O)(=O)N2CCOCC2)c1 Show InChI InChI=1S/C16H16F3N3O3S/c17-16(18,19)12-2-1-3-13(10-12)21-14-4-5-20-11-15(14)26(23,24)22-6-8-25-9-7-22/h1-5,10-11H,6-9H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238123

(CHEMBL4061565)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc2ccccc12 Show InChI InChI=1S/C21H24N4O3S/c1-29(27,28)25-11-9-16(10-12-25)20(26)18-13-22-14-23-21(18)24-19-8-4-6-15-5-2-3-7-17(15)19/h2-8,13-14,16,20,26H,9-12H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238117
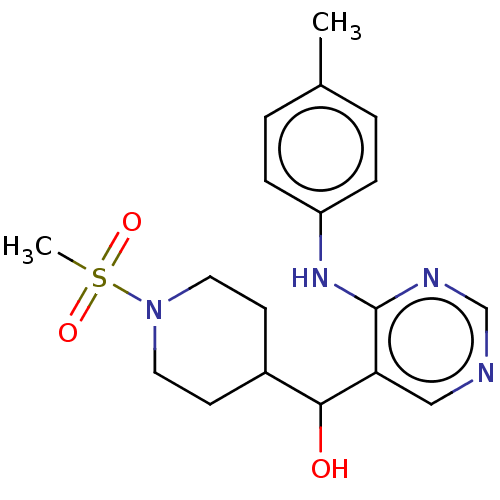
(CHEMBL4092081)Show SMILES Cc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O3S/c1-13-3-5-15(6-4-13)21-18-16(11-19-12-20-18)17(23)14-7-9-22(10-8-14)26(2,24)25/h3-6,11-12,14,17,23H,7-10H2,1-2H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238108

(CHEMBL4070230)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C#N Show InChI InChI=1S/C18H21N5O3S/c1-27(25,26)23-8-6-14(7-9-23)17(24)16-11-20-12-21-18(16)22-15-4-2-13(10-19)3-5-15/h2-5,11-12,14,17,24H,6-9H2,1H3,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238106

(CHEMBL4072943)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc(F)c1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-7-5-12(6-8-22)16(23)15-10-19-11-20-17(15)21-14-4-2-3-13(18)9-14/h2-4,9-12,16,23H,5-8H2,1H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238107

(CHEMBL4064367)Show SMILES COc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O4S/c1-26-15-5-3-14(4-6-15)21-18-16(11-19-12-20-18)17(23)13-7-9-22(10-8-13)27(2,24)25/h3-6,11-13,17,23H,7-10H2,1-2H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238116
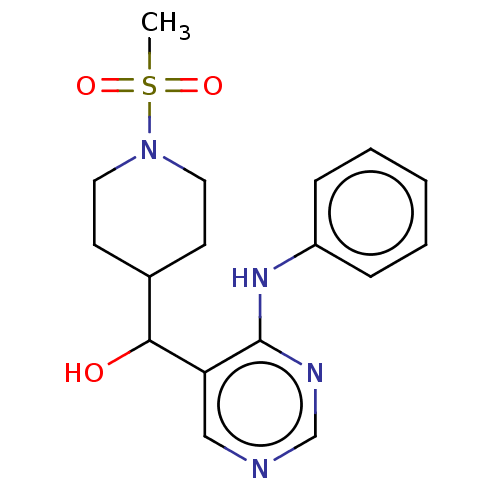
(CHEMBL4079006)Show InChI InChI=1S/C17H22N4O3S/c1-25(23,24)21-9-7-13(8-10-21)16(22)15-11-18-12-19-17(15)20-14-5-3-2-4-6-14/h2-6,11-13,16,22H,7-10H2,1H3,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238109

(CHEMBL4091167)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3S/c1-29(27,28)25-8-6-12(7-9-25)16(26)15-10-22-11-23-17(15)24-14-4-2-13(3-5-14)18(19,20)21/h2-5,10-12,16,26H,6-9H2,1H3,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238117

(CHEMBL4092081)Show SMILES Cc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O3S/c1-13-3-5-15(6-4-13)21-18-16(11-19-12-20-18)17(23)14-7-9-22(10-8-14)26(2,24)25/h3-6,11-12,14,17,23H,7-10H2,1-2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238102
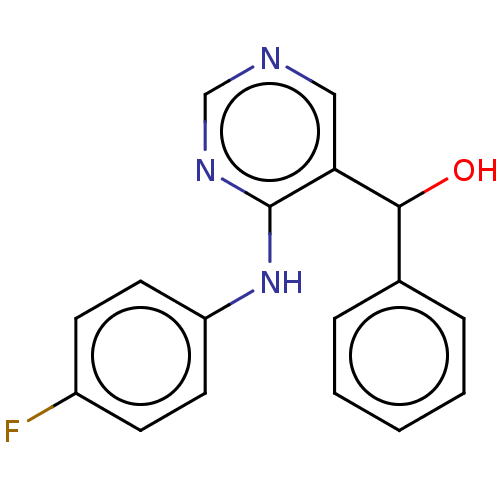
(CHEMBL4065476)Show InChI InChI=1S/C17H14FN3O/c18-13-6-8-14(9-7-13)21-17-15(10-19-11-20-17)16(22)12-4-2-1-3-5-12/h1-11,16,22H,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50333455

