 Found 70 hits with Last Name = 'morikis' and Initial = 'd'
Found 70 hits with Last Name = 'morikis' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388999
(CHEMBL2064013)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:105.121,80.89,61.70,36.43,19.23,4.4,127.132,140.145,wD:91.105,72.78,47.59,27.32,8.15,135.141,(37.4,-43.99,;37.39,-45.53,;38.73,-46.31,;36.05,-46.3,;36.05,-47.84,;34.71,-48.6,;33.38,-47.83,;33.39,-46.29,;32.04,-48.59,;32.04,-50.13,;33.37,-50.91,;33.36,-52.45,;34.69,-53.23,;34.68,-54.77,;33.35,-55.53,;36.02,-55.54,;30.71,-47.82,;29.38,-48.59,;29.37,-50.13,;28.05,-47.81,;28.05,-46.27,;29.38,-45.5,;30.72,-46.28,;29.39,-43.97,;26.7,-48.58,;25.37,-47.8,;25.38,-46.26,;24.04,-48.56,;24.03,-50.11,;25.36,-50.88,;25.36,-52.42,;24.02,-53.19,;26.69,-53.2,;22.71,-47.79,;21.38,-48.56,;21.37,-50.09,;20.04,-47.78,;20.05,-46.25,;21.38,-45.47,;21.39,-43.94,;22.72,-43.17,;22.73,-41.63,;21.4,-40.85,;24.07,-40.86,;18.7,-48.55,;17.37,-47.77,;17.38,-46.23,;16.04,-48.54,;16.03,-50.08,;17.36,-50.85,;18.77,-50.23,;19.8,-51.38,;19.03,-52.71,;19.49,-54.17,;18.46,-55.32,;16.95,-54.99,;16.48,-53.53,;17.52,-52.38,;14.71,-47.76,;13.37,-48.52,;13.36,-50.07,;12.03,-47.75,;12.04,-46.21,;13.38,-45.44,;13.39,-43.9,;14.72,-43.14,;14.73,-41.6,;13.4,-40.82,;16.07,-40.83,;10.7,-48.51,;9.37,-47.74,;9.38,-46.19,;8.03,-48.5,;8.02,-50.04,;9.36,-50.82,;10.69,-50.06,;9.35,-52.36,;6.7,-47.73,;5.36,-48.5,;5.36,-50.03,;4.03,-47.72,;4.04,-46.18,;5.37,-45.41,;5.38,-43.88,;6.72,-43.11,;6.73,-41.57,;5.4,-40.79,;8.06,-40.8,;2.7,-48.49,;1.37,-47.71,;1.37,-46.17,;.03,-48.47,;.02,-50.01,;1.36,-50.79,;2.77,-50.17,;3.79,-51.32,;3.01,-52.65,;3.48,-54.12,;2.45,-55.25,;.94,-54.93,;.47,-53.46,;1.51,-52.33,;-1.3,-47.7,;-2.64,-48.46,;-2.64,-50,;-3.97,-47.69,;-3.96,-46.15,;-2.63,-45.38,;-1.22,-46.01,;-.19,-44.87,;-.96,-43.54,;-.47,-42.07,;-1.5,-40.92,;-3.01,-41.24,;-3.49,-42.7,;-2.46,-43.85,;-5.3,-48.46,;-6.64,-47.69,;-7.96,-48.47,;-6.64,-46.16,;37.38,-48.61,;37.37,-50.15,;38.71,-47.85,;40.04,-48.63,;41.39,-47.86,;41.39,-46.32,;42.72,-48.64,;44.05,-47.87,;44.06,-46.33,;45.4,-45.56,;45.4,-44.03,;46.73,-46.34,;45.38,-48.64,;45.38,-50.18,;46.72,-47.88,;48.05,-48.65,;48.05,-50.2,;49.39,-47.89,;49.39,-46.35,;50.72,-48.66,;52.05,-47.9,;52.06,-46.36,;53.4,-45.6,;53.4,-44.06,;54.74,-43.29,;54.75,-41.75,;53.41,-40.98,;56.09,-40.99,;53.38,-48.68,;54.72,-47.91,;53.38,-50.22,)| Show InChI InChI=1S/C95H143N35O21/c1-47(2)36-66(78(138)116-46-75(134)119-67(37-48(3)4)84(144)117-49(5)77(137)125-65(90(150)151)28-17-35-112-95(106)107)126-80(140)61(25-14-32-109-92(100)101)122-89(149)72(42-74(97)133)130-83(143)64(29-30-76(135)136)124-79(139)60(24-13-31-108-91(98)99)120-86(146)69(39-52-44-114-58-22-11-8-19-55(52)58)127-81(141)62(26-15-33-110-93(102)103)123-88(148)71(41-73(96)132)129-82(142)63(27-16-34-111-94(104)105)121-87(147)70(40-53-45-115-59-23-12-9-20-56(53)59)128-85(145)68(118-50(6)131)38-51-43-113-57-21-10-7-18-54(51)57/h7-12,18-23,43-45,47-49,60-72,113-115H,13-17,24-42,46H2,1-6H3,(H2,96,132)(H2,97,133)(H,116,138)(H,117,144)(H,118,131)(H,119,134)(H,120,146)(H,121,147)(H,122,149)(H,123,148)(H,124,139)(H,125,137)(H,126,140)(H,127,141)(H,128,145)(H,129,142)(H,130,143)(H,135,136)(H,150,151)(H4,98,99,108)(H4,100,101,109)(H4,102,103,110)(H4,104,105,111)(H4,106,107,112)/t49-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389002
(CHEMBL2064017)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:107.123,89.91,122.129,67.76,42.49,29.33,12.16,4.4,133.138,wD:93.107,78.87,53.65,37.37,20.25,128.134,(51.04,2.49,;51.04,.95,;52.38,.19,;49.71,.18,;49.71,-1.36,;48.38,-2.14,;47.04,-1.38,;47.04,.17,;45.72,-2.15,;44.38,-1.37,;43.04,-2.16,;43.05,-3.68,;41.71,-1.38,;41.71,.15,;43.04,.92,;43.04,2.46,;44.37,.15,;40.38,-2.15,;39.04,-1.39,;39.04,.15,;37.71,-2.16,;37.71,-3.7,;39.05,-4.47,;39.05,-6.01,;40.39,-6.77,;40.39,-8.31,;36.37,-1.39,;35.04,-2.17,;35.05,-3.71,;33.71,-1.41,;33.71,.14,;35.03,.91,;36.37,.15,;35.03,2.45,;32.37,-2.18,;31.04,-1.41,;31.03,.13,;29.71,-2.18,;29.71,-3.72,;28.37,-1.41,;27.05,-2.19,;27.05,-3.73,;25.71,-1.42,;25.71,.11,;27.03,.88,;27.03,2.43,;28.36,3.21,;28.36,4.75,;27.02,5.51,;29.69,5.52,;24.38,-2.19,;23.04,-1.43,;23.04,.12,;21.71,-2.2,;21.71,-3.74,;23.05,-4.51,;24.45,-3.87,;25.49,-5.02,;24.72,-6.35,;25.2,-7.82,;24.18,-8.97,;22.67,-8.65,;22.19,-7.19,;23.22,-6.04,;20.38,-1.43,;19.05,-2.21,;19.05,-3.75,;17.71,-1.44,;17.71,.1,;19.04,.87,;19.03,2.4,;20.36,3.17,;20.36,4.72,;19.02,5.49,;21.69,5.49,;16.38,-2.21,;15.04,-1.45,;15.04,.09,;13.71,-2.22,;13.72,-3.76,;15.05,-4.53,;15.05,-6.07,;16.39,-6.83,;16.39,-8.37,;15.06,-9.14,;17.73,-9.14,;12.37,-1.45,;11.04,-2.23,;11.04,-3.76,;9.7,-1.45,;8.38,-2.23,;7.04,-1.46,;7.04,.07,;5.71,-2.24,;5.71,-3.78,;7.05,-4.54,;8.45,-3.92,;9.49,-5.06,;8.72,-6.39,;9.2,-7.86,;8.17,-9.01,;6.67,-8.69,;6.19,-7.22,;7.21,-6.08,;4.38,-1.47,;3.05,-2.25,;3.05,-3.79,;1.71,-1.48,;1.7,.07,;3.03,.84,;4.44,.21,;5.47,1.36,;4.7,2.69,;5.17,4.16,;4.13,5.3,;2.63,4.97,;2.15,3.51,;3.2,2.37,;.39,-2.25,;-.94,-1.49,;-2.27,-2.26,;-.95,.05,;9.7,.08,;8.36,.85,;11.04,.85,;51.04,-2.13,;51.05,-3.67,;52.38,-1.36,;53.72,-2.12,;53.72,-3.67,;55.04,-1.35,;55.04,.19,;56.38,-2.11,;57.71,-1.34,;57.7,.19,;59.04,.97,;59.04,2.51,;60.36,3.28,;60.36,4.82,;59.03,5.59,;61.7,5.6,;59.05,-2.11,;60.38,-1.33,;59.05,-3.65,)| Show InChI InChI=1S/C93H141N31O20/c1-47(2)37-67(78(132)110-46-73(127)114-68(38-48(3)4)83(137)112-49(5)76(130)119-66(89(143)144)31-20-36-106-93(101)102)121-81(135)62(27-15-16-32-94)116-86(140)72(42-74(128)129)120-77(131)50(6)111-79(133)63(28-17-33-103-90(95)96)117-85(139)70(40-54-44-108-60-25-13-10-22-57(54)60)122-82(136)64(29-18-34-104-91(97)98)115-80(134)65(30-19-35-105-92(99)100)118-88(142)75(51(7)125)124-87(141)71(41-55-45-109-61-26-14-11-23-58(55)61)123-84(138)69(113-52(8)126)39-53-43-107-59-24-12-9-21-56(53)59/h9-14,21-26,43-45,47-51,62-72,75,107-109,125H,15-20,27-42,46,94H2,1-8H3,(H,110,132)(H,111,133)(H,112,137)(H,113,126)(H,114,127)(H,115,134)(H,116,140)(H,117,139)(H,118,142)(H,119,130)(H,120,131)(H,121,135)(H,122,136)(H,123,138)(H,124,141)(H,128,129)(H,143,144)(H4,95,96,103)(H4,97,98,104)(H4,99,100,105)(H4,101,102,106)/t49-,50-,51+,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,75-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389005
(CHEMBL2064014)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:106.122,88.90,121.128,66.75,41.48,29.33,12.16,4.4,135.140,wD:92.106,77.86,52.64,37.37,124.131,20.25,130.136,(47.41,2.03,;47.43,.49,;48.77,-.28,;46.1,-.29,;46.11,-1.83,;44.78,-2.61,;43.45,-1.85,;43.44,-.31,;42.12,-2.62,;40.77,-1.87,;39.45,-2.65,;39.46,-4.18,;38.11,-1.88,;38.1,-.35,;39.43,.44,;39.42,1.98,;40.77,-.33,;36.79,-2.66,;35.44,-1.9,;35.43,-.36,;34.11,-2.67,;34.12,-4.22,;35.46,-4.98,;35.47,-6.52,;36.81,-7.28,;36.82,-8.82,;32.78,-1.92,;31.45,-2.7,;31.46,-4.24,;30.11,-1.94,;30.1,-.4,;31.43,.38,;32.77,-.38,;31.42,1.92,;28.79,-2.71,;27.44,-1.95,;27.43,-.41,;26.11,-2.73,;24.77,-1.98,;23.45,-2.75,;23.46,-4.29,;22.11,-1.99,;22.1,-.46,;23.43,.33,;23.42,1.87,;24.74,2.65,;24.73,4.18,;23.4,4.95,;26.06,4.97,;20.79,-2.77,;19.44,-2,;19.43,-.47,;18.11,-2.78,;18.12,-4.33,;19.47,-5.09,;20.86,-4.45,;21.91,-5.59,;21.14,-6.93,;21.63,-8.39,;20.61,-9.54,;19.1,-9.23,;18.61,-7.76,;19.63,-6.62,;16.77,-2.03,;15.44,-2.8,;15.46,-4.34,;14.11,-2.04,;14.1,-.51,;15.43,.28,;15.41,1.81,;16.74,2.59,;16.73,4.13,;15.4,4.89,;18.06,4.92,;12.79,-2.82,;11.44,-2.06,;11.43,-.52,;10.11,-2.84,;10.12,-4.38,;11.47,-5.15,;11.47,-6.68,;12.81,-7.44,;12.83,-8.98,;11.5,-9.76,;14.15,-9.75,;8.77,-2.08,;7.45,-2.85,;7.46,-4.4,;6.11,-2.1,;4.78,-2.88,;3.44,-2.11,;3.43,-.58,;2.12,-2.89,;2.13,-4.43,;3.46,-5.2,;4.86,-4.56,;5.9,-5.7,;5.15,-7.03,;5.63,-8.49,;4.61,-9.65,;3.09,-9.34,;2.61,-7.87,;3.64,-6.73,;.77,-2.13,;-.56,-2.91,;-.54,-4.45,;-1.89,-2.15,;-1.9,-.62,;-.57,.17,;.84,-.45,;1.86,.7,;1.08,2.03,;1.54,3.5,;.51,4.64,;-.99,4.3,;-1.47,2.84,;-.42,1.7,;-3.22,-2.93,;-4.55,-2.17,;-5.87,-2.94,;-4.56,-.63,;6.1,-.57,;4.75,.21,;7.42,.22,;26.12,-4.27,;24.79,-5.05,;27.46,-5.04,;47.44,-2.59,;47.46,-4.13,;48.77,-1.81,;50.12,-2.57,;50.13,-4.11,;51.45,-1.79,;51.44,-.25,;52.79,-2.55,;54.12,-1.78,;54.1,-.24,;55.43,.54,;55.42,2.09,;56.74,2.87,;56.73,4.4,;55.4,5.17,;58.06,5.18,;55.44,-2.54,;56.77,-1.76,;55.46,-4.07,)| Show InChI InChI=1S/C94H143N31O21/c1-47(2)37-67(78(133)111-46-73(129)114-68(38-48(3)4)83(138)112-49(5)77(132)119-66(90(145)146)31-20-36-107-94(102)103)120-80(135)62(27-15-16-32-95)116-86(141)72(42-74(130)131)123-89(144)76(51(7)127)124-82(137)65(30-19-35-106-93(100)101)117-85(140)70(40-54-44-109-60-25-13-10-22-57(54)60)121-81(136)63(28-17-33-104-91(96)97)115-79(134)64(29-18-34-105-92(98)99)118-88(143)75(50(6)126)125-87(142)71(41-55-45-110-61-26-14-11-23-58(55)61)122-84(139)69(113-52(8)128)39-53-43-108-59-24-12-9-21-56(53)59/h9-14,21-26,43-45,47-51,62-72,75-76,108-110,126-127H,15-20,27-42,46,95H2,1-8H3,(H,111,133)(H,112,138)(H,113,128)(H,114,129)(H,115,134)(H,116,141)(H,117,140)(H,118,143)(H,119,132)(H,120,135)(H,121,136)(H,122,139)(H,123,144)(H,124,137)(H,125,142)(H,130,131)(H,145,146)(H4,96,97,104)(H4,98,99,105)(H4,100,101,106)(H4,102,103,107)/t49-,50+,51+,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,75-,76-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389000
(CHEMBL2064015)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:107.123,89.91,122.129,67.76,42.49,29.34,12.16,4.4,136.141,wD:93.107,78.87,53.65,38.38,125.132,20.25,131.137,(45.2,-15.66,;45.17,-17.2,;46.5,-17.99,;43.83,-17.95,;43.8,-19.49,;42.46,-20.24,;41.14,-19.45,;41.16,-17.91,;39.79,-20.2,;38.47,-19.4,;37.13,-20.16,;37.1,-21.7,;35.8,-19.37,;35.83,-17.82,;37.17,-17.07,;37.2,-15.54,;38.5,-17.87,;34.46,-20.12,;33.14,-19.32,;33.16,-17.79,;31.79,-20.07,;31.77,-21.61,;33.09,-22.4,;33.07,-23.95,;34.39,-24.73,;34.36,-26.28,;30.47,-19.28,;29.13,-20.03,;29.11,-21.57,;27.81,-19.24,;27.83,-17.7,;29.18,-16.95,;29.2,-15.41,;27.88,-14.62,;30.54,-14.66,;26.47,-19.99,;25.14,-19.2,;25.16,-17.66,;23.79,-19.95,;22.48,-19.15,;21.13,-19.91,;21.11,-21.45,;19.81,-19.12,;19.83,-17.58,;21.18,-16.82,;21.2,-15.29,;22.55,-14.54,;22.57,-13,;21.25,-12.21,;23.92,-12.25,;18.46,-19.87,;17.14,-19.07,;17.16,-17.53,;15.79,-19.82,;15.77,-21.36,;17.1,-22.15,;18.51,-21.55,;19.52,-22.7,;18.73,-24.03,;19.18,-25.5,;18.13,-26.63,;16.63,-26.28,;16.18,-24.81,;17.23,-23.69,;14.47,-19.03,;13.13,-19.78,;13.11,-21.32,;11.81,-18.99,;11.83,-17.45,;13.18,-16.7,;13.2,-15.16,;14.55,-14.41,;14.57,-12.88,;13.25,-12.08,;15.92,-12.12,;10.46,-19.74,;9.14,-18.95,;9.16,-17.4,;7.79,-19.7,;7.77,-21.23,;9.09,-22.03,;9.07,-23.56,;10.39,-24.35,;10.37,-25.9,;9.02,-26.65,;11.69,-26.68,;6.47,-18.9,;5.13,-19.65,;5.11,-21.2,;3.81,-18.87,;2.46,-19.62,;1.15,-18.82,;1.17,-17.29,;-.2,-19.57,;-.23,-21.11,;1.09,-21.9,;2.51,-21.29,;3.52,-22.45,;2.73,-23.78,;3.18,-25.25,;2.13,-26.37,;.63,-26.03,;.18,-24.56,;1.23,-23.44,;-1.53,-18.78,;-2.87,-19.52,;-2.89,-21.07,;-4.19,-18.74,;-4.17,-17.2,;-2.82,-16.45,;-1.43,-17.1,;-.37,-15.97,;-1.12,-14.63,;-.63,-13.17,;-1.64,-12.01,;-3.15,-12.3,;-3.65,-13.76,;-2.63,-14.92,;-5.54,-19.49,;-6.87,-18.72,;-8.2,-19.5,;-6.88,-17.18,;3.83,-17.32,;2.51,-16.53,;5.18,-16.57,;23.77,-21.48,;22.42,-22.24,;25.09,-22.28,;45.12,-20.28,;45.1,-21.82,;46.47,-19.53,;47.79,-20.32,;47.77,-21.87,;49.14,-19.57,;49.16,-18.04,;50.46,-20.37,;51.8,-19.62,;51.83,-18.08,;53.17,-17.32,;53.2,-15.79,;54.54,-15.04,;54.57,-13.5,;53.25,-12.71,;55.91,-12.75,;53.12,-20.41,;54.47,-19.66,;53.1,-21.95,)| Show InChI InChI=1S/C95H145N31O21/c1-48(2)39-69(79(134)112-47-74(130)115-70(40-49(3)4)85(140)113-50(5)78(133)121-68(91(146)147)31-20-38-108-95(103)104)122-81(136)63(27-15-16-34-96)116-83(138)67(32-33-75(131)132)120-90(145)76(51(6)127)125-84(139)66(30-19-37-107-94(101)102)118-87(142)72(42-55-45-110-61-25-13-10-22-58(55)61)123-82(137)64(28-17-35-105-92(97)98)117-80(135)65(29-18-36-106-93(99)100)119-89(144)77(52(7)128)126-88(143)73(43-56-46-111-62-26-14-11-23-59(56)62)124-86(141)71(114-53(8)129)41-54-44-109-60-24-12-9-21-57(54)60/h9-14,21-26,44-46,48-52,63-73,76-77,109-111,127-128H,15-20,27-43,47,96H2,1-8H3,(H,112,134)(H,113,140)(H,114,129)(H,115,130)(H,116,138)(H,117,135)(H,118,142)(H,119,144)(H,120,145)(H,121,133)(H,122,136)(H,123,137)(H,124,141)(H,125,139)(H,126,143)(H,131,132)(H,146,147)(H4,97,98,105)(H4,99,100,106)(H4,101,102,107)(H4,103,104,108)/t50-,51+,52+,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,76-,77-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389001
(CHEMBL2064016)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:106.122,88.90,121.128,66.75,41.48,29.33,12.16,4.4,132.137,wD:92.106,77.86,52.64,20.25,127.133,(46.53,-33.52,;46.54,-35.05,;47.87,-35.82,;45.2,-35.82,;45.2,-37.36,;43.87,-38.13,;42.54,-37.36,;42.54,-35.82,;41.21,-38.14,;39.87,-37.37,;38.53,-38.13,;38.54,-39.67,;37.2,-37.37,;37.2,-35.83,;38.54,-35.05,;38.54,-33.51,;39.87,-35.83,;35.87,-38.13,;34.54,-37.36,;34.53,-35.83,;33.2,-38.13,;33.2,-39.67,;34.53,-40.44,;34.54,-41.98,;35.87,-42.75,;35.87,-44.29,;31.87,-37.36,;30.54,-38.13,;30.54,-39.67,;29.2,-37.37,;29.2,-35.84,;30.53,-35.05,;31.86,-35.83,;30.53,-33.53,;27.87,-38.13,;26.53,-37.36,;26.53,-35.82,;25.2,-38.14,;23.87,-37.36,;22.54,-38.14,;22.54,-39.68,;21.2,-37.37,;21.2,-35.83,;22.53,-35.05,;22.53,-33.52,;23.86,-32.75,;23.86,-31.21,;22.53,-30.44,;25.2,-30.43,;19.87,-38.14,;18.54,-37.37,;18.54,-35.83,;17.2,-38.14,;17.2,-39.68,;18.54,-40.45,;19.94,-39.82,;20.97,-40.96,;20.21,-42.3,;20.68,-43.76,;19.65,-44.91,;18.15,-44.59,;17.67,-43.12,;18.7,-41.98,;15.87,-37.37,;14.54,-38.14,;14.54,-39.68,;13.21,-37.37,;13.21,-35.83,;14.53,-35.06,;14.53,-33.52,;15.87,-32.75,;15.86,-31.21,;14.53,-30.44,;17.2,-30.44,;11.87,-38.14,;10.53,-37.37,;10.53,-35.83,;9.2,-38.15,;9.2,-39.68,;10.54,-40.45,;10.54,-41.99,;11.87,-42.76,;11.87,-44.3,;10.54,-45.07,;13.2,-45.07,;7.87,-37.37,;6.53,-38.14,;6.53,-39.68,;5.2,-37.37,;3.87,-38.14,;2.54,-37.37,;2.54,-35.83,;1.2,-38.15,;1.21,-39.69,;2.54,-40.45,;3.94,-39.83,;4.97,-40.97,;4.2,-42.3,;4.68,-43.77,;3.65,-44.91,;2.15,-44.6,;1.67,-43.13,;2.69,-41.98,;-.13,-37.37,;-1.47,-38.15,;-1.47,-39.69,;-2.8,-37.38,;-2.8,-35.84,;-1.47,-35.06,;-.06,-35.7,;.97,-34.55,;.2,-33.21,;.67,-31.75,;-.36,-30.61,;-1.86,-30.93,;-2.34,-32.39,;-1.31,-33.54,;-4.13,-38.14,;-5.46,-37.38,;-6.78,-38.15,;-5.46,-35.84,;5.2,-35.84,;3.87,-35.06,;6.54,-35.06,;46.53,-38.13,;46.53,-39.67,;47.87,-37.36,;49.21,-38.13,;49.2,-39.67,;50.54,-37.35,;50.53,-35.83,;51.87,-38.13,;53.21,-37.35,;53.21,-35.82,;54.53,-35.05,;54.53,-33.51,;55.87,-32.74,;55.87,-31.19,;54.53,-30.43,;57.2,-30.43,;54.54,-38.14,;55.87,-37.36,;54.53,-39.66,)| Show InChI InChI=1S/C92H139N31O20/c1-47(2)36-66(78(132)110-46-72(126)113-67(37-48(3)4)82(136)111-49(5)76(130)119-65(88(142)143)30-19-35-105-92(100)101)120-80(134)62(26-14-15-31-93)117-85(139)71(41-74(128)129)114-73(127)45-109-77(131)61(27-16-32-102-89(94)95)115-84(138)69(39-53-43-107-59-24-12-9-21-56(53)59)121-81(135)63(28-17-33-103-90(96)97)116-79(133)64(29-18-34-104-91(98)99)118-87(141)75(50(6)124)123-86(140)70(40-54-44-108-60-25-13-10-22-57(54)60)122-83(137)68(112-51(7)125)38-52-42-106-58-23-11-8-20-55(52)58/h8-13,20-25,42-44,47-50,61-71,75,106-108,124H,14-19,26-41,45-46,93H2,1-7H3,(H,109,131)(H,110,132)(H,111,136)(H,112,125)(H,113,126)(H,114,127)(H,115,138)(H,116,133)(H,117,139)(H,118,141)(H,119,130)(H,120,134)(H,121,135)(H,122,137)(H,123,140)(H,128,129)(H,142,143)(H4,94,95,102)(H4,96,97,103)(H4,98,99,104)(H4,100,101,105)/t49-,50+,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,75-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388998
(CHEMBL2064012)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:99.114,63.69,40.47,29.31,12.16,4.4,122.126,wD:85.98,72.78,51.60,35.35,20.25,117.122,(47,-28.76,;47,-30.3,;48.34,-31.08,;45.67,-31.07,;45.66,-32.61,;44.33,-33.38,;43.01,-32.61,;43,-31.07,;41.67,-33.37,;40.33,-32.61,;39,-33.38,;39,-34.91,;37.66,-32.6,;37.67,-31.07,;39,-30.29,;39.01,-28.75,;40.34,-31.07,;36.34,-33.37,;35,-32.6,;35,-31.06,;33.66,-33.36,;33.66,-34.91,;34.99,-35.68,;34.99,-37.22,;36.33,-37.99,;36.32,-39.53,;32.34,-32.6,;31,-33.37,;31,-34.91,;29.67,-32.6,;29.67,-31.06,;31.01,-30.28,;28.33,-33.37,;27,-32.59,;26.99,-31.06,;25.66,-33.36,;25.66,-34.9,;24.33,-32.6,;22.99,-33.36,;22.99,-34.9,;21.67,-32.59,;21.67,-31.06,;23,-30.28,;23.01,-28.74,;24.33,-27.97,;24.33,-26.44,;23.01,-25.66,;25.67,-25.66,;20.33,-33.36,;19,-32.58,;18.99,-31.05,;17.66,-33.35,;17.66,-34.89,;19,-35.67,;20.33,-34.89,;21.66,-35.67,;21.67,-37.21,;22.99,-37.98,;20.33,-37.97,;18.99,-37.21,;16.33,-32.58,;14.99,-33.35,;14.99,-34.9,;13.66,-32.59,;13.66,-31.04,;15,-30.27,;15,-28.74,;16.33,-27.97,;16.33,-26.42,;12.33,-33.35,;10.99,-32.58,;10.99,-31.04,;9.66,-33.35,;9.66,-34.88,;11,-35.66,;10.99,-37.2,;12.33,-37.97,;12.33,-39.51,;8.33,-32.58,;6.99,-33.34,;6.99,-34.88,;5.66,-32.58,;4.32,-33.34,;2.99,-32.57,;2.99,-31.03,;1.67,-33.34,;1.66,-34.88,;2.99,-35.65,;4.39,-35.03,;5.43,-36.17,;4.66,-37.5,;5.13,-38.97,;4.1,-40.12,;2.59,-39.8,;2.12,-38.33,;3.16,-37.19,;.33,-32.57,;-1.01,-33.34,;-1.01,-34.87,;-2.34,-32.57,;-2.34,-31.03,;-1.01,-30.26,;.4,-30.89,;1.43,-29.75,;.66,-28.41,;1.14,-26.94,;.11,-25.8,;-1.39,-26.12,;-1.88,-27.58,;-.84,-28.73,;-3.68,-33.33,;-5.01,-32.57,;-6.33,-33.34,;-5.01,-31.04,;47,-33.38,;46.99,-34.92,;48.33,-32.61,;49.67,-33.38,;49.67,-34.92,;51,-32.62,;51.01,-31.07,;52.34,-33.38,;53.67,-32.62,;53.67,-31.08,;55,-30.31,;55,-28.77,;56.34,-28,;56.34,-26.46,;55.01,-25.69,;57.67,-25.69,;55,-33.39,;56.33,-32.62,;55,-34.92,)| Show InChI InChI=1S/C88H136N26O19/c1-48(2)38-66(76(122)100-46-73(119)106-67(39-49(3)4)82(128)103-50(5)74(120)110-65(86(132)133)28-19-37-97-88(94)95)111-80(126)63(26-14-17-35-91)109-85(131)71(47-115)114-75(121)51(6)102-78(124)64(27-18-36-96-87(92)93)108-83(129)68(40-53-29-31-56(117)32-30-53)112-81(127)62(25-13-16-34-90)107-79(125)61(24-12-15-33-89)105-72(118)45-101-77(123)69(41-54-43-98-59-22-10-8-20-57(54)59)113-84(130)70(104-52(7)116)42-55-44-99-60-23-11-9-21-58(55)60/h8-11,20-23,29-32,43-44,48-51,61-71,98-99,115,117H,12-19,24-28,33-42,45-47,89-91H2,1-7H3,(H,100,122)(H,101,123)(H,102,124)(H,103,128)(H,104,116)(H,105,118)(H,106,119)(H,107,125)(H,108,129)(H,109,131)(H,110,120)(H,111,126)(H,112,127)(H,113,130)(H,114,121)(H,132,133)(H4,92,93,96)(H4,94,95,97)/t50-,51-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389004
(CHEMBL2064021)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:105.109,87.89,117.124,65.74,40.47,29.31,12.16,4.4,128.133,wD:91.105,76.85,51.63,35.35,20.25,123.129,(43.63,-17.18,;43.63,-18.73,;44.96,-19.49,;42.29,-19.5,;42.29,-21.03,;40.96,-21.8,;39.62,-21.03,;39.62,-19.49,;38.29,-21.8,;36.96,-21.02,;35.62,-21.8,;35.62,-23.34,;34.28,-21.02,;34.29,-19.48,;35.63,-18.71,;35.63,-17.18,;36.96,-19.49,;32.96,-21.8,;31.62,-21.02,;31.62,-19.48,;30.28,-21.79,;30.29,-23.32,;31.61,-24.1,;31.61,-25.64,;32.95,-26.41,;32.94,-27.95,;28.95,-21.02,;27.62,-21.79,;27.62,-23.33,;26.29,-21.01,;26.29,-19.48,;27.62,-18.71,;24.96,-21.78,;23.62,-21.01,;23.63,-19.47,;22.28,-21.79,;22.29,-23.32,;20.95,-21.02,;19.62,-21.79,;19.62,-23.33,;18.29,-21.03,;18.28,-19.48,;19.62,-18.71,;19.61,-17.17,;20.94,-16.4,;20.94,-14.86,;19.61,-14.09,;22.27,-14.08,;16.96,-21.8,;15.62,-21.03,;15.62,-19.49,;14.29,-21.8,;14.29,-23.34,;15.63,-24.11,;17.04,-23.48,;18.07,-24.62,;17.29,-25.96,;17.78,-27.42,;16.75,-28.57,;15.24,-28.25,;14.76,-26.79,;15.79,-25.64,;12.95,-21.04,;11.62,-21.81,;11.62,-23.35,;10.29,-21.04,;10.28,-19.51,;11.61,-18.73,;11.61,-17.19,;12.94,-16.41,;12.94,-14.88,;11.61,-14.11,;14.27,-14.1,;8.95,-21.82,;7.62,-21.05,;7.62,-19.51,;6.29,-21.83,;6.29,-23.36,;7.62,-24.13,;7.63,-25.67,;8.96,-26.43,;8.97,-27.97,;7.64,-28.75,;10.3,-28.74,;4.95,-21.05,;3.62,-21.83,;3.62,-23.37,;2.28,-21.06,;.95,-21.84,;-.38,-21.07,;-.39,-19.53,;-1.71,-21.84,;-1.71,-23.38,;-.38,-24.15,;1.03,-23.52,;2.06,-24.66,;1.3,-26,;1.77,-27.45,;.75,-28.6,;-.76,-28.29,;-1.24,-26.82,;-.21,-25.68,;-3.05,-21.07,;-4.38,-21.84,;-4.38,-23.39,;-5.72,-21.08,;-7.05,-21.85,;-5.72,-19.54,;-4.39,-18.77,;-2.98,-19.39,;-1.95,-18.24,;-2.73,-16.91,;-2.25,-15.45,;-3.29,-14.3,;-4.79,-14.63,;-5.27,-16.09,;-4.23,-17.23,;2.28,-19.52,;.95,-18.76,;3.61,-18.75,;43.62,-21.81,;43.62,-23.34,;44.96,-21.04,;46.29,-21.81,;46.29,-23.35,;47.62,-21.04,;47.62,-19.5,;48.95,-21.81,;50.29,-21.04,;50.3,-19.5,;51.62,-18.73,;51.62,-17.2,;52.96,-16.42,;52.96,-14.88,;51.63,-14.1,;54.3,-14.11,;51.63,-21.81,;52.95,-21.04,;51.62,-23.36,)| Show InChI InChI=1S/C90H139N31O18/c1-46(2)36-66(76(128)108-44-71(124)111-67(37-47(3)4)81(133)110-48(5)73(125)116-65(86(138)139)30-19-35-104-90(99)100)118-79(131)61(26-14-15-31-91)114-84(136)70(45-122)120-74(126)49(6)109-77(129)62(27-16-32-101-87(93)94)113-82(134)68(39-52-42-106-59-24-12-9-21-55(52)59)119-80(132)63(28-17-33-102-88(95)96)112-78(130)64(29-18-34-103-89(97)98)115-85(137)72(50(7)123)121-83(135)69(40-53-43-107-60-25-13-10-22-56(53)60)117-75(127)57(92)38-51-41-105-58-23-11-8-20-54(51)58/h8-13,20-25,41-43,46-50,57,61-70,72,105-107,122-123H,14-19,26-40,44-45,91-92H2,1-7H3,(H,108,128)(H,109,129)(H,110,133)(H,111,124)(H,112,130)(H,113,134)(H,114,136)(H,115,137)(H,116,125)(H,117,127)(H,118,131)(H,119,132)(H,120,126)(H,121,135)(H,138,139)(H4,93,94,101)(H4,95,96,102)(H4,97,98,103)(H4,99,100,104)/t48-,49-,50+,57-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389003
(CHEMBL2064018)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:109.125,87.93,65.74,40.47,29.31,12.16,4.4,132.137,wD:95.109,76.85,51.63,35.35,20.25,127.133,(46.4,-15.18,;46.39,-16.72,;47.73,-17.49,;45.05,-17.49,;45.05,-19.03,;43.72,-19.8,;42.38,-19.02,;42.39,-17.48,;41.05,-19.8,;39.72,-19.02,;38.38,-19.78,;38.38,-21.32,;37.05,-19.01,;37.05,-17.48,;38.39,-16.7,;38.39,-15.16,;39.72,-17.48,;35.71,-19.78,;34.38,-19.01,;34.38,-17.47,;33.04,-19.77,;33.04,-21.31,;34.37,-22.09,;34.37,-23.63,;35.7,-24.4,;35.7,-25.94,;31.71,-19,;30.38,-19.77,;30.37,-21.31,;29.04,-18.99,;29.05,-17.46,;30.38,-16.69,;27.7,-19.77,;26.37,-18.99,;26.38,-17.45,;25.04,-19.76,;25.03,-21.3,;23.71,-18.99,;22.37,-19.75,;22.37,-21.29,;21.04,-18.98,;21.04,-17.44,;22.38,-16.67,;22.38,-15.13,;23.71,-14.37,;23.72,-12.82,;22.38,-12.05,;25.06,-12.05,;19.71,-19.75,;18.38,-18.97,;18.38,-17.43,;17.03,-19.74,;17.03,-21.28,;18.36,-22.05,;19.77,-21.43,;20.8,-22.58,;20.03,-23.91,;20.5,-25.38,;19.47,-26.52,;17.96,-26.19,;17.49,-24.73,;18.52,-23.59,;15.7,-18.97,;14.37,-19.73,;14.37,-21.28,;13.04,-18.96,;13.04,-17.43,;14.37,-16.65,;14.38,-15.11,;15.71,-14.35,;15.71,-12.81,;14.38,-12.03,;17.05,-12.04,;11.7,-19.73,;10.36,-18.96,;10.37,-17.41,;9.03,-19.72,;9.03,-21.26,;10.36,-22.04,;10.36,-23.58,;11.69,-24.35,;11.69,-25.89,;10.35,-26.66,;13.02,-26.66,;7.7,-18.95,;6.36,-19.72,;6.36,-21.26,;5.03,-18.94,;5.03,-17.4,;6.37,-16.64,;7.7,-17.42,;6.37,-15.1,;3.7,-19.72,;2.36,-18.94,;2.37,-17.4,;1.04,-19.7,;1.03,-21.24,;2.37,-22.02,;3.77,-21.4,;4.79,-22.55,;4.02,-23.88,;4.49,-25.34,;3.46,-26.48,;1.97,-26.16,;1.47,-24.69,;2.52,-23.56,;-.3,-18.93,;-1.64,-19.7,;-1.64,-21.24,;-2.97,-18.93,;-2.96,-17.38,;-1.64,-16.62,;-.24,-17.25,;.8,-16.1,;.03,-14.77,;.52,-13.3,;-.51,-12.16,;-2.02,-12.48,;-2.5,-13.94,;-1.48,-15.08,;-4.3,-19.7,;-5.63,-18.93,;-6.96,-19.71,;-5.63,-17.4,;46.38,-19.8,;46.38,-21.34,;47.72,-19.04,;49.06,-19.81,;49.05,-21.35,;50.39,-19.04,;50.39,-17.5,;51.72,-19.81,;53.06,-19.05,;53.06,-17.51,;54.4,-16.74,;54.4,-15.2,;55.73,-14.43,;55.74,-12.89,;54.4,-12.12,;57.08,-12.13,;54.39,-19.82,;55.73,-19.05,;54.38,-21.36,)| Show InChI InChI=1S/C92H139N31O20/c1-47(2)36-66(77(131)109-45-73(126)113-67(37-48(3)4)82(136)111-49(5)75(129)118-65(88(142)143)30-19-35-105-92(100)101)119-80(134)61(26-14-15-31-93)117-87(141)72(46-124)123-76(130)50(6)110-78(132)62(27-16-32-102-89(94)95)115-84(138)69(39-53-43-107-59-24-12-9-21-56(53)59)120-81(135)64(29-18-34-104-91(98)99)114-79(133)63(28-17-33-103-90(96)97)116-86(140)71(41-74(127)128)122-85(139)70(40-54-44-108-60-25-13-10-22-57(54)60)121-83(137)68(112-51(7)125)38-52-42-106-58-23-11-8-20-55(52)58/h8-13,20-25,42-44,47-50,61-72,106-108,124H,14-19,26-41,45-46,93H2,1-7H3,(H,109,131)(H,110,132)(H,111,136)(H,112,125)(H,113,126)(H,114,133)(H,115,138)(H,116,140)(H,117,141)(H,118,129)(H,119,134)(H,120,135)(H,121,137)(H,122,139)(H,123,130)(H,127,128)(H,142,143)(H4,94,95,102)(H4,96,97,103)(H4,98,99,104)(H4,100,101,105)/t49-,50-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388997
(CHEMBL2064011)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:103.118,85.86,118.124,63.71,40.47,29.31,12.16,4.4,129.133,wD:89.102,74.82,51.60,35.35,20.25,124.129,(47.73,-15.06,;47.74,-16.6,;49.09,-17.36,;46.42,-17.39,;46.43,-18.92,;45.11,-19.7,;43.77,-18.95,;43.75,-17.4,;42.44,-19.73,;41.1,-18.96,;39.78,-19.75,;39.78,-21.29,;38.43,-18.99,;38.43,-17.45,;39.75,-16.67,;39.74,-15.13,;41.09,-17.43,;37.11,-19.78,;35.77,-19.01,;35.75,-17.48,;34.44,-19.79,;34.46,-21.33,;35.79,-22.09,;35.81,-23.64,;37.15,-24.39,;37.16,-25.93,;33.1,-19.04,;31.78,-19.82,;31.79,-21.36,;30.44,-19.06,;30.43,-17.52,;31.75,-16.74,;29.11,-19.84,;27.77,-19.08,;27.75,-17.54,;26.44,-19.87,;26.46,-21.4,;25.1,-19.1,;23.78,-19.89,;23.79,-21.43,;22.44,-19.13,;22.43,-17.59,;23.75,-16.81,;23.74,-15.27,;25.07,-14.49,;25.05,-12.95,;23.72,-12.19,;26.38,-12.16,;21.11,-19.91,;19.77,-19.15,;19.75,-17.61,;18.44,-19.93,;18.46,-21.47,;19.8,-22.23,;21.12,-21.45,;22.46,-22.2,;22.48,-23.75,;23.82,-24.5,;21.15,-24.53,;19.81,-23.76,;17.1,-19.17,;15.78,-19.95,;15.79,-21.49,;14.44,-19.19,;14.43,-17.66,;15.75,-16.88,;15.74,-15.34,;17.07,-14.55,;17.05,-13.02,;15.72,-12.26,;18.38,-12.23,;13.11,-19.98,;11.77,-19.22,;11.75,-17.68,;10.44,-20,;10.46,-21.54,;11.8,-22.3,;11.81,-23.84,;13.15,-24.59,;13.17,-26.13,;11.84,-26.92,;14.5,-26.89,;9.1,-19.24,;7.78,-20.02,;7.79,-21.57,;6.44,-19.27,;5.11,-20.05,;3.78,-19.29,;3.76,-17.75,;2.45,-20.07,;2.46,-21.6,;3.8,-22.37,;5.2,-21.73,;6.24,-22.86,;5.48,-24.2,;5.97,-25.66,;4.95,-26.81,;3.44,-26.51,;2.95,-25.05,;3.98,-23.9,;1.1,-19.31,;-.22,-20.09,;-.21,-21.63,;-1.56,-19.33,;-1.57,-17.8,;-.25,-17.01,;1.16,-17.63,;2.19,-16.47,;1.41,-15.15,;1.86,-13.68,;.83,-12.54,;-.67,-12.88,;-1.14,-14.34,;-.1,-15.48,;-2.89,-20.11,;-4.22,-19.35,;-5.55,-20.12,;-4.23,-17.81,;6.43,-17.72,;5.08,-16.97,;7.75,-16.94,;47.77,-19.68,;47.78,-21.22,;49.1,-18.9,;50.44,-19.66,;50.45,-21.2,;51.77,-18.87,;51.75,-17.34,;53.1,-19.64,;54.43,-18.86,;54.42,-17.31,;55.74,-16.53,;55.73,-15,;57.06,-14.21,;57.04,-12.67,;55.71,-11.92,;58.37,-11.9,;55.77,-19.61,;57.1,-18.83,;55.78,-21.15,)| Show InChI InChI=1S/C90H140N30O20/c1-46(2)37-65(75(128)106-44-71(125)110-66(38-47(3)4)80(133)108-48(5)73(126)115-64(86(139)140)27-18-36-103-90(98)99)116-78(131)60(23-13-14-32-91)113-84(137)70(45-121)119-74(127)49(6)107-76(129)61(24-15-33-100-87(92)93)112-81(134)67(39-52-28-30-55(124)31-29-52)117-79(132)62(25-16-34-101-88(94)95)111-77(130)63(26-17-35-102-89(96)97)114-85(138)72(50(7)122)120-83(136)69(41-54-43-105-59-22-12-10-20-57(54)59)118-82(135)68(109-51(8)123)40-53-42-104-58-21-11-9-19-56(53)58/h9-12,19-22,28-31,42-43,46-50,60-70,72,104-105,121-122,124H,13-18,23-27,32-41,44-45,91H2,1-8H3,(H,106,128)(H,107,129)(H,108,133)(H,109,123)(H,110,125)(H,111,130)(H,112,134)(H,113,137)(H,114,138)(H,115,126)(H,116,131)(H,117,132)(H,118,135)(H,119,127)(H,120,136)(H,139,140)(H4,92,93,100)(H4,94,95,101)(H4,96,97,102)(H4,98,99,103)/t48-,49-,50+,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388995
(CHEMBL2064019)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:110.126,87.94,65.74,40.47,29.31,12.16,4.4,133.138,wD:96.110,76.85,51.63,35.35,20.25,128.134,(43.19,-33.09,;43.2,-34.64,;44.54,-35.39,;41.87,-35.41,;41.88,-36.95,;40.55,-37.72,;39.21,-36.96,;39.2,-35.43,;37.89,-37.74,;36.55,-36.97,;35.21,-37.75,;35.22,-39.29,;33.88,-36.99,;33.87,-35.45,;35.2,-34.67,;35.19,-33.14,;36.54,-35.43,;32.55,-37.77,;31.21,-37,;31.2,-35.46,;29.88,-37.78,;29.89,-39.32,;31.22,-40.08,;31.23,-41.62,;32.57,-42.39,;32.58,-43.93,;28.54,-37.02,;27.21,-37.79,;27.22,-39.33,;25.87,-37.03,;25.87,-35.49,;27.19,-34.71,;24.54,-37.81,;23.2,-37.04,;23.2,-35.51,;21.88,-37.82,;21.88,-39.36,;20.54,-37.06,;19.21,-37.84,;19.22,-39.38,;17.87,-37.08,;17.86,-35.54,;19.19,-34.75,;19.18,-33.22,;20.51,-32.44,;20.5,-30.9,;19.16,-30.14,;21.83,-30.13,;16.55,-37.85,;15.21,-37.09,;15.2,-35.55,;13.87,-37.87,;13.88,-39.41,;15.22,-40.17,;16.62,-39.54,;17.66,-40.67,;16.9,-42.01,;17.38,-43.47,;16.36,-44.63,;14.85,-44.31,;14.36,-42.85,;15.39,-41.7,;12.54,-37.1,;11.21,-37.88,;11.22,-39.42,;9.87,-37.12,;9.86,-35.58,;11.19,-34.8,;11.18,-33.26,;12.51,-32.48,;12.5,-30.94,;11.16,-30.18,;13.84,-30.16,;8.54,-37.89,;7.2,-37.13,;7.19,-35.59,;5.87,-37.91,;5.88,-39.45,;7.22,-40.21,;7.23,-41.75,;8.56,-42.52,;8.57,-44.06,;7.24,-44.83,;9.91,-44.82,;4.53,-37.15,;3.2,-37.92,;3.21,-39.46,;1.86,-37.16,;1.85,-35.62,;3.19,-34.84,;3.18,-33.31,;1.84,-32.54,;4.51,-32.52,;.54,-37.94,;-.8,-37.17,;-.81,-35.63,;-2.13,-37.96,;-2.12,-39.5,;-.78,-40.25,;.62,-39.62,;1.65,-40.76,;.89,-42.1,;1.37,-43.56,;.35,-44.71,;-1.15,-44.4,;-1.64,-42.94,;-.62,-41.79,;-3.46,-37.19,;-4.79,-37.97,;-4.79,-39.51,;-6.14,-37.2,;-6.15,-35.67,;-4.82,-34.88,;-3.41,-35.5,;-2.38,-34.36,;-3.16,-33.02,;-2.69,-31.55,;-3.73,-30.42,;-5.23,-30.75,;-5.7,-32.21,;-4.67,-33.35,;-7.47,-37.98,;-8.8,-37.22,;-10.12,-37.99,;-8.8,-35.68,;43.22,-37.71,;43.22,-39.24,;44.55,-36.94,;45.89,-37.7,;45.89,-39.24,;47.21,-36.92,;47.2,-35.38,;48.55,-37.68,;49.88,-36.91,;49.88,-35.36,;51.2,-34.59,;51.19,-33.05,;52.52,-32.26,;52.51,-30.74,;51.18,-29.97,;53.85,-29.95,;51.22,-37.67,;52.55,-36.89,;51.23,-39.21,)| Show InChI InChI=1S/C93H141N31O20/c1-48(2)38-68(78(132)110-46-74(127)114-69(39-49(3)4)84(138)112-50(5)76(130)120-67(89(143)144)30-19-37-106-93(101)102)121-81(135)62(26-14-15-33-94)118-88(142)73(47-125)124-77(131)51(6)111-79(133)63(27-16-34-103-90(95)96)117-86(140)71(41-54-44-108-60-24-12-9-21-57(54)60)122-82(136)65(29-18-36-105-92(99)100)115-80(134)64(28-17-35-104-91(97)98)116-83(137)66(31-32-75(128)129)119-87(141)72(42-55-45-109-61-25-13-10-22-58(55)61)123-85(139)70(113-52(7)126)40-53-43-107-59-23-11-8-20-56(53)59/h8-13,20-25,43-45,48-51,62-73,107-109,125H,14-19,26-42,46-47,94H2,1-7H3,(H,110,132)(H,111,133)(H,112,138)(H,113,126)(H,114,127)(H,115,134)(H,116,137)(H,117,140)(H,118,142)(H,119,141)(H,120,130)(H,121,135)(H,122,136)(H,123,139)(H,124,131)(H,128,129)(H,143,144)(H4,95,96,103)(H4,97,98,104)(H4,99,100,105)(H4,101,102,106)/t50-,51-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388996
(CHEMBL2063898)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:105.121,87.89,120.127,65.74,40.47,29.31,12.16,4.4,131.136,wD:91.105,76.85,51.63,35.35,20.25,126.132,(45.71,2.34,;45.71,.8,;47.04,.03,;44.38,.03,;44.37,-1.5,;43.04,-2.28,;41.7,-1.5,;41.71,.04,;40.37,-2.27,;39.04,-1.5,;37.71,-2.27,;37.7,-3.81,;36.37,-1.5,;36.38,.04,;37.71,.81,;37.71,2.35,;39.04,.04,;35.04,-2.27,;33.7,-1.49,;33.7,.04,;32.37,-2.26,;32.37,-3.8,;33.7,-4.57,;33.7,-6.12,;35.03,-6.89,;35.03,-8.43,;31.04,-1.49,;29.7,-2.26,;29.7,-3.8,;28.37,-1.49,;28.37,.05,;29.71,.82,;27.04,-2.26,;25.7,-1.49,;25.71,.06,;24.37,-2.26,;24.37,-3.8,;23.04,-1.49,;21.71,-2.26,;21.71,-3.81,;20.37,-1.5,;20.37,.04,;21.7,.82,;21.7,2.35,;23.03,3.13,;23.02,4.67,;21.69,5.43,;24.36,5.45,;19.04,-2.27,;17.71,-1.5,;17.7,.03,;16.38,-2.28,;16.37,-3.81,;17.71,-4.58,;19.12,-3.95,;20.15,-5.09,;19.38,-6.43,;19.86,-7.89,;18.84,-9.04,;17.32,-8.72,;16.85,-7.26,;17.87,-6.11,;15.03,-1.51,;13.7,-2.28,;13.71,-3.82,;12.37,-1.51,;12.37,.02,;13.7,.8,;13.69,2.34,;15.03,3.11,;15.02,4.65,;13.69,5.42,;16.35,5.42,;11.04,-2.29,;9.7,-1.52,;9.7,.02,;8.37,-2.3,;8.37,-3.83,;9.71,-4.6,;9.72,-6.14,;11.05,-6.9,;11.05,-8.45,;9.72,-9.22,;12.38,-9.21,;7.03,-1.53,;5.7,-2.3,;5.71,-3.84,;4.37,-1.54,;3.04,-2.31,;1.7,-1.54,;1.7,-0,;.37,-2.31,;.38,-3.85,;1.71,-4.62,;3.12,-3.99,;4.15,-5.13,;3.38,-6.47,;3.86,-7.93,;2.83,-9.07,;1.33,-8.76,;.84,-7.3,;1.88,-6.15,;-.97,-1.55,;-2.3,-2.32,;-2.3,-3.86,;-3.63,-1.55,;-3.64,-.02,;-2.31,.76,;-.9,.14,;.13,1.29,;-.64,2.61,;-.17,4.08,;-1.2,5.22,;-2.71,4.9,;-3.18,3.44,;-2.15,2.3,;-4.97,-2.33,;-6.3,-1.56,;-7.63,-2.34,;-6.31,-.02,;4.36,.01,;3.03,.77,;5.7,.78,;45.71,-2.28,;45.71,-3.82,;47.04,-1.51,;48.37,-2.28,;48.37,-3.82,;49.71,-1.51,;49.71,.03,;51.04,-2.29,;52.37,-1.52,;52.38,.02,;53.71,.8,;53.71,2.33,;55.04,3.1,;55.05,4.64,;53.72,5.43,;56.38,5.42,;53.71,-2.29,;55.04,-1.51,;53.7,-3.83,)| Show InChI InChI=1S/C92H141N31O19/c1-47(2)37-67(77(130)109-45-73(127)113-68(38-48(3)4)82(135)111-49(5)75(128)118-66(88(141)142)31-20-36-105-92(100)101)119-80(133)62(27-15-16-32-93)116-86(139)72(46-124)122-76(129)50(6)110-78(131)63(28-17-33-102-89(94)95)115-84(137)70(40-54-43-107-60-25-13-10-22-57(54)60)120-81(134)64(29-18-34-103-90(96)97)114-79(132)65(30-19-35-104-91(98)99)117-87(140)74(51(7)125)123-85(138)71(41-55-44-108-61-26-14-11-23-58(55)61)121-83(136)69(112-52(8)126)39-53-42-106-59-24-12-9-21-56(53)59/h9-14,21-26,42-44,47-51,62-72,74,106-108,124-125H,15-20,27-41,45-46,93H2,1-8H3,(H,109,130)(H,110,131)(H,111,135)(H,112,126)(H,113,127)(H,114,132)(H,115,137)(H,116,139)(H,117,140)(H,118,128)(H,119,133)(H,120,134)(H,121,136)(H,122,129)(H,123,138)(H,141,142)(H4,94,95,102)(H4,96,97,103)(H4,98,99,104)(H4,100,101,105)/t49-,50-,51+,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389006
(CHEMBL2064020)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:107.123,87.91,65.74,40.47,29.31,12.16,4.4,130.135,wD:93.107,76.85,51.63,35.35,20.25,125.131,(42.57,1.47,;42.59,-.07,;43.93,-.82,;41.26,-.85,;41.27,-2.4,;39.95,-3.19,;38.61,-2.41,;38.59,-.89,;37.29,-3.2,;35.95,-2.45,;34.62,-3.23,;34.63,-4.77,;33.27,-2.48,;33.26,-.94,;34.59,-.15,;34.57,1.38,;35.93,-.9,;31.95,-3.26,;30.61,-2.5,;30.59,-.97,;29.28,-3.28,;29.3,-4.82,;30.64,-5.58,;30.66,-7.12,;32,-7.88,;32.01,-9.42,;27.94,-2.53,;26.62,-3.32,;26.63,-4.85,;25.28,-2.55,;25.26,-1.02,;26.58,-.24,;23.95,-3.34,;22.6,-2.58,;22.6,-1.05,;21.28,-3.37,;21.3,-4.91,;19.94,-2.61,;18.62,-3.41,;18.63,-4.94,;17.28,-2.64,;17.26,-1.11,;18.58,-.32,;18.57,1.21,;19.89,2,;19.87,3.54,;18.53,4.29,;21.2,4.32,;15.96,-3.43,;14.61,-2.68,;14.6,-1.14,;13.28,-3.46,;13.3,-5,;14.64,-5.75,;16.04,-5.11,;17.08,-6.24,;16.33,-7.58,;16.82,-9.04,;15.8,-10.2,;14.29,-9.9,;13.8,-8.44,;14.82,-7.28,;11.94,-2.7,;10.62,-3.49,;10.64,-5.03,;9.28,-2.73,;9.26,-1.19,;10.58,-.41,;10.57,1.13,;11.89,1.92,;11.87,3.45,;10.53,4.2,;13.2,4.23,;7.95,-3.51,;6.61,-2.76,;6.59,-1.22,;5.28,-3.54,;5.3,-5.08,;6.64,-5.84,;6.66,-7.38,;8,-8.13,;8.01,-9.67,;6.69,-10.45,;9.36,-10.43,;3.94,-2.79,;2.61,-3.57,;2.63,-5.11,;1.27,-2.82,;1.26,-1.27,;2.58,-.49,;-.05,-3.6,;-1.39,-2.84,;-1.41,-1.3,;-2.72,-3.63,;-2.7,-5.17,;-1.36,-5.92,;.04,-5.28,;1.08,-6.42,;.32,-7.76,;.82,-9.22,;-.2,-10.37,;-1.71,-10.07,;-2.2,-8.61,;-1.19,-7.45,;-4.06,-2.87,;-5.39,-3.66,;-5.37,-5.2,;-6.73,-2.9,;-6.74,-1.36,;-5.42,-.57,;-4.01,-1.18,;-2.99,-.03,;-3.77,1.3,;-3.31,2.77,;-4.36,3.9,;-5.85,3.56,;-6.32,2.09,;-5.27,.96,;-8.05,-3.68,;-9.38,-2.92,;-10.71,-3.69,;-9.39,-1.39,;42.61,-3.15,;42.63,-4.69,;43.94,-2.37,;45.29,-3.12,;45.3,-4.66,;46.61,-2.34,;46.59,-.8,;47.95,-3.09,;49.28,-2.31,;49.27,-.77,;50.59,.02,;50.57,1.55,;51.89,2.35,;51.88,3.88,;50.53,4.63,;53.21,4.66,;50.62,-3.07,;51.94,-2.28,;50.63,-4.61,)| Show InChI InChI=1S/C91H139N31O19/c1-47(2)36-66(76(129)108-44-73(126)112-67(37-48(3)4)81(134)110-49(5)74(127)117-65(87(140)141)30-19-35-104-91(99)100)118-79(132)61(26-14-15-31-92)115-85(138)71(45-123)121-75(128)50(6)109-77(130)62(27-16-32-101-88(93)94)114-83(136)69(39-53-42-106-59-24-12-9-21-56(53)59)119-80(133)64(29-18-34-103-90(97)98)113-78(131)63(28-17-33-102-89(95)96)116-86(139)72(46-124)122-84(137)70(40-54-43-107-60-25-13-10-22-57(54)60)120-82(135)68(111-51(7)125)38-52-41-105-58-23-11-8-20-55(52)58/h8-13,20-25,41-43,47-50,61-72,105-107,123-124H,14-19,26-40,44-46,92H2,1-7H3,(H,108,129)(H,109,130)(H,110,134)(H,111,125)(H,112,126)(H,113,131)(H,114,136)(H,115,138)(H,116,139)(H,117,127)(H,118,132)(H,119,133)(H,120,135)(H,121,128)(H,122,137)(H,140,141)(H4,93,94,101)(H4,95,96,102)(H4,97,98,103)(H4,99,100,104)/t49-,50-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant compound preincubat... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159043
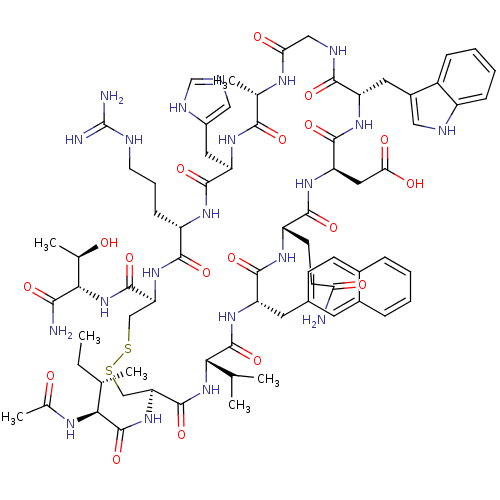
(Ac-I[CV(2Nal)QDWGAHRC]T-NH2 | CHEMBL427664)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C73H101N21O18S2/c1-8-36(4)59(84-39(7)96)72(112)92-54-33-114-113-32-53(70(110)94-60(38(6)95)61(75)101)91-64(104)47(18-13-23-79-73(76)77)85-67(107)51(27-44-30-78-34-82-44)87-62(102)37(5)83-56(98)31-81-63(103)50(26-43-29-80-46-17-12-11-16-45(43)46)88-68(108)52(28-57(99)100)89-65(105)48(21-22-55(74)97)86-66(106)49(90-71(111)58(35(2)3)93-69(54)109)25-40-19-20-41-14-9-10-15-42(41)24-40/h9-12,14-17,19-20,24,29-30,34-38,47-54,58-60,80,95H,8,13,18,21-23,25-28,31-33H2,1-7H3,(H2,74,97)(H2,75,101)(H,78,82)(H,81,103)(H,83,98)(H,84,96)(H,85,107)(H,86,106)(H,87,102)(H,88,108)(H,89,105)(H,90,111)(H,91,104)(H,92,112)(H,93,109)(H,94,110)(H,99,100)(H4,76,77,79)/t36-,37-,38+,47-,48-,49-,50-,51+,52+,53-,54-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159050
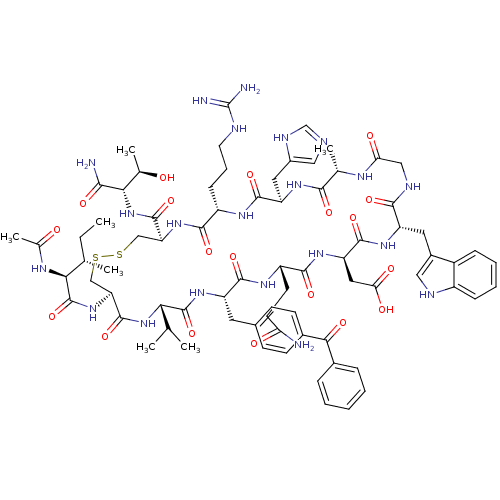
(Ac-I[CV(Bpa)QDWGAHRC]T-NH2 | CHEMBL430275)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C76H103N21O19S2/c1-8-38(4)61(87-41(7)99)75(116)95-56-35-118-117-34-55(73(114)97-62(40(6)98)64(78)105)94-67(108)49(19-14-26-82-76(79)80)88-70(111)53(29-46-32-81-36-85-46)90-65(106)39(5)86-58(101)33-84-66(107)52(28-45-31-83-48-18-13-12-17-47(45)48)91-71(112)54(30-59(102)103)92-68(109)50(24-25-57(77)100)89-69(110)51(93-74(115)60(37(2)3)96-72(56)113)27-42-20-22-44(23-21-42)63(104)43-15-10-9-11-16-43/h9-13,15-18,20-23,31-32,36-40,49-56,60-62,83,98H,8,14,19,24-30,33-35H2,1-7H3,(H2,77,100)(H2,78,105)(H,81,85)(H,84,107)(H,86,101)(H,87,99)(H,88,111)(H,89,110)(H,90,106)(H,91,112)(H,92,109)(H,93,115)(H,94,108)(H,95,116)(H,96,113)(H,97,114)(H,102,103)(H4,79,80,82)/t38-,39-,40+,49-,50-,51-,52-,53+,54+,55-,56-,60-,61-,62-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159049
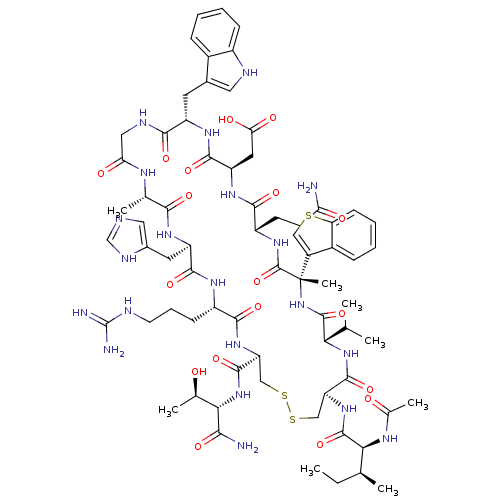
(Ac-I[CV(Bta)QDWGAHRC]T-NH2 | CHEMBL439706)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@](C)(NC(=O)[C@@H](NC1=O)C(C)C)c1csc2ccccc12)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C71H99N21O18S3/c1-9-34(4)56(82-37(7)94)67(108)88-50-31-113-112-30-49(66(107)91-57(36(6)93)58(73)99)87-61(102)44(18-14-22-77-70(74)75)83-63(104)47(24-39-27-76-32-80-39)84-59(100)35(5)81-53(96)28-79-60(101)46(23-38-26-78-43-17-12-10-15-40(38)43)85-64(105)48(25-54(97)98)86-62(103)45(20-21-52(72)95)89-69(110)71(8,42-29-111-51-19-13-11-16-41(42)51)92-68(109)55(33(2)3)90-65(50)106/h10-13,15-17,19,26-27,29,32-36,44-50,55-57,78,93H,9,14,18,20-25,28,30-31H2,1-8H3,(H2,72,95)(H2,73,99)(H,76,80)(H,79,101)(H,81,96)(H,82,94)(H,83,104)(H,84,100)(H,85,105)(H,86,103)(H,87,102)(H,88,108)(H,89,110)(H,90,106)(H,91,107)(H,92,109)(H,97,98)(H4,74,75,77)/t34-,35-,36+,44-,45-,46-,47+,48+,49-,50-,55-,56-,57-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159068
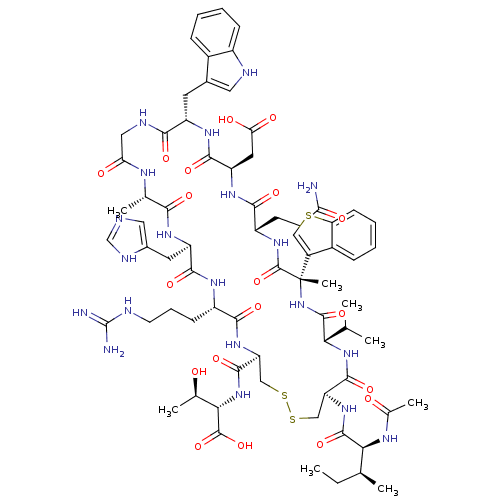
(Ac-I[CV(Bta)QDWGAHRC]T | CHEMBL408836)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@](C)(NC(=O)[C@@H](NC1=O)C(C)C)c1csc2ccccc12)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H98N20O19S3/c1-9-34(4)56(81-37(7)93)66(106)87-50-31-113-112-30-49(65(105)90-57(36(6)92)68(108)109)86-60(100)44(18-14-22-76-70(73)74)82-62(102)47(24-39-27-75-32-79-39)83-58(98)35(5)80-53(95)28-78-59(99)46(23-38-26-77-43-17-12-10-15-40(38)43)84-63(103)48(25-54(96)97)85-61(101)45(20-21-52(72)94)88-69(110)71(8,42-29-111-51-19-13-11-16-41(42)51)91-67(107)55(33(2)3)89-64(50)104/h10-13,15-17,19,26-27,29,32-36,44-50,55-57,77,92H,9,14,18,20-25,28,30-31H2,1-8H3,(H2,72,94)(H,75,79)(H,78,99)(H,80,95)(H,81,93)(H,82,102)(H,83,98)(H,84,103)(H,85,101)(H,86,100)(H,87,106)(H,88,110)(H,89,104)(H,90,105)(H,91,107)(H,96,97)(H,108,109)(H4,73,74,76)/t34-,35-,36+,44-,45-,46-,47+,48+,49-,50-,55-,56-,57-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159063
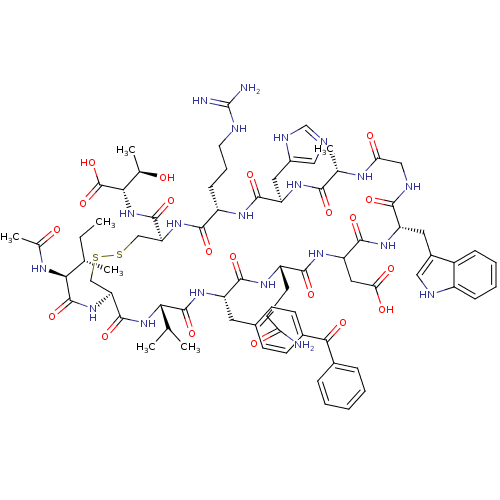
(Ac-I[CV(Bpa)QDWGAHRC]T | CHEMBL263817)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)C(CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C76H102N20O20S2/c1-8-38(4)61(86-41(7)98)74(114)94-56-35-118-117-34-55(72(112)96-62(40(6)97)75(115)116)93-66(106)49(19-14-26-81-76(78)79)87-69(109)53(29-46-32-80-36-84-46)89-64(104)39(5)85-58(100)33-83-65(105)52(28-45-31-82-48-18-13-12-17-47(45)48)90-70(110)54(30-59(101)102)91-67(107)50(24-25-57(77)99)88-68(108)51(92-73(113)60(37(2)3)95-71(56)111)27-42-20-22-44(23-21-42)63(103)43-15-10-9-11-16-43/h9-13,15-18,20-23,31-32,36-40,49-56,60-62,82,97H,8,14,19,24-30,33-35H2,1-7H3,(H2,77,99)(H,80,84)(H,83,105)(H,85,100)(H,86,98)(H,87,109)(H,88,108)(H,89,104)(H,90,110)(H,91,107)(H,92,113)(H,93,106)(H,94,114)(H,95,111)(H,96,112)(H,101,102)(H,115,116)(H4,78,79,81)/t38-,39-,40+,49-,50-,51-,52-,53+,54?,55-,56-,60-,61-,62-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388995

(CHEMBL2064019)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:110.126,87.94,65.74,40.47,29.31,12.16,4.4,133.138,wD:96.110,76.85,51.63,35.35,20.25,128.134,(43.19,-33.09,;43.2,-34.64,;44.54,-35.39,;41.87,-35.41,;41.88,-36.95,;40.55,-37.72,;39.21,-36.96,;39.2,-35.43,;37.89,-37.74,;36.55,-36.97,;35.21,-37.75,;35.22,-39.29,;33.88,-36.99,;33.87,-35.45,;35.2,-34.67,;35.19,-33.14,;36.54,-35.43,;32.55,-37.77,;31.21,-37,;31.2,-35.46,;29.88,-37.78,;29.89,-39.32,;31.22,-40.08,;31.23,-41.62,;32.57,-42.39,;32.58,-43.93,;28.54,-37.02,;27.21,-37.79,;27.22,-39.33,;25.87,-37.03,;25.87,-35.49,;27.19,-34.71,;24.54,-37.81,;23.2,-37.04,;23.2,-35.51,;21.88,-37.82,;21.88,-39.36,;20.54,-37.06,;19.21,-37.84,;19.22,-39.38,;17.87,-37.08,;17.86,-35.54,;19.19,-34.75,;19.18,-33.22,;20.51,-32.44,;20.5,-30.9,;19.16,-30.14,;21.83,-30.13,;16.55,-37.85,;15.21,-37.09,;15.2,-35.55,;13.87,-37.87,;13.88,-39.41,;15.22,-40.17,;16.62,-39.54,;17.66,-40.67,;16.9,-42.01,;17.38,-43.47,;16.36,-44.63,;14.85,-44.31,;14.36,-42.85,;15.39,-41.7,;12.54,-37.1,;11.21,-37.88,;11.22,-39.42,;9.87,-37.12,;9.86,-35.58,;11.19,-34.8,;11.18,-33.26,;12.51,-32.48,;12.5,-30.94,;11.16,-30.18,;13.84,-30.16,;8.54,-37.89,;7.2,-37.13,;7.19,-35.59,;5.87,-37.91,;5.88,-39.45,;7.22,-40.21,;7.23,-41.75,;8.56,-42.52,;8.57,-44.06,;7.24,-44.83,;9.91,-44.82,;4.53,-37.15,;3.2,-37.92,;3.21,-39.46,;1.86,-37.16,;1.85,-35.62,;3.19,-34.84,;3.18,-33.31,;1.84,-32.54,;4.51,-32.52,;.54,-37.94,;-.8,-37.17,;-.81,-35.63,;-2.13,-37.96,;-2.12,-39.5,;-.78,-40.25,;.62,-39.62,;1.65,-40.76,;.89,-42.1,;1.37,-43.56,;.35,-44.71,;-1.15,-44.4,;-1.64,-42.94,;-.62,-41.79,;-3.46,-37.19,;-4.79,-37.97,;-4.79,-39.51,;-6.14,-37.2,;-6.15,-35.67,;-4.82,-34.88,;-3.41,-35.5,;-2.38,-34.36,;-3.16,-33.02,;-2.69,-31.55,;-3.73,-30.42,;-5.23,-30.75,;-5.7,-32.21,;-4.67,-33.35,;-7.47,-37.98,;-8.8,-37.22,;-10.12,-37.99,;-8.8,-35.68,;43.22,-37.71,;43.22,-39.24,;44.55,-36.94,;45.89,-37.7,;45.89,-39.24,;47.21,-36.92,;47.2,-35.38,;48.55,-37.68,;49.88,-36.91,;49.88,-35.36,;51.2,-34.59,;51.19,-33.05,;52.52,-32.26,;52.51,-30.74,;51.18,-29.97,;53.85,-29.95,;51.22,-37.67,;52.55,-36.89,;51.23,-39.21,)| Show InChI InChI=1S/C93H141N31O20/c1-48(2)38-68(78(132)110-46-74(127)114-69(39-49(3)4)84(138)112-50(5)76(130)120-67(89(143)144)30-19-37-106-93(101)102)121-81(135)62(26-14-15-33-94)118-88(142)73(47-125)124-77(131)51(6)111-79(133)63(27-16-34-103-90(95)96)117-86(140)71(41-54-44-108-60-24-12-9-21-57(54)60)122-82(136)65(29-18-36-105-92(99)100)115-80(134)64(28-17-35-104-91(97)98)116-83(137)66(31-32-75(128)129)119-87(141)72(42-55-45-109-61-25-13-10-22-58(55)61)123-85(139)70(113-52(7)126)40-53-43-107-59-23-11-8-20-56(53)59/h8-13,20-25,43-45,48-51,62-73,107-109,125H,14-19,26-42,46-47,94H2,1-7H3,(H,110,132)(H,111,133)(H,112,138)(H,113,126)(H,114,127)(H,115,134)(H,116,137)(H,117,140)(H,118,142)(H,119,141)(H,120,130)(H,121,135)(H,122,136)(H,123,139)(H,124,131)(H,128,129)(H,143,144)(H4,95,96,103)(H4,97,98,104)(H4,99,100,105)(H4,101,102,106)/t50-,51-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in dbcAMP differentiated human U937 cells assessed as inhibition of increase in intracellular calcium compound pr... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159057

