

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125708 (1-(3,5-Dichloro-phenyl)-3-{3-[3-(3,5-dichloro-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125717 (1-{2,4-Dibromo-5-[3-(4-iodo-phenyl)-ureido]-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125729 (3,5-Dichloro-N-{3-[3-(2,4-dibromo-phenyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125714 (3,5-Dichloro-N-{3-[3-(3,5-dichloro-phenyl)-ureido]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125731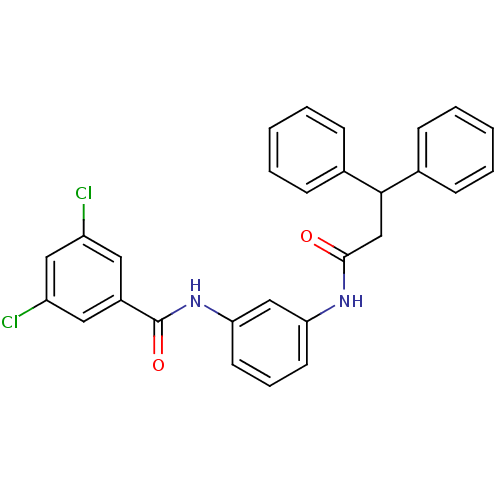 (3,5-Dichloro-N-[3-(3,3-diphenyl-propionylamino)-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125720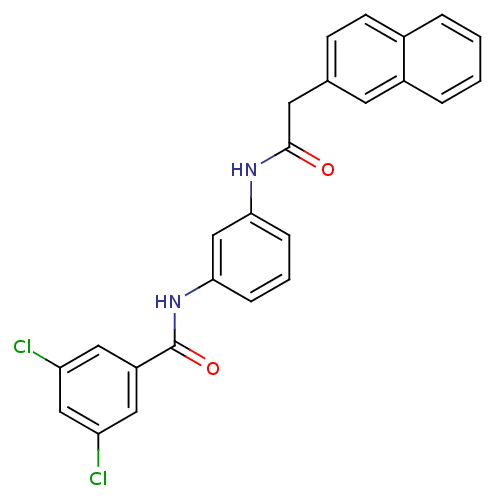 (3,5-Dichloro-N-[3-(2-naphthalen-2-yl-acetylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125716 (3,5-dichloro-N-{3-[(3,5-dichlorobenzoyl)amino]phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125706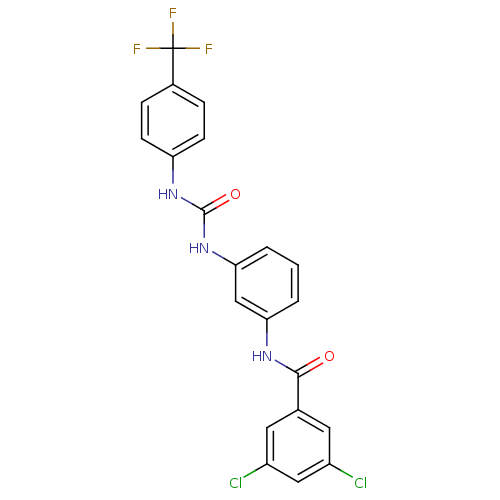 (3,5-Dichloro-N-{3-[3-(4-trifluoromethyl-phenyl)-ur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125718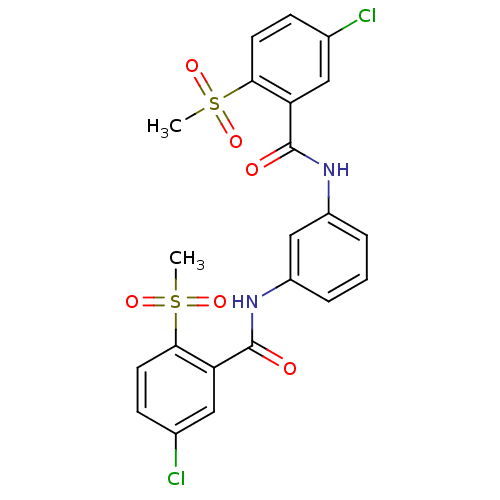 (1,3-di(5-chloro-2-methylsulfonylphenylcarboxamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125721 (3,5-Dichloro-N-[3-(2-naphthalen-1-yl-acetylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125715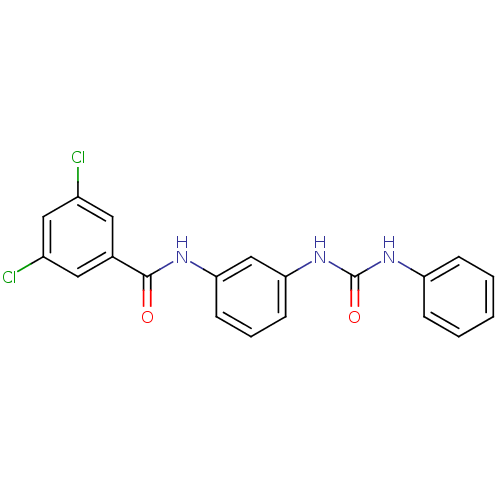 (3,5-Dichloro-N-[3-(3-phenyl-ureido)-phenyl]-benzam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125719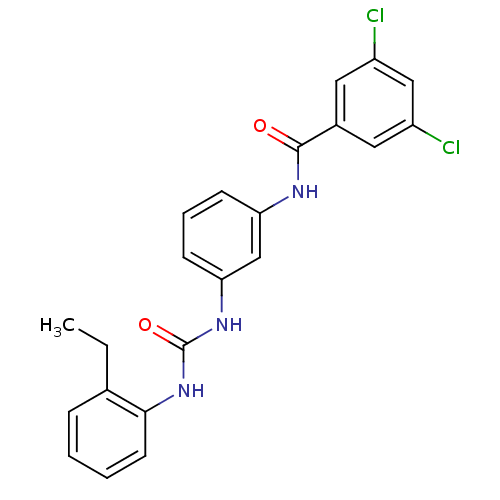 (3,5-Dichloro-N-{3-[3-(2-ethyl-phenyl)-ureido]-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125722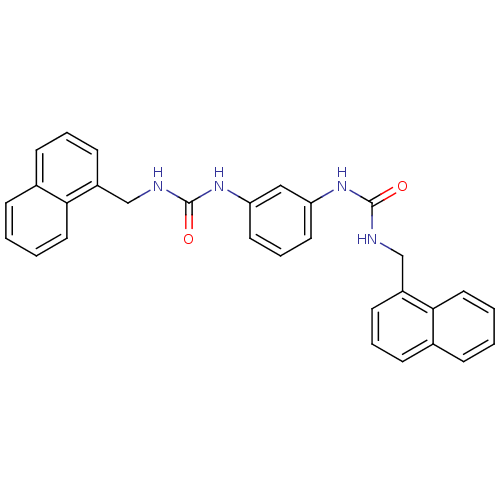 (1-Naphthalen-1-ylmethyl-3-[3-(3-naphthalen-1-ylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125724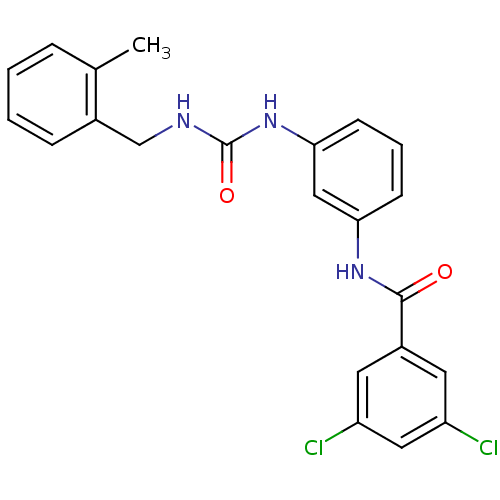 (3,5-Dichloro-N-{3-[3-(2-methyl-benzyl)-ureido]-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125709 (1-{2,4-Dibromo-5-[3-(4-dimethylamino-phenyl)-ureid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125732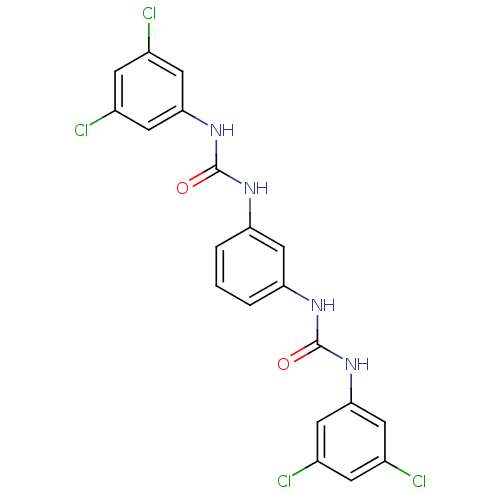 (1-(3,5-Dichloro-phenyl)-3-{3-[3-(3,5-dichloro-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125713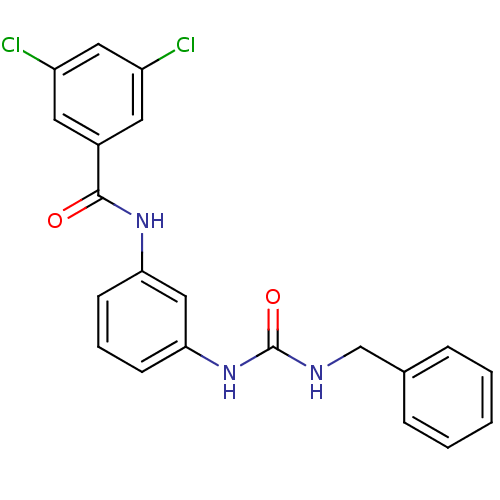 (CHEMBL276713 | N-[3-(3-Benzyl-ureido)-phenyl]-3,5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125728 (CHEMBL13550 | N-(3,5-dichlorophenyl)-N'-{3-[({[(3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125707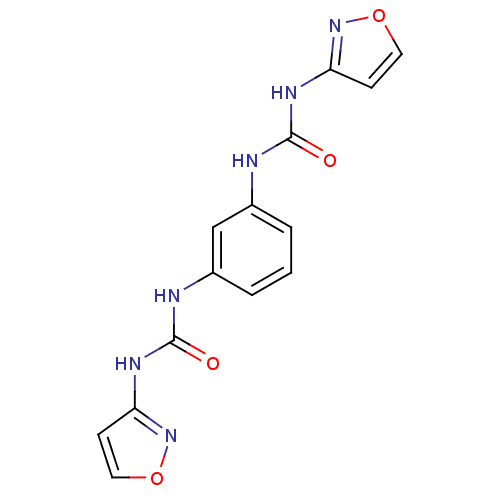 (1-Isoxazol-3-yl-3-[3-(3-isoxazol-3-yl-ureido)-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125727 (1-(3,5-Dichloro-phenyl)-3-{3-[3-(3,5-dichloro-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125710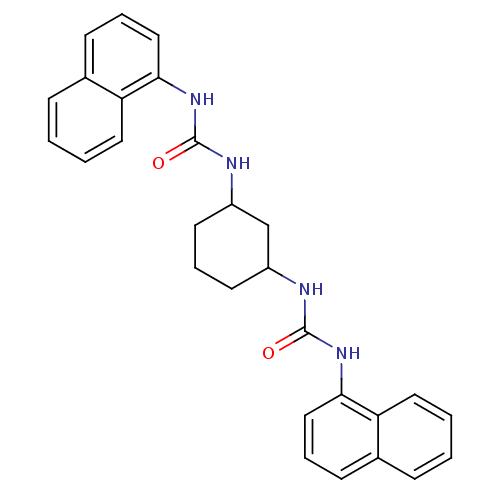 (1-Naphthalen-1-yl-3-[3-(3-naphthalen-1-yl-ureido)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125712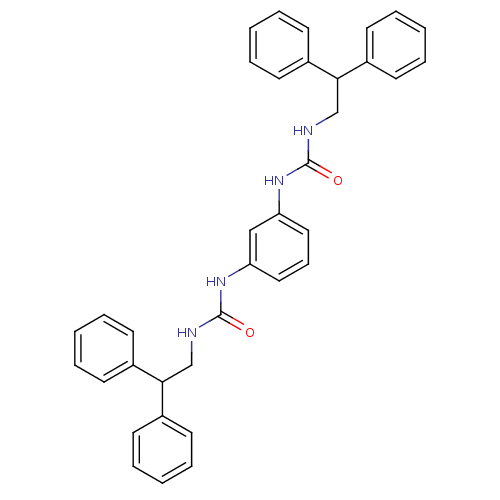 (1-(2,2-Diphenyl-ethyl)-3-{3-[3-(2,2-diphenyl-ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125723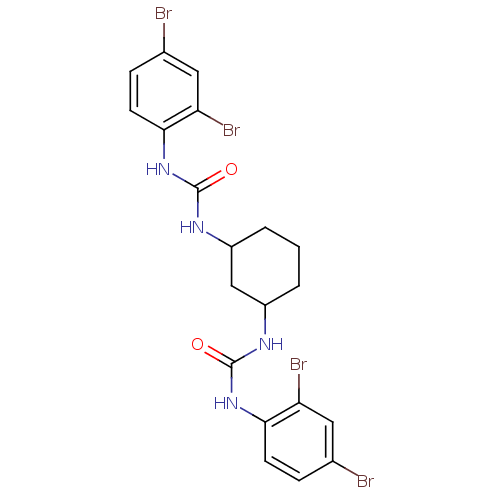 (1-(2,4-Dibromo-phenyl)-3-{3-[3-(2,4-dibromo-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125725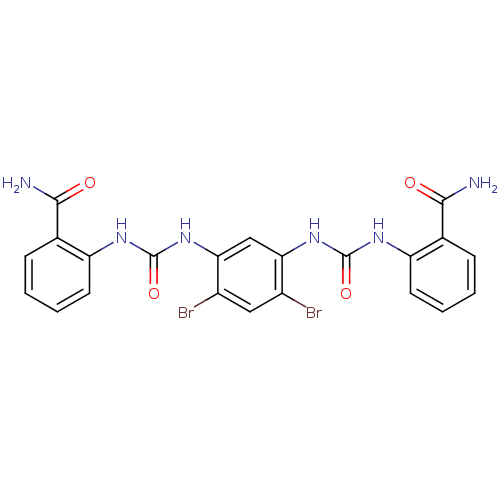 (1,1'-(4,6-dibromo-1,3-phenylene)bis(3-(2-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125730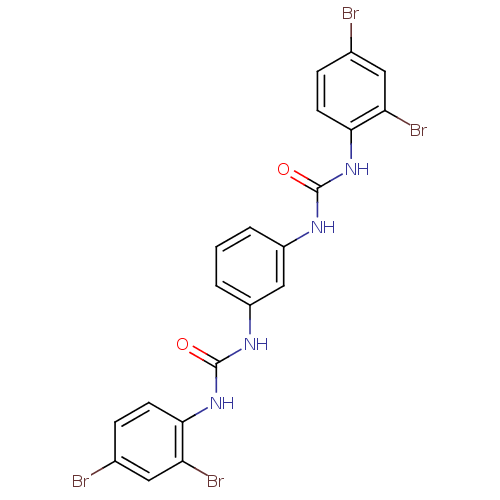 (1-(2,4-Dibromo-phenyl)-3-{3-[3-(2,4-dibromo-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125711 (1-(2,4-Dimethyl-phenyl)-3-{3-[3-(2,4-dimethyl-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Rattus norvegicus) | BDBM50125726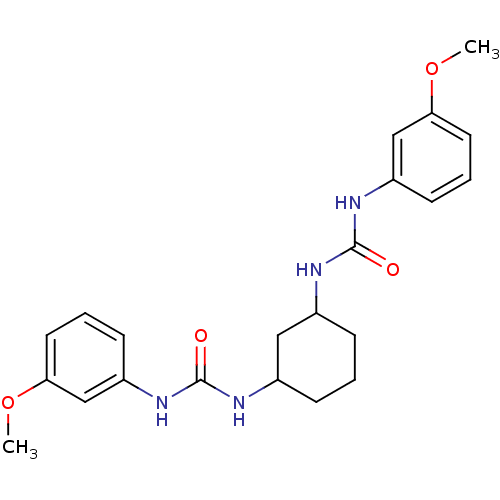 (1-(3-Methoxy-phenyl)-3-{3-[3-(3-methoxy-phenyl)-ur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of the rotamase activity of cyclophilin A (CyPA) | J Med Chem 46: 1112-5 (2003) Article DOI: 10.1021/jm020409u BindingDB Entry DOI: 10.7270/Q2V1244X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50009073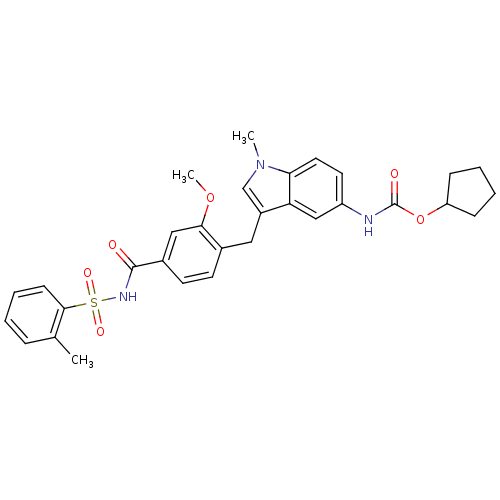 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 710 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088383 (Amlodipine | CHEBI:2668 | Norvasc) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM22876 (CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50101963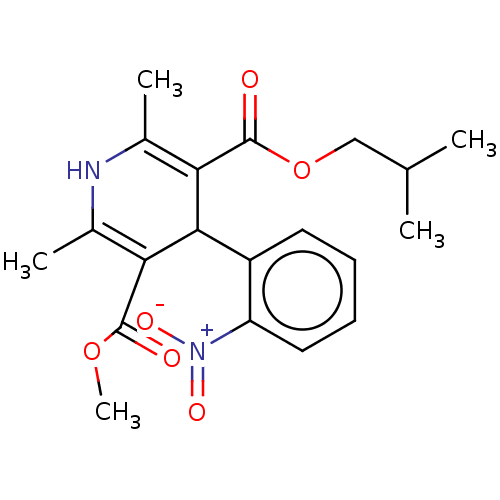 (BAY-K-5552 | CHEBI:76917 | Nisoldipine | Sular) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM115054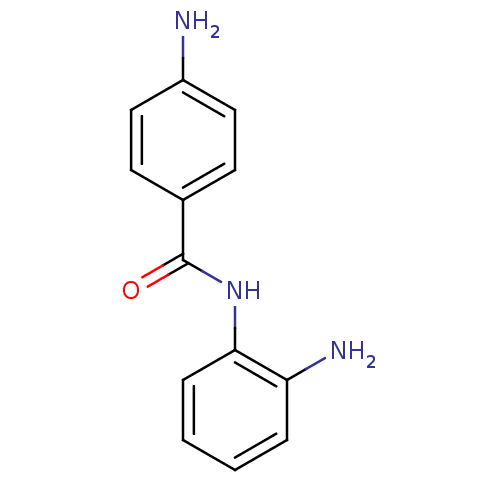 (4-Amino-N-(2-amino-phenyl)-benzamide | 4-amino-N-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM115054 (4-Amino-N-(2-amino-phenyl)-benzamide | 4-amino-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103608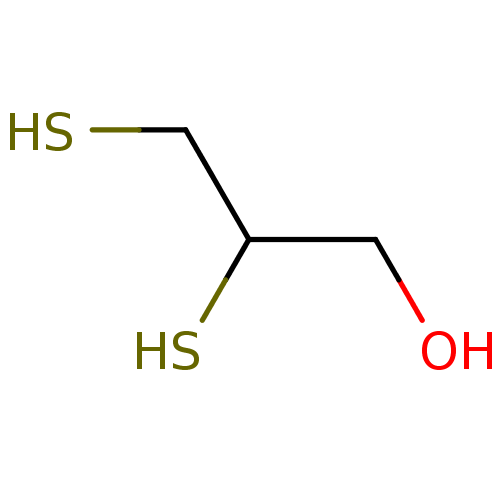 (Anti-Lewisite | CHEBI:64198 | Dimercaprol | Sulfac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103631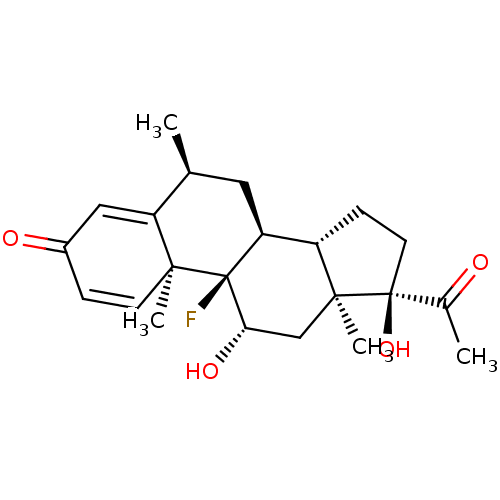 (CHEBI:31625 | Fluor-Op | Fluorometholone | Fml For...