((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-6-8(12)9(13)7(5-11)10-6/h6-13H,2-5H2,1H3/t6-,7-,8-,9-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238116

(CHEMBL4079006)Show InChI InChI=1S/C17H22N4O3S/c1-25(23,24)21-9-7-13(8-10-21)16(22)15-11-18-12-19-17(15)20-14-5-3-2-4-6-14/h2-6,11-13,16,22H,7-10H2,1H3,(H,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238104

(CHEMBL4072871)Show SMILES CCCCC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C21H29FN4O3S/c1-3-4-11-21(27,16-9-12-26(13-10-16)30(2,28)29)19-14-23-15-24-20(19)25-18-7-5-17(22)6-8-18/h5-8,14-16,27H,3-4,9-13H2,1-2H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50182801
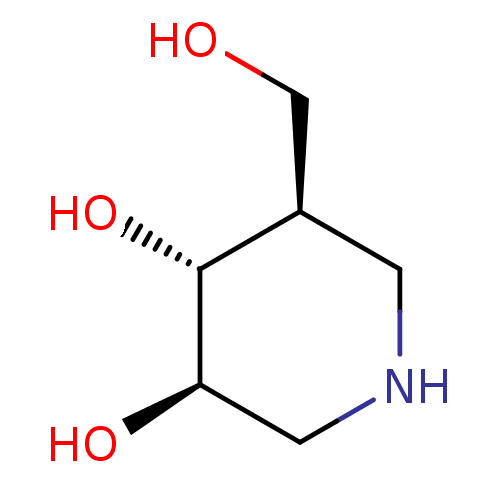
((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of human lysosome beta-glucosidase assessed as production of 4-methylumbelliferone using 4-methylumbelliferyl beta-D-glucoside as substrat... |
Bioorg Med Chem 19: 3558-68 (2011)
Article DOI: 10.1016/j.bmc.2011.04.011
BindingDB Entry DOI: 10.7270/Q2BC3ZWW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238101
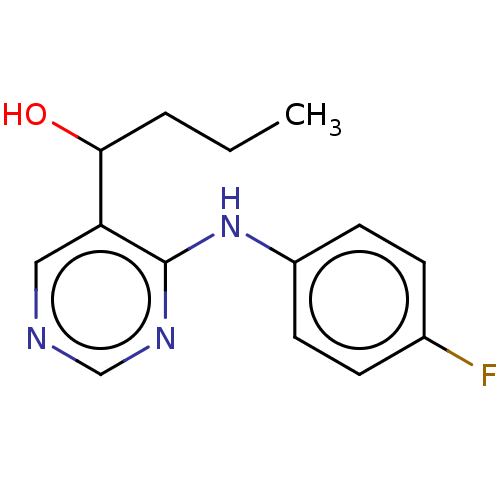
(CHEMBL4104118)Show InChI InChI=1S/C14H16FN3O/c1-2-3-13(19)12-8-16-9-17-14(12)18-11-6-4-10(15)5-7-11/h4-9,13,19H,2-3H2,1H3,(H,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238122
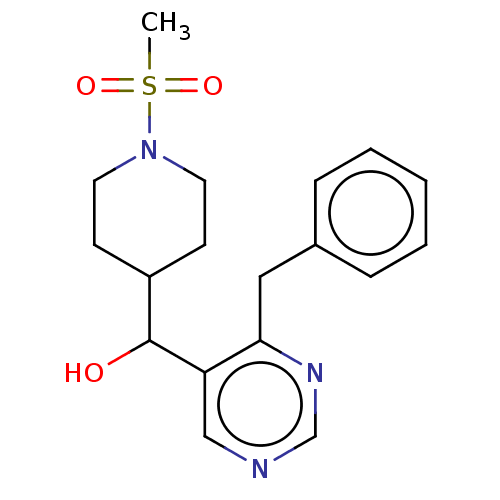
(CHEMBL4083387)Show InChI InChI=1S/C18H23N3O3S/c1-25(23,24)21-9-7-15(8-10-21)18(22)16-12-19-13-20-17(16)11-14-5-3-2-4-6-14/h2-6,12-13,15,18,22H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat adenylate kinase II was determined in the presence of ATP, non competitive inhibition |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238102

(CHEMBL4065476)Show InChI InChI=1S/C17H14FN3O/c18-13-6-8-14(9-7-13)21-17-15(10-19-11-20-17)16(22)12-4-2-1-3-5-12/h1-11,16,22H,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238113

(CHEMBL4067686)Show SMILES CS(=O)(=O)N1CCC(CC1)C(=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H19FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12H,6-9H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238123

(CHEMBL4061565)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc2ccccc12 Show InChI InChI=1S/C21H24N4O3S/c1-29(27,28)25-11-9-16(10-12-25)20(26)18-13-22-14-23-21(18)24-19-8-4-6-15-5-2-3-7-17(15)19/h2-8,13-14,16,20,26H,9-12H2,1H3,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50333457
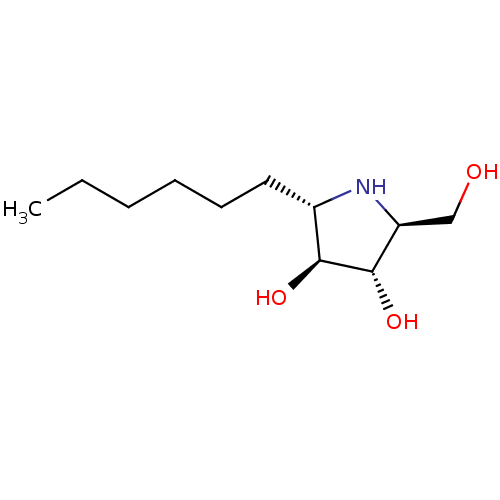
((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...)Show SMILES CCCCCC[C@@H]1N[C@@H](CO)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-6-8-10(14)11(15)9(7-13)12-8/h8-15H,2-7H2,1H3/t8-,9-,10-,11-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM18363
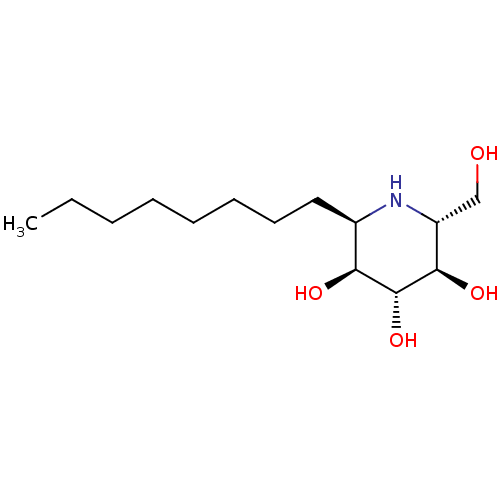
((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...)Show SMILES CCCCCCCC[C@H]1N[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H29NO4/c1-2-3-4-5-6-7-8-10-12(17)14(19)13(18)11(9-16)15-10/h10-19H,2-9H2,1H3/t10-,11-,12+,13-,14-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal isomaltase using isomoltose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50263044
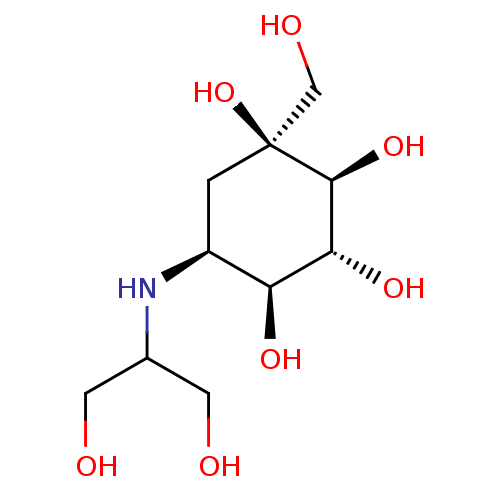
(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal maltase using moltose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Lactase/phlorizin hydrolase
(Rattus norvegicus) | BDBM50182801

((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5-,6-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal lactase assessed as production of p-nitrophenol by spectrophotometry |
Bioorg Med Chem 19: 3558-68 (2011)
Article DOI: 10.1016/j.bmc.2011.04.011
BindingDB Entry DOI: 10.7270/Q2BC3ZWW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238104

(CHEMBL4072871)Show SMILES CCCCC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C21H29FN4O3S/c1-3-4-11-21(27,16-9-12-26(13-10-16)30(2,28)29)19-14-23-15-24-20(19)25-18-7-5-17(22)6-8-18/h5-8,14-16,27H,3-4,9-13H2,1-2H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50333455

((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-6-8(12)9(13)7(5-11)10-6/h6-13H,2-5H2,1H3/t6-,7-,8-,9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal maltase using moltose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238119

(CHEMBL4075207)Show SMILES CS(=O)(=O)N1CCC(C1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C16H19FN4O3S/c1-25(23,24)21-7-6-11(9-21)15(22)14-8-18-10-19-16(14)20-13-4-2-12(17)3-5-13/h2-5,8,10-11,15,22H,6-7,9H2,1H3,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat adenylate kinase II was determined in the presence of AMP, competitive inhibition |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data