(Ac-I[CV(2Igl)QDWGAHRC]T-NH2 | CHEMBL437270)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)C(C)C)C1CCc2ccccc12)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C71H101N21O18S2/c1-8-34(4)56(82-37(7)94)69(109)89-51-31-112-111-30-50(67(107)91-57(36(6)93)59(73)99)88-62(102)45(18-13-23-77-71(74)75)83-64(104)48(25-40-28-76-32-80-40)85-60(100)35(5)81-53(96)29-79-61(101)47(24-39-27-78-44-17-12-11-16-42(39)44)86-65(105)49(26-54(97)98)87-63(103)46(21-22-52(72)95)84-70(110)58(43-20-19-38-14-9-10-15-41(38)43)92-68(108)55(33(2)3)90-66(51)106/h9-12,14-17,27-28,32-36,43,45-51,55-58,78,93H,8,13,18-26,29-31H2,1-7H3,(H2,72,95)(H2,73,99)(H,76,80)(H,79,101)(H,81,96)(H,82,94)(H,83,104)(H,84,110)(H,85,100)(H,86,105)(H,87,103)(H,88,102)(H,89,109)(H,90,106)(H,91,107)(H,92,108)(H,97,98)(H4,74,75,77)/t34-,35-,36+,43?,45-,46-,47-,48+,49+,50-,51-,55-,56-,57-,58-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159069

(Ac-I[CV(2Nal)QDWGAHRC]T | CHEMBL437245)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C73H100N20O19S2/c1-8-36(4)59(83-39(7)95)71(110)91-54-33-114-113-32-53(69(108)93-60(38(6)94)72(111)112)90-63(102)47(18-13-23-78-73(75)76)84-66(105)51(27-44-30-77-34-81-44)86-61(100)37(5)82-56(97)31-80-62(101)50(26-43-29-79-46-17-12-11-16-45(43)46)87-67(106)52(28-57(98)99)88-64(103)48(21-22-55(74)96)85-65(104)49(89-70(109)58(35(2)3)92-68(54)107)25-40-19-20-41-14-9-10-15-42(41)24-40/h9-12,14-17,19-20,24,29-30,34-38,47-54,58-60,79,94H,8,13,18,21-23,25-28,31-33H2,1-7H3,(H2,74,96)(H,77,81)(H,80,101)(H,82,97)(H,83,95)(H,84,105)(H,85,104)(H,86,100)(H,87,106)(H,88,103)(H,89,109)(H,90,102)(H,91,110)(H,92,107)(H,93,108)(H,98,99)(H,111,112)(H4,75,76,78)/t36-,37-,38+,47-,48-,49-,50-,51+,52+,53-,54-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159054
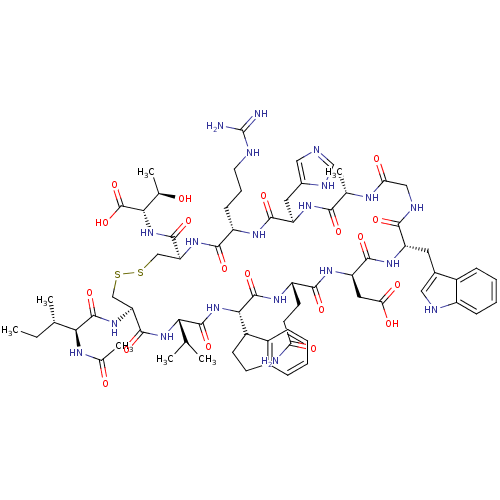
(Ac-I[CV(2Igl)QDWGAHRC]T | CHEMBL445418)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)C(C)C)C1CCc2ccccc12)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H100N20O19S2/c1-8-34(4)56(81-37(7)93)68(107)88-51-31-112-111-30-50(66(105)90-57(36(6)92)70(109)110)87-61(100)45(18-13-23-76-71(73)74)82-63(102)48(25-40-28-75-32-79-40)84-59(98)35(5)80-53(95)29-78-60(99)47(24-39-27-77-44-17-12-11-16-42(39)44)85-64(103)49(26-54(96)97)86-62(101)46(21-22-52(72)94)83-69(108)58(43-20-19-38-14-9-10-15-41(38)43)91-67(106)55(33(2)3)89-65(51)104/h9-12,14-17,27-28,32-36,43,45-51,55-58,77,92H,8,13,18-26,29-31H2,1-7H3,(H2,72,94)(H,75,79)(H,78,99)(H,80,95)(H,81,93)(H,82,102)(H,83,108)(H,84,98)(H,85,103)(H,86,101)(H,87,100)(H,88,107)(H,89,104)(H,90,105)(H,91,106)(H,96,97)(H,109,110)(H4,73,74,76)/t34-,35-,36+,43?,45-,46-,47-,48+,49+,50-,51-,55-,56-,57-,58-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159047

(Ac-I[CVWQDWG(Abu)HRC]T-NH2 | CHEMBL414282)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)C(CC)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C72H101N21O19S2/c1-8-35(5)58(82-37(7)95)70(110)91-53-32-114-113-31-52(68(108)93-59(36(6)94)71(111)112)90-62(102)46(19-14-22-77-72(74)75)84-65(105)50(25-40-29-76-33-81-40)87-61(101)43(9-2)83-55(97)30-80-60(100)48(23-38-27-78-44-17-12-10-15-41(38)44)86-66(106)51(26-56(98)99)88-63(103)47(20-21-54(73)96)85-64(104)49(89-69(109)57(34(3)4)92-67(53)107)24-39-28-79-45-18-13-11-16-42(39)45/h10-13,15-18,27-29,33-36,43,46-53,57-59,78-79,94H,8-9,14,19-26,30-32H2,1-7H3,(H2,73,96)(H,76,81)(H,80,100)(H,82,95)(H,83,97)(H,84,105)(H,85,104)(H,86,106)(H,87,101)(H,88,103)(H,89,109)(H,90,102)(H,91,110)(H,92,107)(H,93,108)(H,98,99)(H,111,112)(H4,74,75,77)/t35-,36+,43?,46+,47-,48-,49+,50-,51+,52+,53-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159046
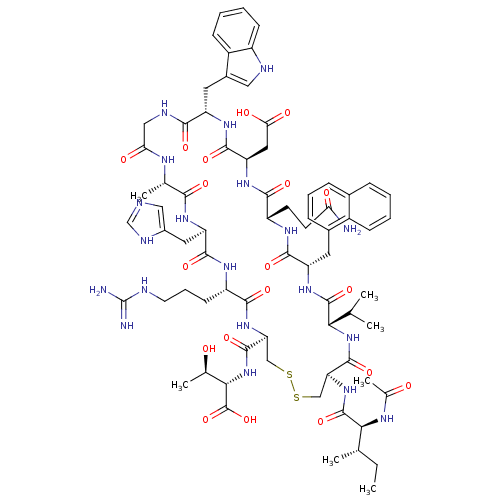
(Ac-I[CV(1Nal)QDWGAHRC]T | CHEMBL262797)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C73H100N20O19S2/c1-8-36(4)59(83-39(7)95)71(110)91-54-33-114-113-32-53(69(108)93-60(38(6)94)72(111)112)90-63(102)47(21-14-24-78-73(75)76)84-66(105)51(27-43-30-77-34-81-43)86-61(100)37(5)82-56(97)31-80-62(101)49(26-42-29-79-46-20-12-11-19-45(42)46)87-67(106)52(28-57(98)99)88-64(103)48(22-23-55(74)96)85-65(104)50(89-70(109)58(35(2)3)92-68(54)107)25-41-17-13-16-40-15-9-10-18-44(40)41/h9-13,15-20,29-30,34-38,47-54,58-60,79,94H,8,14,21-28,31-33H2,1-7H3,(H2,74,96)(H,77,81)(H,80,101)(H,82,97)(H,83,95)(H,84,105)(H,85,104)(H,86,100)(H,87,106)(H,88,103)(H,89,109)(H,90,102)(H,91,110)(H,92,107)(H,93,108)(H,98,99)(H,111,112)(H4,75,76,78)/t36-,37-,38+,47-,48-,49-,50-,51+,52+,53-,54-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159071