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088492 (Bumecaine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103611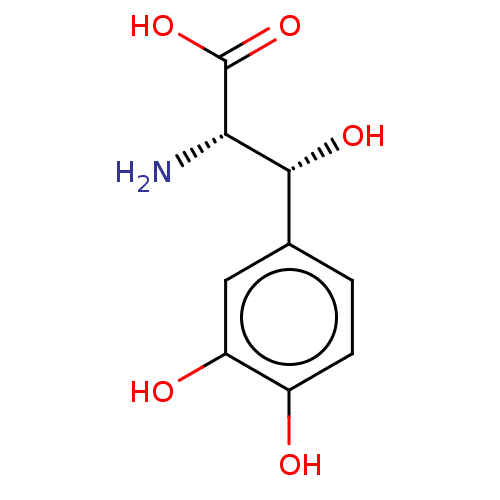 (CHEBI:31524 | DOPS | Droxidopa | L-DOPS) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103636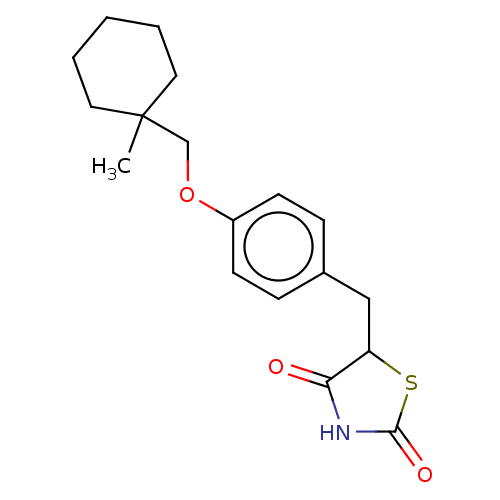 (ADD-3878 | CHEBI:64227 | Ciglitazone | U-63287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50065387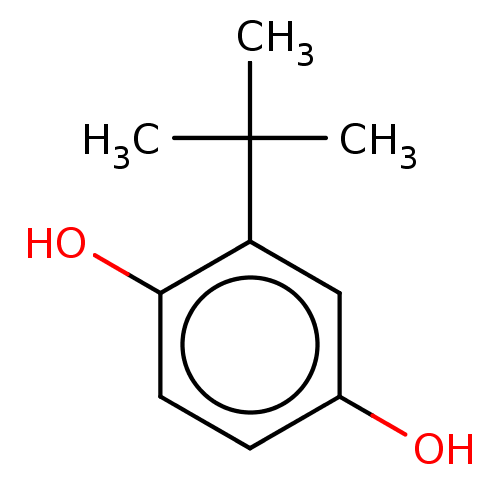 (CHEBI:78886 | E319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103628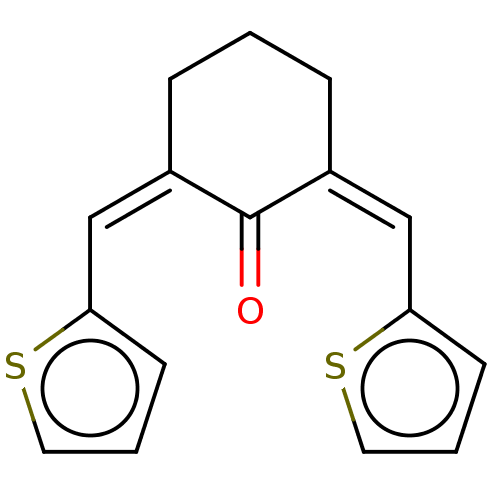 (CHEMBL1702784) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103612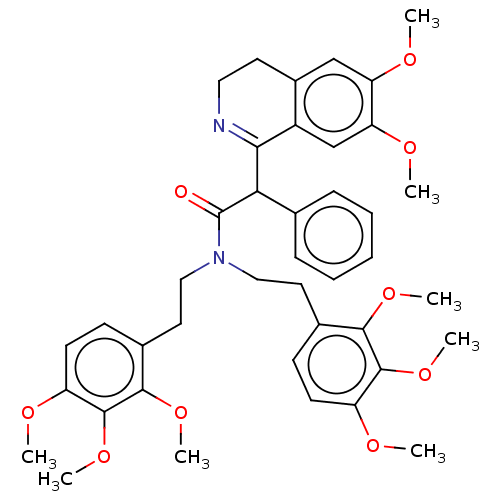 (CHEMBL2356116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50088492 (Bumecaine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50067593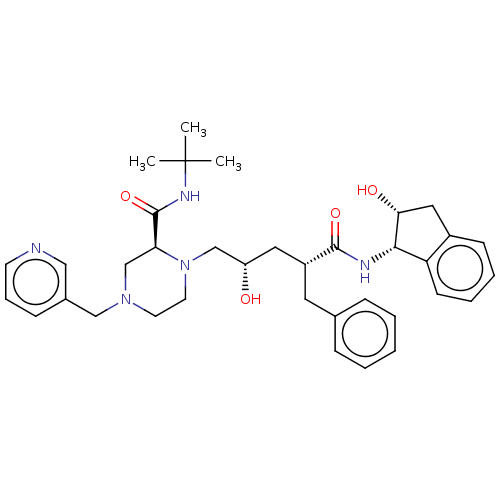 (CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50301375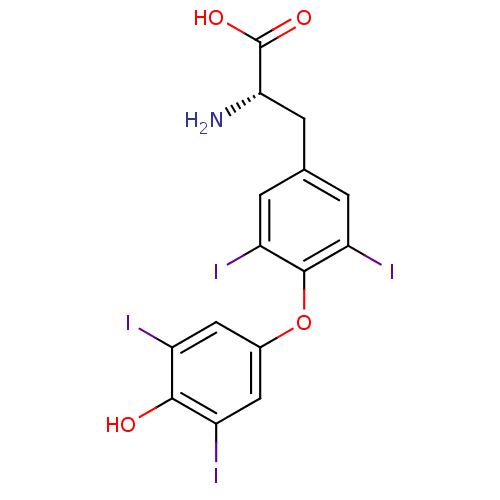 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50370232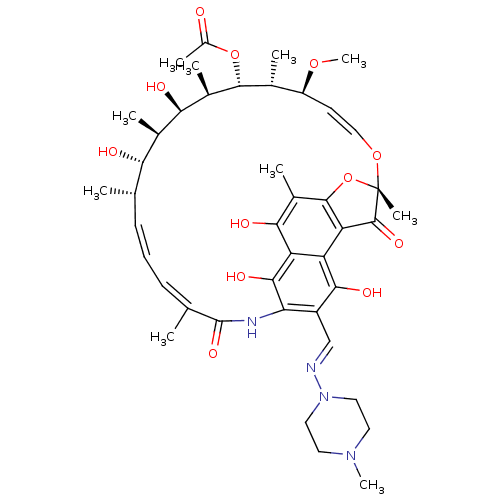 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50008984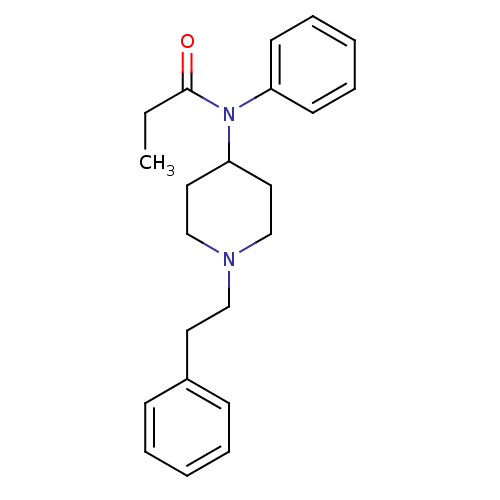 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM78576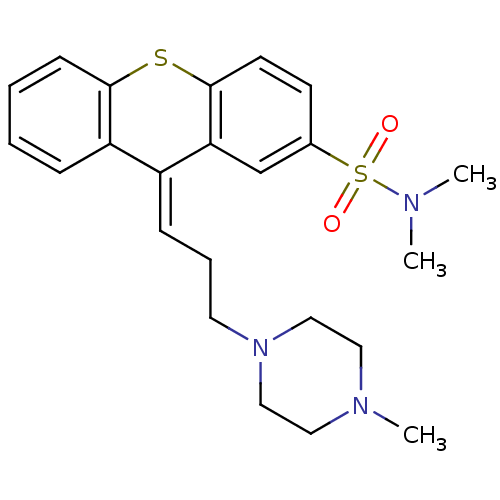 (CIS-THIOTHIXENE | MLS000028463 | SMR000058396 | TH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM32073 ((7R,11S)-11-methyl-7,15,17-tris(oxidanyl)-12-oxabi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50375318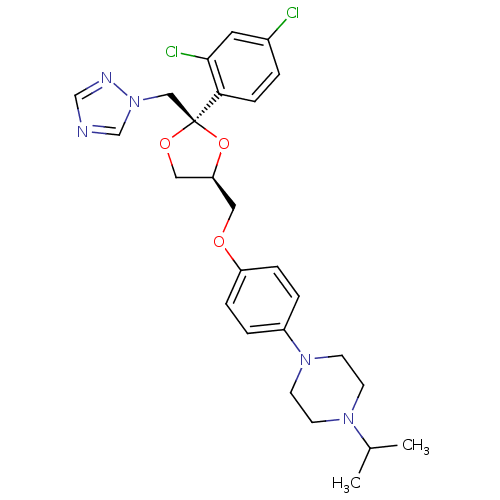 (Fungistat | Gyno-Terazol | Panlomyc | R-42470 | TE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |