

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM13211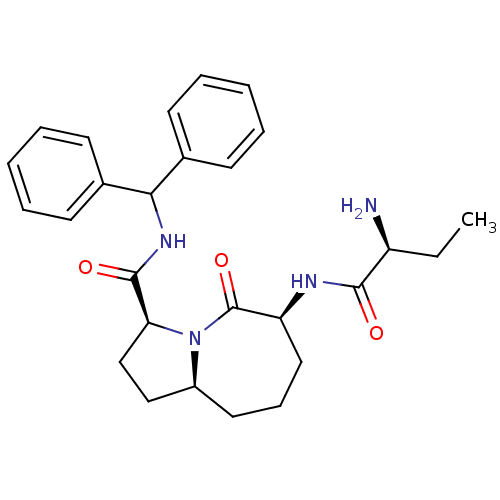 ((3S,6S,9aS)-6-[(2S)-2-aminobutanamido]-N-(diphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.1 | 460 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | Bioorg Med Chem 17: 5834-56 (2009) Article DOI: 10.1016/j.bmc.2009.07.009 BindingDB Entry DOI: 10.7270/Q28W3BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM13212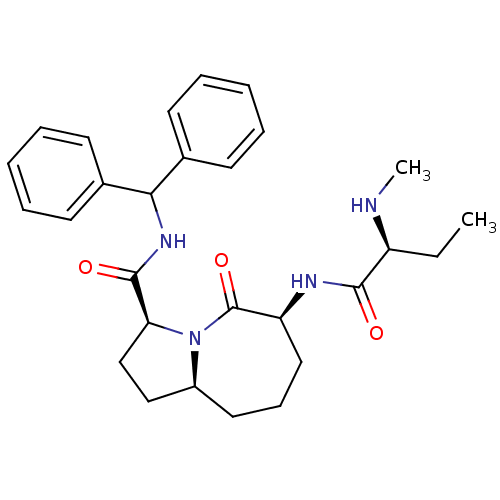 ((3S,6S,9aS)-N-(diphenylmethyl)-6-[(2S)-2-(methylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | -40.9 | 530 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | Bioorg Med Chem 17: 5834-56 (2009) Article DOI: 10.1016/j.bmc.2009.07.009 BindingDB Entry DOI: 10.7270/Q28W3BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50237601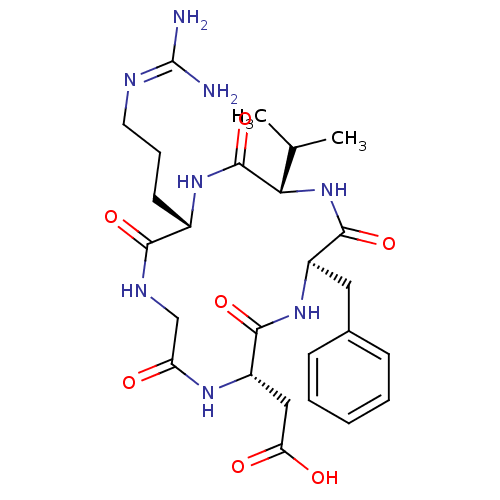 (CHEMBL411941 | CycloRGDfV | [(2S,5R,8S,11S)-5-Benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50235980 (2-((2S,5R,8S,11S)-5-benzyl-11-(3-guanidinopropyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50177887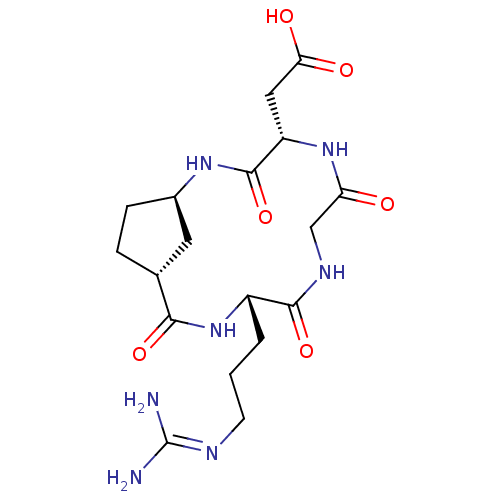 (CHEMBL269913 | CHEMBL556402 | c-[-Arg-Gly-Asp-Acpc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50069316 (CHEMBL3403549 | US9688661, 1-[2-Chloro-5-(trifluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of recombinant epitope-tagged JAK2 (808 to 1132) (unknown origin) using LPLDKDYYVVREPGQ as substrate by beta-countiing analysis in presenc... | Bioorg Med Chem 23: 2387-407 (2015) Article DOI: 10.1016/j.bmc.2015.03.059 BindingDB Entry DOI: 10.7270/Q2Q52RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50069322 (CHEMBL3403545) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of recombinant epitope-tagged JAK2 (808 to 1132) (unknown origin) using LPLDKDYYVVREPGQ as substrate by beta-countiing analysis in presenc... | Bioorg Med Chem 23: 2387-407 (2015) Article DOI: 10.1016/j.bmc.2015.03.059 BindingDB Entry DOI: 10.7270/Q2Q52RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50069326 (CHEMBL3403541 | US9688661, 1-(5-Chloro-2-methylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of recombinant epitope-tagged JAK2 (808 to 1132) (unknown origin) using LPLDKDYYVVREPGQ as substrate by beta-countiing analysis in presenc... | Bioorg Med Chem 23: 2387-407 (2015) Article DOI: 10.1016/j.bmc.2015.03.059 BindingDB Entry DOI: 10.7270/Q2Q52RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50370689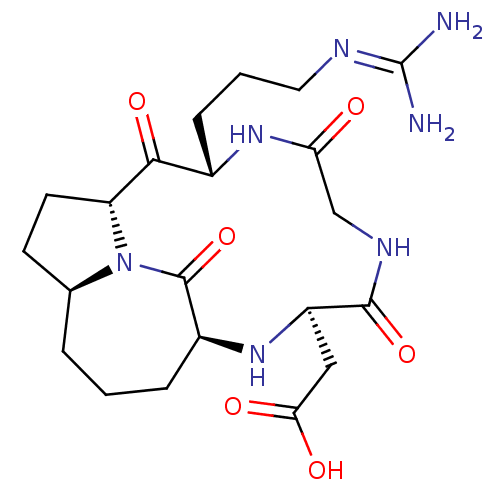 (CHEMBL392303 | ST-1646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50370689 (CHEMBL392303 | ST-1646) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alphavbeta3 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50177887 (CHEMBL269913 | CHEMBL556402 | c-[-Arg-Gly-Asp-Acpc...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alphavbeta3 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50497782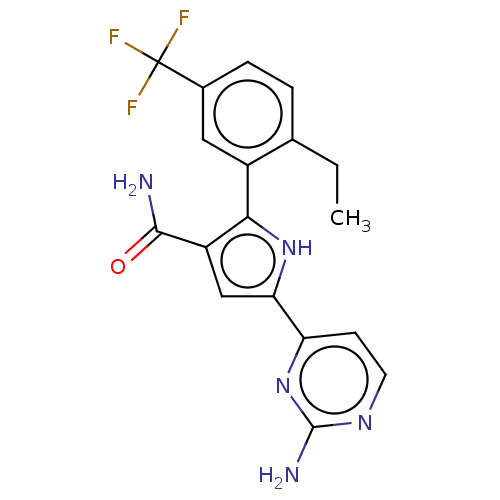 (CHEMBL3330130) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of recombinant human JAK2 (808-1132 residues) using LPLDKDYYVVREPGQ as substrate by radiometric