(Ac-I[CVWQDWGAHRC]dT | CHEMBL269247)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@H](C)O)C(O)=O Show InChI InChI=1S/C71H99N21O19S2/c1-8-34(4)57(82-37(7)94)69(109)90-52-31-113-112-30-51(67(107)92-58(36(6)93)70(110)111)89-61(101)45(18-13-21-76-71(73)74)83-64(104)49(24-40-28-75-32-80-40)85-59(99)35(5)81-54(96)29-79-60(100)47(22-38-26-77-43-16-11-9-14-41(38)43)86-65(105)50(25-55(97)98)87-62(102)46(19-20-53(72)95)84-63(103)48(88-68(108)56(33(2)3)91-66(52)106)23-39-27-78-44-17-12-10-15-42(39)44/h9-12,14-17,26-28,32-36,45-52,56-58,77-78,93H,8,13,18-25,29-31H2,1-7H3,(H2,72,95)(H,75,80)(H,79,100)(H,81,96)(H,82,94)(H,83,104)(H,84,103)(H,85,99)(H,86,105)(H,87,102)(H,88,108)(H,89,101)(H,90,109)(H,91,106)(H,92,107)(H,97,98)(H,110,111)(H4,73,74,76)/t34-,35-,36-,45-,46-,47-,48+,49+,50+,51-,52-,56-,57-,58-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159041

(Ac-I[CVYQDWGAHRC]T-NH2 | CHEMBL268084)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C69H99N21O19S2/c1-8-33(4)55(80-36(7)92)68(109)88-50-30-111-110-29-49(66(107)90-56(35(6)91)57(71)98)87-60(101)43(14-11-21-75-69(72)73)81-63(104)47(24-39-27-74-31-78-39)83-58(99)34(5)79-52(95)28-77-59(100)46(23-38-26-76-42-13-10-9-12-41(38)42)84-64(105)48(25-53(96)97)85-61(102)44(19-20-51(70)94)82-62(103)45(22-37-15-17-40(93)18-16-37)86-67(108)54(32(2)3)89-65(50)106/h9-10,12-13,15-18,26-27,31-35,43-50,54-56,76,91,93H,8,11,14,19-25,28-30H2,1-7H3,(H2,70,94)(H2,71,98)(H,74,78)(H,77,100)(H,79,95)(H,80,92)(H,81,104)(H,82,103)(H,83,99)(H,84,105)(H,85,102)(H,86,108)(H,87,101)(H,88,109)(H,89,106)(H,90,107)(H,96,97)(H4,72,73,75)/t33-,34-,35+,43-,44-,45+,46-,47+,48+,49-,50-,54-,55-,56-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50070207
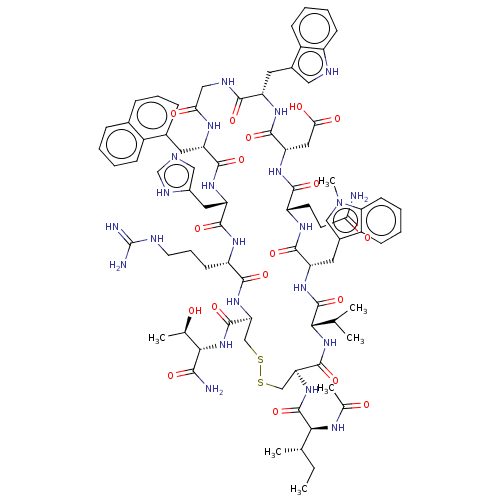
(CHEMBL3408038)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cn(C)c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of C3 cleavage in human serum assessed as reduction in C5b-9 formation compound preincubated for 15 mins measured 1 hr post LPS stimulatio... |
J Med Chem 58: 814-26 (2015)
Article DOI: 10.1021/jm501345y
BindingDB Entry DOI: 10.7270/Q2474CKZ |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50070207

(CHEMBL3408038)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2cn(C)c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of C3 cleavage in human serum assessed as reduction in C3b formation compound preincubated for 15 mins measured 1 hr post LPS stimulation ... |
J Med Chem 58: 814-26 (2015)
Article DOI: 10.1021/jm501345y
BindingDB Entry DOI: 10.7270/Q2474CKZ |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159044
(Ac-I[CVTQDWGHHRC]T-NH2 | CHEMBL414283)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C67H99N23O19S2/c1-8-31(4)52(79-34(7)93)65(108)87-47-27-111-110-26-46(63(106)89-53(32(5)91)55(69)98)86-57(100)40(14-11-17-74-67(70)71)81-60(103)44(20-37-24-73-29-78-37)84-59(102)43(19-36-23-72-28-77-36)80-49(95)25-76-56(99)42(18-35-22-75-39-13-10-9-12-38(35)39)83-61(104)45(21-50(96)97)85-58(101)41(15-16-48(68)94)82-66(109)54(33(6)92)90-64(107)51(30(2)3)88-62(47)105/h9-10,12-13,22-24,28-33,40-47,51-54,75,91-92H,8,11,14-21,25-27H2,1-7H3,(H2,68,94)(H2,69,98)(H,72,77)(H,73,78)(H,76,99)(H,79,93)(H,80,95)(H,81,103)(H,82,109)(H,83,104)(H,84,102)(H,85,101)(H,86,100)(H,87,108)(H,88,105)(H,89,106)(H,90,107)(H,96,97)(H4,70,71,74)/t31-,32+,33+,40-,41-,42-,43-,44+,45+,46-,47-,51-,52-,53-,54+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159048

(Ac-I[CVFQDWGHHRC]T-NH2 | CHEMBL265872)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C72H101N23O18S2/c1-7-36(4)58(84-38(6)97)71(113)93-53-32-115-114-31-52(69(111)95-59(37(5)96)60(74)102)92-62(104)45(18-13-21-79-72(75)76)86-66(108)50(25-42-29-78-34-83-42)89-65(107)49(24-41-28-77-33-82-41)85-55(99)30-81-61(103)48(23-40-27-80-44-17-12-11-16-43(40)44)88-67(109)51(26-56(100)101)90-63(105)46(19-20-54(73)98)87-64(106)47(22-39-14-9-8-10-15-39)91-70(112)57(35(2)3)94-68(53)110/h8-12,14-17,27-29,33-37,45-53,57-59,80,96H,7,13,18-26,30-32H2,1-6H3,(H2,73,98)(H2,74,102)(H,77,82)(H,78,83)(H,81,103)(H,84,97)(H,85,99)(H,86,108)(H,87,106)(H,88,109)(H,89,107)(H,90,105)(H,91,112)(H,92,104)(H,93,113)(H,94,110)(H,95,111)(H,100,101)(H4,75,76,79)/t36-,37+,45-,46-,47+,48-,49-,50+,51+,52-,53-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159060

(Ac-I[CVWQDWGWHRC]T-NH2 | CHEMBL415601)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C79H105N23O18S2/c1-7-39(4)65(91-41(6)104)78(120)100-60-36-122-121-35-59(76(118)102-66(40(5)103)67(81)109)99-69(111)52(21-14-24-85-79(82)83)93-73(115)57(28-45-33-84-37-90-45)96-71(113)55(26-43-31-87-50-19-12-9-16-47(43)50)92-62(106)34-89-68(110)54(25-42-30-86-49-18-11-8-15-46(42)49)95-74(116)58(29-63(107)108)97-70(112)53(22-23-61(80)105)94-72(114)56(98-77(119)64(38(2)3)101-75(60)117)27-44-32-88-51-20-13-10-17-48(44)51/h8-13,15-20,30-33,37-40,52-60,64-66,86-88,103H,7,14,21-29,34-36H2,1-6H3,(H2,80,105)(H2,81,109)(H,84,90)(H,89,110)(H,91,104)(H,92,106)(H,93,115)(H,94,114)(H,95,116)(H,96,113)(H,97,112)(H,98,119)(H,99,111)(H,100,120)(H,101,117)(H,102,118)(H,107,108)(H4,82,83,85)/t39-,40+,52-,53-,54-,55-,56+,57+,58+,59-,60-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159066
(Ac-I[CVSQDWGHHRC]T-NH2 | CHEMBL415619)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](CO)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C66H97N23O19S2/c1-7-31(4)52(78-33(6)92)65(108)87-47-27-110-109-26-46(63(106)89-53(32(5)91)54(68)97)86-56(99)39(13-10-16-73-66(69)70)80-59(102)43(19-36-23-72-29-77-36)83-58(101)42(18-35-22-71-28-76-35)79-49(94)24-75-55(98)41(17-34-21-74-38-12-9-8-11-37(34)38)82-60(103)44(20-50(95)96)84-57(100)40(14-15-48(67)93)81-61(104)45(25-90)85-64(107)51(30(2)3)88-62(47)105/h8-9,11-12,21-23,28-32,39-47,51-53,74,90-91H,7,10,13-20,24-27H2,1-6H3,(H2,67,93)(H2,68,97)(H,71,76)(H,72,77)(H,75,98)(H,78,92)(H,79,94)(H,80,102)(H,81,104)(H,82,103)(H,83,101)(H,84,100)(H,85,107)(H,86,99)(H,87,108)(H,88,105)(H,89,106)(H,95,96)(H4,69,70,73)/t31-,32+,39-,40-,41-,42-,43+,44+,45+,46-,47-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159059
(Ac-I[CVHQDWGHHRC]T-NH2 | CHEMBL429695)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C69H99N25O18S2/c1-7-33(4)55(83-35(6)96)68(112)92-50-28-114-113-27-49(66(110)94-56(34(5)95)57(71)101)91-59(103)42(13-10-16-77-69(72)73)85-62(106)46(19-38-24-75-30-81-38)88-61(105)45(18-37-23-74-29-80-37)84-52(98)26-79-58(102)44(17-36-22-78-41-12-9-8-11-40(36)41)87-64(108)48(21-53(99)100)89-60(104)43(14-15-51(70)97)86-63(107)47(20-39-25-76-31-82-39)90-67(111)54(32(2)3)93-65(50)109/h8-9,11-12,22-25,29-34,42-50,54-56,78,95H,7,10,13-21,26-28H2,1-6H3,(H2,70,97)(H2,71,101)(H,74,80)(H,75,81)(H,76,82)(H,79,102)(H,83,96)(H,84,98)(H,85,106)(H,86,107)(H,87,108)(H,88,105)(H,89,104)(H,90,111)(H,91,103)(H,92,112)(H,93,109)(H,94,110)(H,99,100)(H4,72,73,77)/t33-,34+,42-,43-,44-,45-,46+,47+,48+,49-,50-,54-,55-,56-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159055
(Ac-I[CVWQDWGHHRC]T-NH2 | CHEMBL411957)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C74H102N24O18S2/c1-7-36(4)60(87-38(6)100)73(116)96-55-32-118-117-31-54(71(114)98-61(37(5)99)62(76)105)95-64(107)47(17-12-20-81-74(77)78)89-68(111)52(24-42-29-80-34-86-42)92-67(110)51(23-41-28-79-33-85-41)88-57(102)30-84-63(106)49(21-39-26-82-45-15-10-8-13-43(39)45)91-69(112)53(25-58(103)104)93-65(108)48(18-19-56(75)101)90-66(109)50(94-72(115)59(35(2)3)97-70(55)113)22-40-27-83-46-16-11-9-14-44(40)46/h8-11,13-16,26-29,33-37,47-55,59-61,82-83,99H,7,12,17-25,30-32H2,1-6H3,(H2,75,101)(H2,76,105)(H,79,85)(H,80,86)(H,84,106)(H,87,100)(H,88,102)(H,89,111)(H,90,109)(H,91,112)(H,92,110)(H,93,108)(H,94,115)(H,95,107)(H,96,116)(H,97,113)(H,98,114)(H,103,104)(H4,77,78,81)/t36-,37+,47-,48-,49-,50+,51-,52+,53+,54-,55-,59-,60-,61-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389003

(CHEMBL2064018)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:109.125,87.93,65.74,40.47,29.31,12.16,4.4,132.137,wD:95.109,76.85,51.63,35.35,20.25,127.133,(46.4,-15.18,;46.39,-16.72,;47.73,-17.49,;45.05,-17.49,;45.05,-19.03,;43.72,-19.8,;42.38,-19.02,;42.39,-17.48,;41.05,-19.8,;39.72,-19.02,;38.38,-19.78,;38.38,-21.32,;37.05,-19.01,;37.05,-17.48,;38.39,-16.7,;38.39,-15.16,;39.72,-17.48,;35.71,-19.78,;34.38,-19.01,;34.38,-17.47,;33.04,-19.77,;33.04,-21.31,;34.37,-22.09,;34.37,-23.63,;35.7,-24.4,;35.7,-25.94,;31.71,-19,;30.38,-19.77,;30.37,-21.31,;29.04,-18.99,;29.05,-17.46,;30.38,-16.69,;27.7,-19.77,;26.37,-18.99,;26.38,-17.45,;25.04,-19.76,;25.03,-21.3,;23.71,-18.99,;22.37,-19.75,;22.37,-21.29,;21.04,-18.98,;21.04,-17.44,;22.38,-16.67,;22.38,-15.13,;23.71,-14.37,;23.72,-12.82,;22.38,-12.05,;25.06,-12.05,;19.71,-19.75,;18.38,-18.97,;18.38,-17.43,;17.03,-19.74,;17.03,-21.28,;18.36,-22.05,;19.77,-21.43,;20.8,-22.58,;20.03,-23.91,;20.5,-25.38,;19.47,-26.52,;17.96,-26.19,;17.49,-24.73,;18.52,-23.59,;15.7,-18.97,;14.37,-19.73,;14.37,-21.28,;13.04,-18.96,;13.04,-17.43,;14.37,-16.65,;14.38,-15.11,;15.71,-14.35,;15.71,-12.81,;14.38,-12.03,;17.05,-12.04,;11.7,-19.73,;10.36,-18.96,;10.37,-17.41,;9.03,-19.72,;9.03,-21.26,;10.36,-22.04,;10.36,-23.58,;11.69,-24.35,;11.69,-25.89,;10.35,-26.66,;13.02,-26.66,;7.7,-18.95,;6.36,-19.72,;6.36,-21.26,;5.03,-18.94,;5.03,-17.4,;6.37,-16.64,;7.7,-17.42,;6.37,-15.1,;3.7,-19.72,;2.36,-18.94,;2.37,-17.4,;1.04,-19.7,;1.03,-21.24,;2.37,-22.02,;3.77,-21.4,;4.79,-22.55,;4.02,-23.88,;4.49,-25.34,;3.46,-26.48,;1.97,-26.16,;1.47,-24.69,;2.52,-23.56,;-.3,-18.93,;-1.64,-19.7,;-1.64,-21.24,;-2.97,-18.93,;-2.96,-17.38,;-1.64,-16.62,;-.24,-17.25,;.8,-16.1,;.03,-14.77,;.52,-13.3,;-.51,-12.16,;-2.02,-12.48,;-2.5,-13.94,;-1.48,-15.08,;-4.3,-19.7,;-5.63,-18.93,;-6.96,-19.71,;-5.63,-17.4,;46.38,-19.8,;46.38,-21.34,;47.72,-19.04,;49.06,-19.81,;49.05,-21.35,;50.39,-19.04,;50.39,-17.5,;51.72,-19.81,;53.06,-19.05,;53.06,-17.51,;54.4,-16.74,;54.4,-15.2,;55.73,-14.43,;55.74,-12.89,;54.4,-12.12,;57.08,-12.13,;54.39,-19.82,;55.73,-19.05,;54.38,-21.36,)| Show InChI InChI=1S/C92H139N31O20/c1-47(2)36-66(77(131)109-45-73(126)113-67(37-48(3)4)82(136)111-49(5)75(129)118-65(88(142)143)30-19-35-105-92(100)101)119-80(134)61(26-14-15-31-93)117-87(141)72(46-124)123-76(130)50(6)110-78(132)62(27-16-32-102-89(94)95)115-84(138)69(39-53-43-107-59-24-12-9-21-56(53)59)120-81(135)64(29-18-34-104-91(98)99)114-79(133)63(28-17-33-103-90(96)97)116-86(140)71(41-74(127)128)122-85(139)70(40-54-44-108-60-25-13-10-22-57(54)60)121-83(137)68(112-51(7)125)38-52-42-106-58-23-11-8-20-55(52)58/h8-13,20-25,42-44,47-50,61-72,106-108,124H,14-19,26-41,45-46,93H2,1-7H3,(H,109,131)(H,110,132)(H,111,136)(H,112,125)(H,113,126)(H,114,133)(H,115,138)(H,116,140)(H,117,141)(H,118,129)(H,119,134)(H,120,135)(H,121,137)(H,122,139)(H,123,130)(H,127,128)(H,142,143)(H4,94,95,102)(H4,96,97,103)(H4,98,99,104)(H4,100,101,105)/t49-,50-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Antagonist activity at C3a receptor in dbcAMP differentiated human U937 cells assessed as inhibition of increase in intracellular calcium compound pr... |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159067

(Ac-I[CV(Yphs)QDWGAHRC]I-NH2 | CHEMBL268083)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(C)=O)[C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2ccc(OP(O)(O)=O)cc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@H](Cc2cnc[nH]2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)C(N)=O Show InChI InChI=1S/C71H104N21O21PS2/c1-9-35(5)57(59(73)98)92-68(107)51-31-115-116-32-52(90-70(109)58(36(6)10-2)82-38(8)93)67(106)91-56(34(3)4)69(108)88-47(24-39-17-19-42(20-18-39)113-114(110,111)112)64(103)84-46(21-22-53(72)94)63(102)87-50(27-55(96)97)66(105)86-48(25-40-28-78-44-15-12-11-14-43(40)44)61(100)79-30-54(95)81-37(7)60(99)85-49(26-41-29-76-33-80-41)65(104)83-45(62(101)89-51)16-13-23-77-71(74)75/h11-12,14-15,17-20,28-29,33-37,45-52,56-58,78H,9-10,13,16,21-27,30-32H2,1-8H3,(H2,72,94)(H2,73,98)(H,76,80)(H,79,100)(H,81,95)(H,82,93)(H,83,104)(H,84,103)(H,85,99)(H,86,105)(H,87,102)(H,88,108)(H,89,101)(H,90,109)(H,91,106)(H,92,107)(H,96,97)(H4,74,75,77)(H2,110,111,112)/t35-,36-,37-,45-,46-,47-,48-,49+,50+,51-,52-,56-,57-,58-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159058
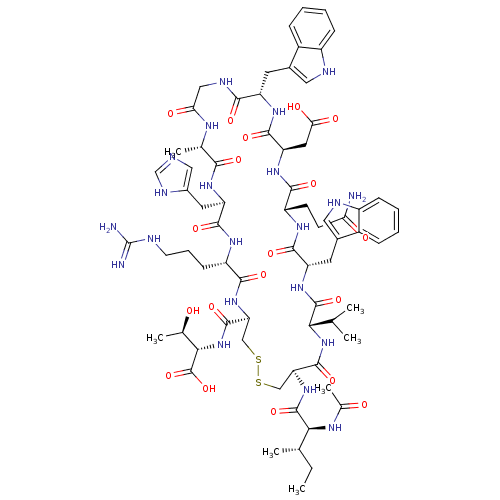
(Ac-I[CV(Dht)QDWGAHRC]T | CHEMBL437340)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H99N21O19S2/c1-8-34(4)57(82-37(7)94)69(109)90-52-31-113-112-30-51(67(107)92-58(36(6)93)70(110)111)89-61(101)45(18-13-21-76-71(73)74)83-64(104)49(24-40-28-75-32-80-40)85-59(99)35(5)81-54(96)29-79-60(100)47(22-38-26-77-43-16-11-9-14-41(38)43)86-65(105)50(25-55(97)98)87-62(102)46(19-20-53(72)95)84-63(103)48(88-68(108)56(33(2)3)91-66(52)106)23-39-27-78-44-17-12-10-15-42(39)44/h9-12,14-17,26-28,32-36,45-52,56-58,77-78,93H,8,13,18-25,29-31H2,1-7H3,(H2,72,95)(H,75,80)(H,79,100)(H,81,96)(H,82,94)(H,83,104)(H,84,103)(H,85,99)(H,86,105)(H,87,102)(H,88,108)(H,89,101)(H,90,109)(H,91,106)(H,92,107)(H,97,98)(H,110,111)(H4,73,74,76)/t34-,35-,36+,45-,46-,47-,48-,49+,50+,51-,52-,56-,57-,58-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159037

(Ac-I[CVWQDWGAHRC]T | CHEMBL413626)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H99N21O19S2/c1-8-34(4)57(82-37(7)94)69(109)90-52-31-113-112-30-51(67(107)92-58(36(6)93)70(110)111)89-61(101)45(18-13-21-76-71(73)74)83-64(104)49(24-40-28-75-32-80-40)85-59(99)35(5)81-54(96)29-79-60(100)47(22-38-26-77-43-16-11-9-14-41(38)43)86-65(105)50(25-55(97)98)87-62(102)46(19-20-53(72)95)84-63(103)48(88-68(108)56(33(2)3)91-66(52)106)23-39-27-78-44-17-12-10-15-42(39)44/h9-12,14-17,26-28,32-36,45-52,56-58,77-78,93H,8,13,18-25,29-31H2,1-7H3,(H2,72,95)(H,75,80)(H,79,100)(H,81,96)(H,82,94)(H,83,104)(H,84,103)(H,85,99)(H,86,105)(H,87,102)(H,88,108)(H,89,101)(H,90,109)(H,91,106)(H,92,107)(H,97,98)(H,110,111)(H4,73,74,76)/t34-,35-,36+,45-,46-,47-,48+,49+,50+,51-,52-,56-,57-,58-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159064

(Ac-I[CVVQDWGAHRC]T-NH2 | CHEMBL269043)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C65H99N21O18S2/c1-10-31(6)51(76-34(9)88)64(104)83-45-27-106-105-26-44(61(101)86-52(33(8)87)53(67)93)82-56(96)39(16-13-19-71-65(68)69)77-58(98)42(21-36-24-70-28-74-36)79-54(94)32(7)75-47(90)25-73-55(95)41(20-35-23-72-38-15-12-11-14-37(35)38)80-59(99)43(22-48(91)92)81-57(97)40(17-18-46(66)89)78-62(102)49(29(2)3)85-63(103)50(30(4)5)84-60(45)100/h11-12,14-15,23-24,28-33,39-45,49-52,72,87H,10,13,16-22,25-27H2,1-9H3,(H2,66,89)(H2,67,93)(H,70,74)(H,73,95)(H,75,90)(H,76,88)(H,77,98)(H,78,102)(H,79,94)(H,80,99)(H,81,97)(H,82,96)(H,83,104)(H,84,100)(H,85,103)(H,86,101)(H,91,92)(H4,68,69,71)/t31-,32-,33+,39-,40-,41-,42+,43+,44-,45-,49+,50-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159061
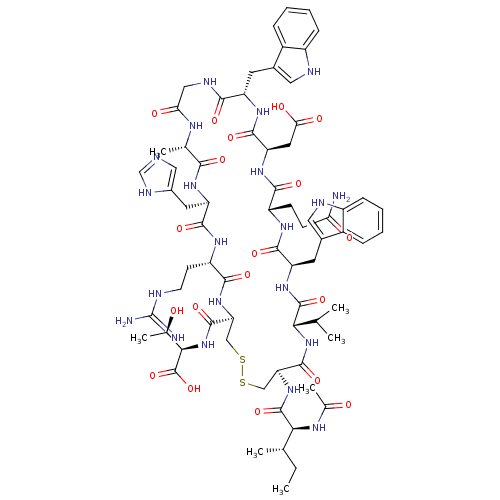
(Ac-dI[CVWQDWGAHRC]T | CHEMBL412730)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C70H97N21O19S2/c1-8-33(4)56(81-36(7)93)68(108)89-51-30-112-111-29-50(66(106)91-57(35(6)92)69(109)110)88-61(101)45(19-20-75-70(72)73)83-63(103)48(23-39-27-74-31-79-39)84-58(98)34(5)80-53(95)28-78-59(99)46(21-37-25-76-42-15-11-9-13-40(37)42)85-64(104)49(24-54(96)97)86-60(100)44(17-18-52(71)94)82-62(102)47(87-67(107)55(32(2)3)90-65(51)105)22-38-26-77-43-16-12-10-14-41(38)43/h9-16,25-27,31-35,44-51,55-57,76-77,92H,8,17-24,28-30H2,1-7H3,(H2,71,94)(H,74,79)(H,78,99)(H,80,95)(H,81,93)(H,82,102)(H,83,103)(H,84,98)(H,85,104)(H,86,100)(H,87,107)(H,88,101)(H,89,108)(H,90,105)(H,91,106)(H,96,97)(H,109,110)(H4,72,73,75)/t33-,34-,35+,44-,45-,46-,47+,48+,49+,50-,51-,55-,56-,57+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159040
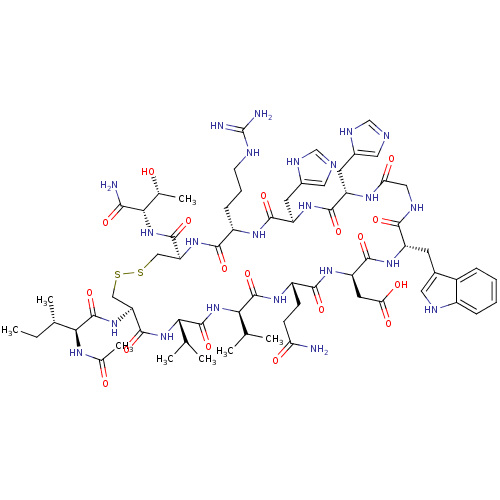
(Ac-I[CVVQDWGHHRC]T-NH2 | CHEMBL409335)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C68H101N23O18S2/c1-9-33(6)54(80-35(8)93)67(109)88-48-28-111-110-27-47(64(106)91-55(34(7)92)56(70)98)87-58(100)41(15-12-18-75-68(71)72)82-61(103)45(21-38-25-74-30-79-38)85-60(102)44(20-37-24-73-29-78-37)81-50(95)26-77-57(99)43(19-36-23-76-40-14-11-10-13-39(36)40)84-62(104)46(22-51(96)97)86-59(101)42(16-17-49(69)94)83-65(107)52(31(2)3)90-66(108)53(32(4)5)89-63(48)105/h10-11,13-14,23-25,29-34,41-48,52-55,76,92H,9,12,15-22,26-28H2,1-8H3,(H2,69,94)(H2,70,98)(H,73,78)(H,74,79)(H,77,99)(H,80,93)(H,81,95)(H,82,103)(H,83,107)(H,84,104)(H,85,102)(H,86,101)(H,87,100)(H,88,109)(H,89,105)(H,90,108)(H,91,106)(H,96,97)(H4,71,72,75)/t33-,34+,41-,42-,43-,44-,45+,46+,47-,48-,52+,53-,54-,55-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159052
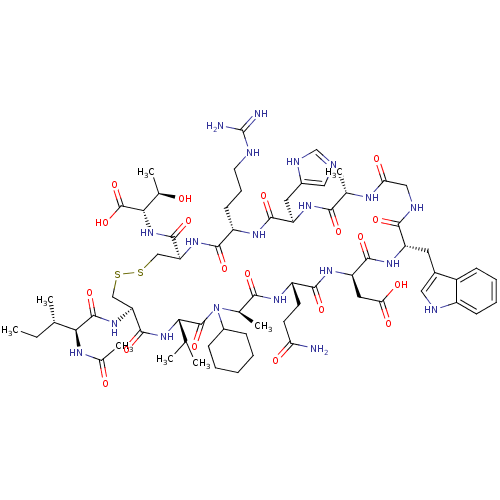
(Ac-I[CV(Cha) QDWGAHRC]T | CHEMBL438586)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](C)N(C2CCCCC2)C(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C69H104N20O19S2/c1-9-34(4)55(79-38(8)91)66(105)86-50-31-110-109-30-49(65(104)88-56(37(7)90)68(107)108)85-60(99)44(20-15-23-74-69(71)72)81-62(101)47(25-40-28-73-32-77-40)82-57(96)35(5)78-52(93)29-76-59(98)46(24-39-27-75-43-19-14-13-18-42(39)43)83-63(102)48(26-53(94)95)84-61(100)45(21-22-51(70)92)80-58(97)36(6)89(41-16-11-10-12-17-41)67(106)54(33(2)3)87-64(50)103/h13-14,18-19,27-28,32-37,41,44-50,54-56,75,90H,9-12,15-17,20-26,29-31H2,1-8H3,(H2,70,92)(H,73,77)(H,76,98)(H,78,93)(H,79,91)(H,80,97)(H,81,101)(H,82,96)(H,83,102)(H,84,100)(H,85,99)(H,86,105)(H,87,103)(H,88,104)(H,94,95)(H,107,108)(H4,71,72,74)/t34-,35-,36+,37+,44-,45-,46-,47+,48+,49-,50-,54-,55-,56-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159056

(CHEMBL265136 | I[CVVQDWGHHRC]T-NH2)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C66H99N23O17S2/c1-8-32(6)50(68)63(104)86-46-27-108-107-26-45(62(103)89-53(33(7)90)54(69)95)85-56(97)39(14-11-17-74-66(70)71)80-59(100)43(20-36-24-73-29-78-36)83-58(99)42(19-35-23-72-28-77-35)79-48(92)25-76-55(96)41(18-34-22-75-38-13-10-9-12-37(34)38)82-60(101)44(21-49(93)94)84-57(98)40(15-16-47(67)91)81-64(105)51(30(2)3)88-65(106)52(31(4)5)87-61(46)102/h9-10,12-13,22-24,28-33,39-46,50-53,75,90H,8,11,14-21,25-27,68H2,1-7H3,(H2,67,91)(H2,69,95)(H,72,77)(H,73,78)(H,76,96)(H,79,92)(H,80,100)(H,81,105)(H,82,101)(H,83,99)(H,84,98)(H,85,97)(H,86,104)(H,87,102)(H,88,106)(H,89,103)(H,93,94)(H4,70,71,74)/t32-,33+,39-,40-,41-,42-,43+,44+,45-,46-,50-,51+,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159051
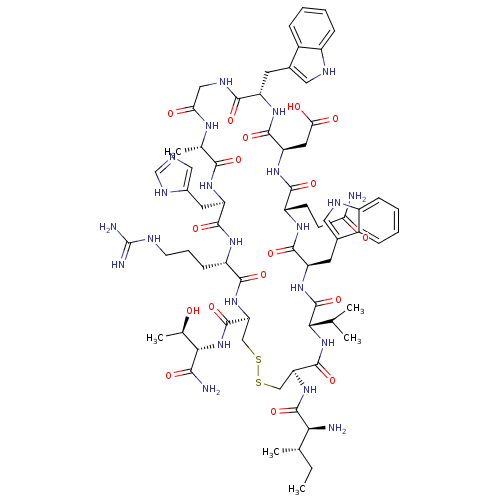
(Ac-I[CVWQDWGAHRC]T-NH2 | CHEMBL411152)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C69H98N22O17S2/c1-7-33(4)54(71)67(107)89-50-30-110-109-29-49(66(106)91-56(35(6)92)57(72)97)88-60(100)43(17-12-20-76-69(73)74)82-63(103)47(23-38-27-75-31-80-38)84-58(98)34(5)81-52(94)28-79-59(99)45(21-36-25-77-41-15-10-8-13-39(36)41)85-64(104)48(24-53(95)96)86-61(101)44(18-19-51(70)93)83-62(102)46(87-68(108)55(32(2)3)90-65(50)105)22-37-26-78-42-16-11-9-14-40(37)42/h8-11,13-16,25-27,31-35,43-50,54-56,77-78,92H,7,12,17-24,28-30,71H2,1-6H3,(H2,70,93)(H2,72,97)(H,75,80)(H,79,99)(H,81,94)(H,82,103)(H,83,102)(H,84,98)(H,85,104)(H,86,101)(H,87,108)(H,88,100)(H,89,107)(H,90,105)(H,91,106)(H,95,96)(H4,73,74,76)/t33-,34-,35+,43-,44-,45-,46+,47+,48+,49-,50-,54-,55-,56-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159039
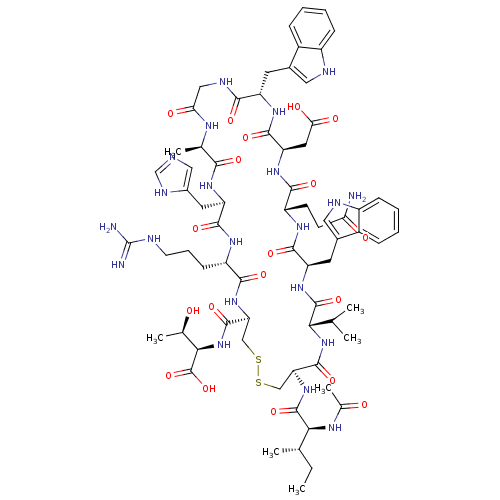
(Ac-I[CVWQDWGdAHRC]T | CHEMBL409250)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H99N21O19S2/c1-8-34(4)57(82-37(7)94)69(109)90-52-31-113-112-30-51(67(107)92-58(36(6)93)70(110)111)89-61(101)45(18-13-21-76-71(73)74)83-64(104)49(24-40-28-75-32-80-40)85-59(99)35(5)81-54(96)29-79-60(100)47(22-38-26-77-43-16-11-9-14-41(38)43)86-65(105)50(25-55(97)98)87-62(102)46(19-20-53(72)95)84-63(103)48(88-68(108)56(33(2)3)91-66(52)106)23-39-27-78-44-17-12-10-15-42(39)44/h9-12,14-17,26-28,32-36,45-52,56-58,77-78,93H,8,13,18-25,29-31H2,1-7H3,(H2,72,95)(H,75,80)(H,79,100)(H,81,96)(H,82,94)(H,83,104)(H,84,103)(H,85,99)(H,86,105)(H,87,102)(H,88,108)(H,89,101)(H,90,109)(H,91,106)(H,92,107)(H,97,98)(H,110,111)(H4,73,74,76)/t34-,35+,36+,45-,46-,47-,48+,49+,50+,51-,52-,56-,57-,58+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159038

(Ac-I[CVWQDWGAdHRC]T | CHEMBL264596)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC1=O)C(C)C)C(=O)N[C@H]([C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H99N21O19S2/c1-8-34(4)57(82-37(7)94)69(109)90-52-31-113-112-30-51(67(107)92-58(36(6)93)70(110)111)89-61(101)45(18-13-21-76-71(73)74)83-64(104)49(24-40-28-75-32-80-40)85-59(99)35(5)81-54(96)29-79-60(100)47(22-38-26-77-43-16-11-9-14-41(38)43)86-65(105)50(25-55(97)98)87-62(102)46(19-20-53(72)95)84-63(103)48(88-68(108)56(33(2)3)91-66(52)106)23-39-27-78-44-17-12-10-15-42(39)44/h9-12,14-17,26-28,32-36,45-52,56-58,77-78,93H,8,13,18-25,29-31H2,1-7H3,(H2,72,95)(H,75,80)(H,79,100)(H,81,96)(H,82,94)(H,83,104)(H,84,103)(H,85,99)(H,86,105)(H,87,102)(H,88,108)(H,89,101)(H,90,109)(H,91,106)(H,92,107)(H,97,98)(H,110,111)(H4,73,74,76)/t34-,35-,36+,45-,46-,47-,48+,49-,50+,51-,52-,56-,57-,58+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159065
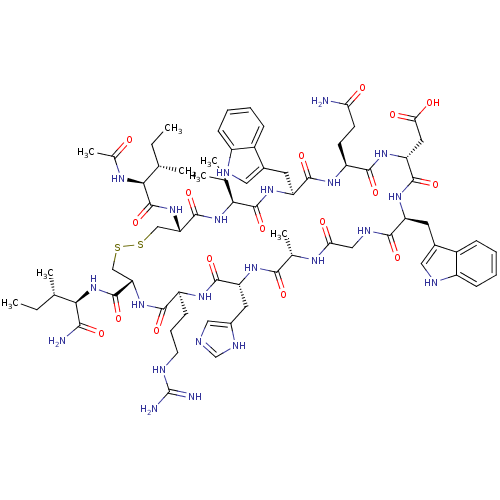
(Ac-I[CVdWQDWGAHRC]I-NH2 | CHEMBL411923)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(C)=O)[C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@H](Cc2cnc[nH]2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)C(N)=O Show InChI InChI=1S/C73H104N22O17S2/c1-9-36(5)59(61(75)101)95-70(110)53-32-113-114-33-54(93-72(112)60(37(6)10-2)85-39(8)96)69(109)94-58(35(3)4)71(111)91-50(25-41-29-81-46-19-14-12-17-44(41)46)66(106)87-48(21-22-55(74)97)65(105)90-52(27-57(99)100)68(108)89-49(24-40-28-80-45-18-13-11-16-43(40)45)63(103)82-31-56(98)84-38(7)62(102)88-51(26-42-30-78-34-83-42)67(107)86-47(64(104)92-53)20-15-23-79-73(76)77/h11-14,16-19,28-30,34-38,47-54,58-60,80-81H,9-10,15,20-27,31-33H2,1-8H3,(H2,74,97)(H2,75,101)(H,78,83)(H,82,103)(H,84,98)(H,85,96)(H,86,107)(H,87,106)(H,88,102)(H,89,108)(H,90,105)(H,91,111)(H,92,104)(H,93,112)(H,94,109)(H,95,110)(H,99,100)(H4,76,77,79)/t36-,37-,38-,47-,48-,49-,50-,51+,52+,53-,54-,58-,59+,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50370556
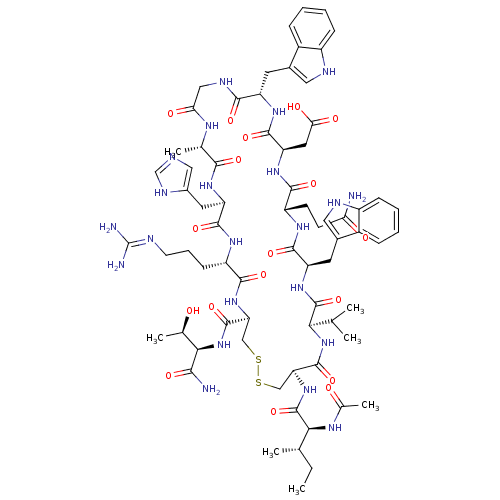
(CHEMBL558435)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](NC1=O)C(C)C)C(=O)N[C@H]([C@@H](C)O)C(N)=O |r,wU:106.111,51.52,4.4,73.76,21.21,42.43,wD:17.108,12.11,65.68,82.85,32.32,96.105,107.114,2.2,(12.88,-32.96,;14.24,-32.24,;14.31,-30.72,;13,-29.88,;15.67,-29.99,;16.97,-30.83,;18.35,-30.13,;18.42,-28.57,;19.65,-30.94,;15.75,-28.44,;17.12,-27.75,;14.44,-27.64,;14.51,-26.1,;16.08,-26.02,;17.54,-25.67,;18.98,-25.07,;20.26,-24.24,;21.36,-23.16,;22.28,-21.91,;22.94,-20.53,;24.37,-21.07,;23.34,-19.05,;24.86,-19.31,;25.4,-20.77,;26.9,-21.04,;27.42,-22.5,;28.92,-22.76,;30.11,-21.8,;29.14,-24.27,;23.49,-17.51,;23.34,-15.98,;24.86,-15.71,;22.94,-14.5,;24.37,-13.95,;25.84,-14.35,;26.87,-13.21,;28.26,-13.81,;28.11,-15.38,;26.61,-15.67,;22.28,-13.1,;21.36,-11.85,;22.54,-10.86,;20.26,-10.8,;21.21,-9.59,;18.98,-9.95,;17.54,-9.33,;18.02,-7.87,;16.08,-8.99,;14.51,-8.92,;12.99,-9.14,;12.66,-7.63,;11.52,-9.62,;10.91,-8.2,;11.3,-6.73,;10.07,-5.81,;10.58,-4.34,;12.11,-4.38,;13.18,-3.24,;14.66,-3.57,;15.12,-5.04,;14.07,-6.18,;12.55,-5.81,;10.18,-10.35,;8.96,-11.3,;7.9,-10.2,;7.96,-12.45,;6.72,-11.56,;6.31,-10.06,;4.81,-9.67,;7.36,-8.97,;7.17,-13.8,;6.62,-15.23,;5.14,-14.83,;6.35,-16.74,;4.82,-16.6,;4.18,-15.21,;2.64,-15.06,;1.98,-13.67,;1.76,-16.33,;6.35,-18.28,;6.62,-19.78,;5.14,-20.2,;7.17,-21.23,;5.77,-21.91,;5.37,-23.38,;6.2,-24.68,;5.21,-25.87,;3.79,-25.3,;2.39,-25.99,;1.1,-25.12,;1.22,-23.6,;2.61,-22.9,;3.89,-23.78,;7.96,-22.55,;8.96,-23.72,;7.9,-24.85,;10.18,-24.66,;11.52,-25.4,;12.99,-25.89,;12.66,-27.38,;9.32,-25.96,;10.02,-27.34,;7.78,-25.87,;22.54,-24.19,;24.08,-24.19,;23.28,-25.51,;22.76,-26.94,;21.21,-27.2,;20.26,-26.02,;20.7,-28.64,;23.72,-28.13,;23.2,-29.58,;25.25,-27.87,)| Show InChI InChI=1S/C71H100N22O18S2/c1-8-34(4)57(83-37(7)95)70(111)91-52-31-113-112-30-51(68(109)93-58(36(6)94)59(73)100)90-62(103)45(18-13-21-77-71(74)75)84-65(106)49(24-40-28-76-32-81-40)86-60(101)35(5)82-54(97)29-80-61(102)47(22-38-26-78-43-16-11-9-14-41(38)43)87-66(107)50(25-55(98)99)88-63(104)46(19-20-53(72)96)85-64(105)48(89-69(110)56(33(2)3)92-67(52)108)23-39-27-79-44-17-12-10-15-42(39)44/h9-12,14-17,26-28,32-36,45-52,56-58,78-79,94H,8,13,18-25,29-31H2,1-7H3,(H2,72,96)(H2,73,100)(H,76,81)(H,80,102)(H,82,97)(H,83,95)(H,84,106)(H,85,105)(H,86,101)(H,87,107)(H,88,104)(H,89,110)(H,90,103)(H,91,111)(H,92,108)(H,93,109)(H,98,99)(H4,74,75,77)/t34-,35-,36+,45-,46-,47-,48+,49+,50+,51-,52-,56+,57-,58+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159053

(Ac-I[CVVQDdWGHHRC]-NH2 | CHEMBL263238)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)C(C)C)C(N)=O Show InChI InChI=1S/C64H94N22O16S2/c1-8-32(6)52(76-33(7)87)63(102)84-46-27-104-103-26-45(53(66)92)83-55(94)39(14-11-17-71-64(67)68)78-58(97)43(20-36-24-70-29-75-36)81-57(96)42(19-35-23-69-28-74-35)77-48(89)25-73-54(93)41(18-34-22-72-38-13-10-9-12-37(34)38)80-59(98)44(21-49(90)91)82-56(95)40(15-16-47(65)88)79-61(100)50(30(2)3)86-62(101)51(31(4)5)85-60(46)99/h9-10,12-13,22-24,28-32,39-46,50-52,72H,8,11,14-21,25-27H2,1-7H3,(H2,65,88)(H2,66,92)(H,69,74)(H,70,75)(H,73,93)(H,76,87)(H,77,89)(H,78,97)(H,79,100)(H,80,98)(H,81,96)(H,82,95)(H,83,94)(H,84,102)(H,85,99)(H,86,101)(H,90,91)(H4,67,68,71)/t32-,39-,40-,41+,42-,43+,44+,45-,46-,50+,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159045
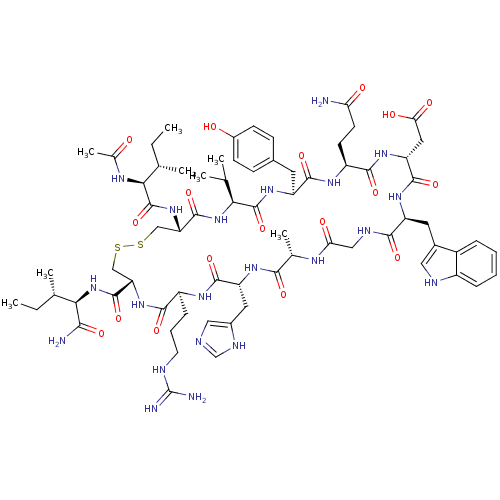
(Ac-I[CVdYQDWGAHRC]I-NH2 | CHEMBL439442)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(C)=O)[C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@H](Cc2cnc[nH]2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)C(N)=O Show InChI InChI=1S/C71H103N21O18S2/c1-9-35(5)57(59(73)99)92-68(108)51-31-111-112-32-52(90-70(110)58(36(6)10-2)82-38(8)93)67(107)91-56(34(3)4)69(109)88-47(24-39-17-19-42(94)20-18-39)64(104)84-46(21-22-53(72)95)63(103)87-50(27-55(97)98)66(106)86-48(25-40-28-78-44-15-12-11-14-43(40)44)61(101)79-30-54(96)81-37(7)60(100)85-49(26-41-29-76-33-80-41)65(105)83-45(62(102)89-51)16-13-23-77-71(74)75/h11-12,14-15,17-20,28-29,33-37,45-52,56-58,78,94H,9-10,13,16,21-27,30-32H2,1-8H3,(H2,72,95)(H2,73,99)(H,76,80)(H,79,101)(H,81,96)(H,82,93)(H,83,105)(H,84,104)(H,85,100)(H,86,106)(H,87,103)(H,88,109)(H,89,102)(H,90,110)(H,91,107)(H,92,108)(H,97,98)(H4,74,75,77)/t35-,36-,37-,45-,46-,47-,48-,49+,50+,51-,52-,56-,57+,58-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |
Complement C3
(Homo sapiens (Human)) | BDBM50159042
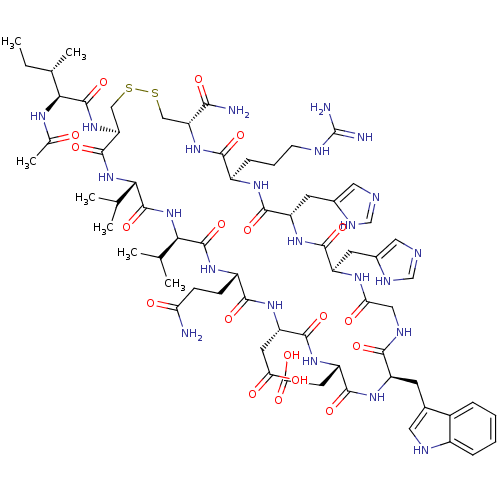
(Ac-I[CVVQdDWGHHRC]-NH2 | CHEMBL273856)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CSSC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CNC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](NC(=O)[C@@H](NC1=O)C(C)C)C(C)C)C(N)=O Show InChI InChI=1S/C68H99N23O19S2/c1-8-33(6)55(80-34(7)92)67(110)89-48-28-112-111-27-47(56(70)99)88-58(101)40(14-11-17-75-68(71)72)82-61(104)44(20-37-25-74-30-79-37)85-60(103)43(19-36-24-73-29-78-36)81-50(94)26-77-57(100)42(18-35-23-76-39-13-10-9-12-38(35)39)84-62(105)46(22-52(97)98)87-63(106)45(21-51(95)96)86-59(102)41(15-16-49(69)93)83-65(108)53(31(2)3)91-66(109)54(32(4)5)90-64(48)107/h9-10,12-13,23-25,29-33,40-48,53-55,76H,8,11,14-22,26-28H2,1-7H3,(H2,69,93)(H2,70,99)(H,73,78)(H,74,79)(H,77,100)(H,80,92)(H,81,94)(H,82,104)(H,83,108)(H,84,105)(H,85,103)(H,86,102)(H,87,106)(H,88,101)(H,89,110)(H,90,107)(H,91,109)(H,95,96)(H,97,98)(H4,71,72,75)/t33-,40+,41-,42+,43+,44-,45-,46-,47+,48-,53+,54-,55-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Riverside
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human complement component C3 activation |
J Med Chem 48: 274-86 (2005)
Article DOI: 10.1021/jm0495531
BindingDB Entry DOI: 10.7270/Q2DR2W81 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