assay in presence of [33P]-gamma-ATP | Bioorg Med Chem 22: 4998-5012 (2014) Article DOI: 10.1016/j.bmc.2014.06.025 BindingDB Entry DOI: 10.7270/Q28G8PP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50069366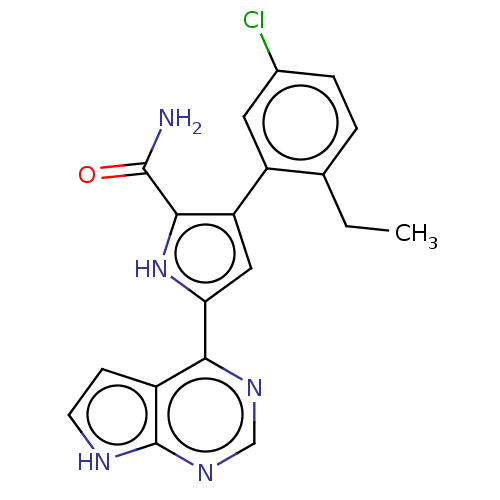 (CHEMBL3403553) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of recombinant epitope-tagged JAK2 (808 to 1132) (unknown origin) using LPLDKDYYVVREPGQ as substrate by beta-countiing analysis in presenc... | Bioorg Med Chem 23: 2387-407 (2015) Article DOI: 10.1016/j.bmc.2015.03.059 BindingDB Entry DOI: 10.7270/Q2Q52RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50497795 (CHEMBL3330129) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of recombinant human JAK2 (808-1132 residues) using LPLDKDYYVVREPGQ as substrate by radiometric assay in presence of [33P]-gamma-ATP | Bioorg Med Chem 22: 4998-5012 (2014) Article DOI: 10.1016/j.bmc.2014.06.025 BindingDB Entry DOI: 10.7270/Q28G8PP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50177884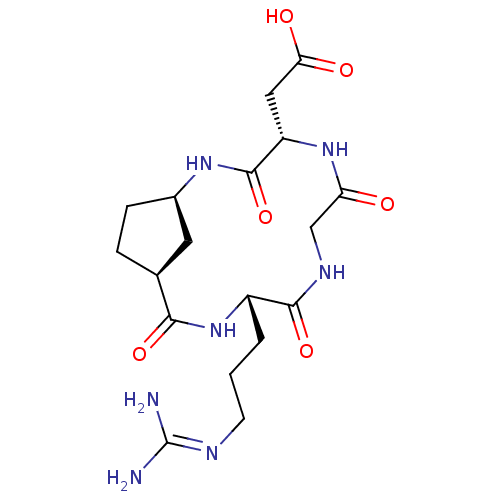 (CHEMBL534934 | c-[-Arg-Gly-Asp-Acpca31-]) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50177878 (CHEMBL557157 | c[-Arg-Gly-Asp-Acpca36-]) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50177884 (CHEMBL534934 | c-[-Arg-Gly-Asp-Acpca31-]) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alphavbeta3 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50177878 (CHEMBL557157 | c[-Arg-Gly-Asp-Acpca36-]) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alphavbeta3 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50177885 (CHEMBL540622 | c-[-Arg-Gly-Asp-Acpca30-]) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50177883 (CHEMBL534713 | c[-Arg-Gly-Asp-Acpca35-]) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alphavbeta3 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50177885 (CHEMBL540622 | c-[-Arg-Gly-Asp-Acpca30-]) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alphavbeta3 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50497793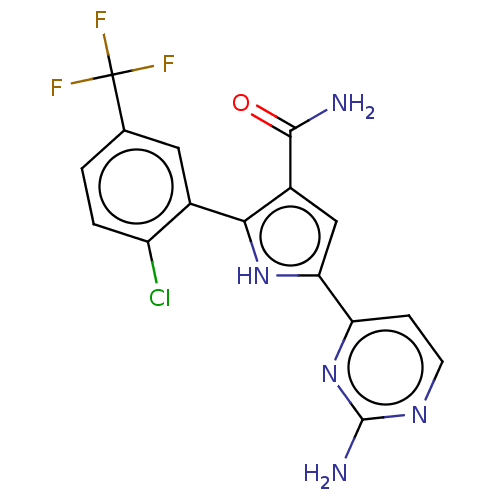 (CHEMBL3330125) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of recombinant human JAK2 (808-1132 residues) using LPLDKDYYVVREPGQ as substrate by radiometric assay in presence of [33P]-gamma-ATP | Bioorg Med Chem 22: 4998-5012 (2014) Article DOI: 10.1016/j.bmc.2014.06.025 BindingDB Entry DOI: 10.7270/Q28G8PP6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50069324 (CHEMBL3403543) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of recombinant epitope-tagged JAK2 (808 to 1132) (unknown origin) using LPLDKDYYVVREPGQ as substrate by beta-countiing analysis in presenc... | Bioorg Med Chem 23: 2387-407 (2015) Article DOI: 10.1016/j.bmc.2015.03.059 BindingDB Entry DOI: 10.7270/Q2Q52RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50069310 (CHEMBL3403552) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of recombinant epitope-tagged JAK2 (808 to 1132) (unknown origin) using LPLDKDYYVVREPGQ as substrate by beta-countiing analysis in presenc... | Bioorg Med Chem 23: 2387-407 (2015) Article DOI: 10.1016/j.bmc.2015.03.059 BindingDB Entry DOI: 10.7270/Q2Q52RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50177881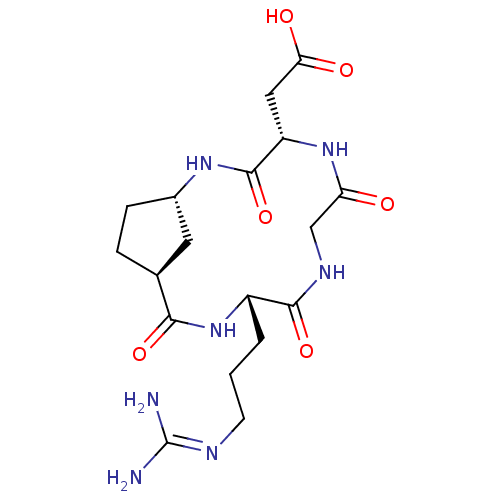 (CHEMBL534712 | c-[-Arg-Gly-Asp-Acpca33-]) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alphavbeta3 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50497783 (CHEMBL3330124) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of recombinant human JAK2 (808-1132 residues) using LPLDKDYYVVREPGQ as substrate by radiometric assay in presence of [33P]-gamma-ATP | Bioorg Med Chem 22: 4998-5012 (2014) Article DOI: 10.1016/j.bmc.2014.06.025 BindingDB Entry DOI: 10.7270/Q28G8PP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50069323 (CHEMBL3403544) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of recombinant epitope-tagged JAK2 (808 to 1132) (unknown origin) using LPLDKDYYVVREPGQ as substrate by beta-countiing analysis in presenc... | Bioorg Med Chem 23: 2387-407 (2015) Article DOI: 10.1016/j.bmc.2015.03.059 BindingDB Entry DOI: 10.7270/Q2Q52RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50497799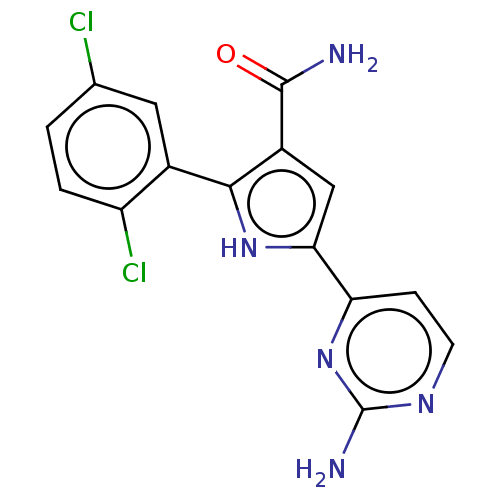 (CHEMBL3330122) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of recombinant human JAK2 (808-1132 residues) using LPLDKDYYVVREPGQ as substrate by radiometric assay in presence of [33P]-gamma-ATP | Bioorg Med Chem 22: 4998-5012 (2014) Article DOI: 10.1016/j.bmc.2014.06.025 BindingDB Entry DOI: 10.7270/Q28G8PP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50177883 (CHEMBL534713 | c[-Arg-Gly-Asp-Acpca35-]) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 [540-1115] (Homo sapiens (Human)) | BDBM482228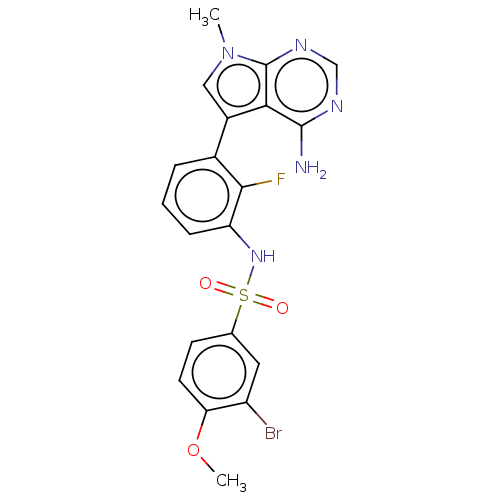 (N-[3-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C2518M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 [540-1115] (Homo sapiens (Human)) | BDBM482163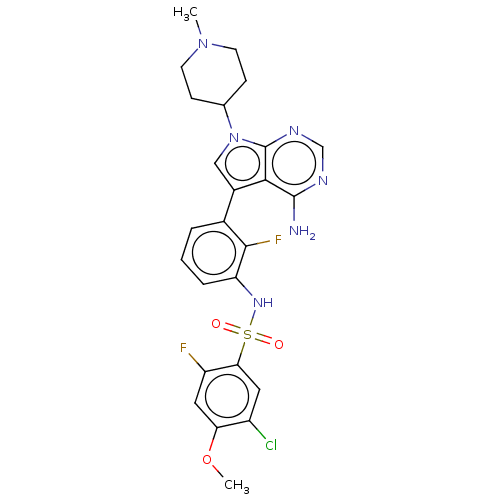 (N-[3-(4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C2518M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 [540-1115] (Homo sapiens (Human)) | BDBM482162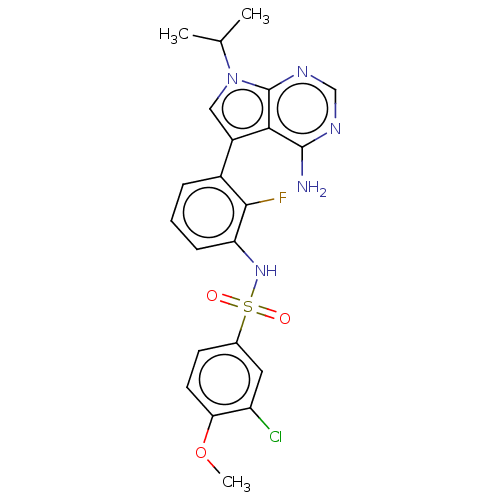 (N-[3-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C2518M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482162 (N-[3-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482163 (N-[3-(4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482164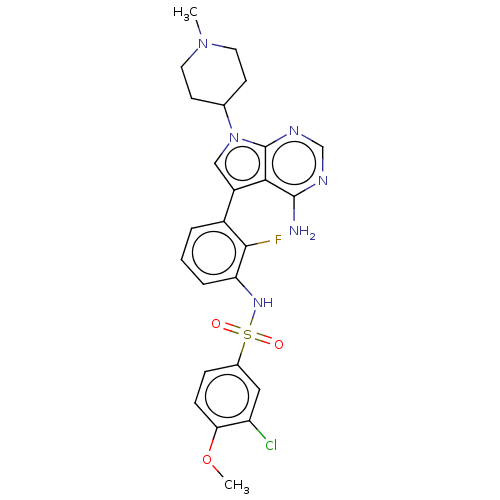 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482166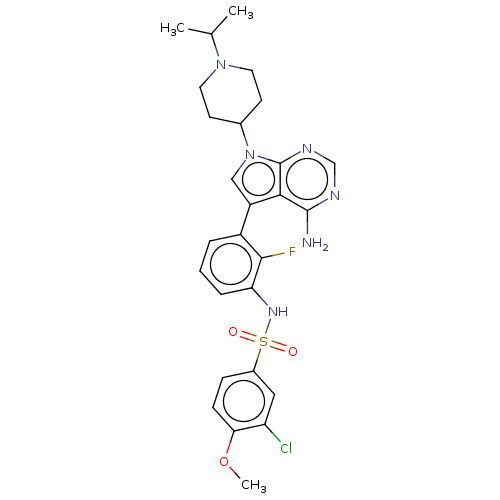 (N-{3-[4-Amino-7-(1-isopropyl-piperidin-4-yl)-7H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482167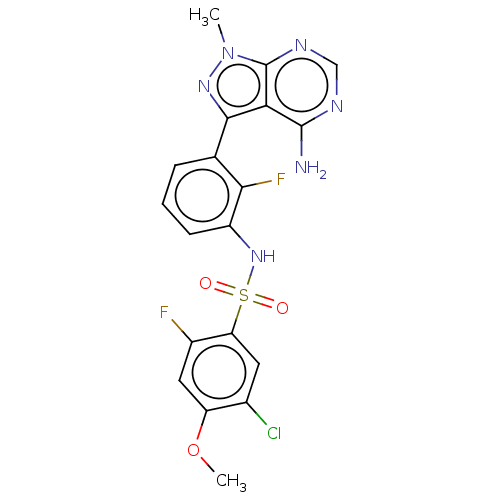 (N-[3-(4-Amino-1-methyl-1H-pyrazolo[3,4-d]pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482171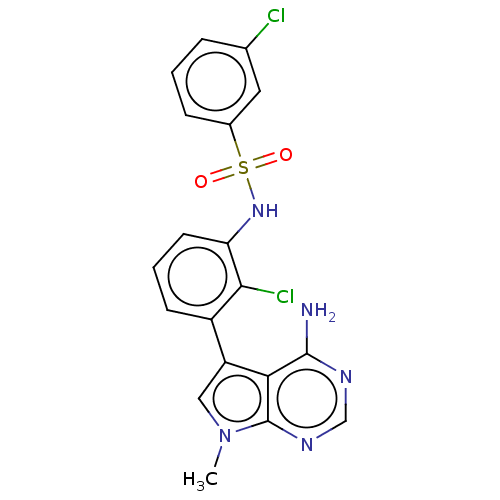 (N-[3-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482183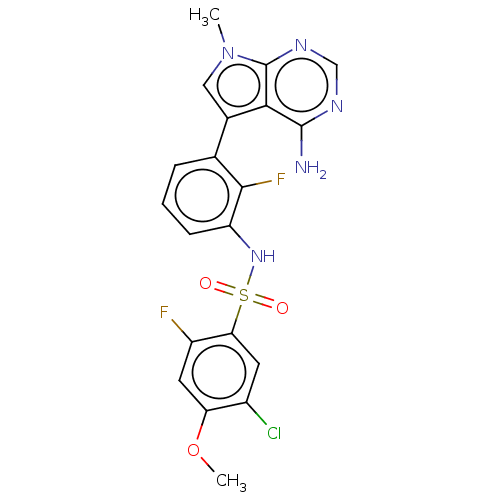 (N-[3-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482186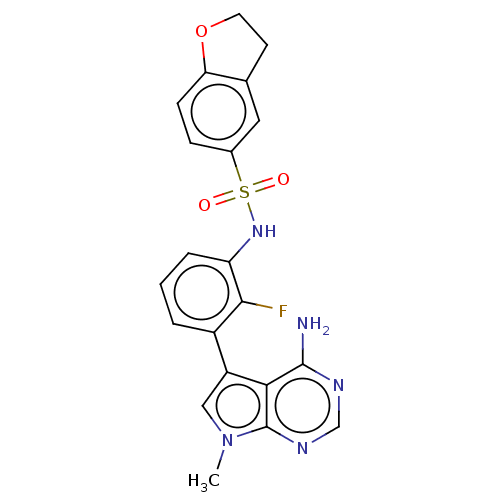 (2,3-Dihydro-benzofuran-5-sulfonic acid [3-(4-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482193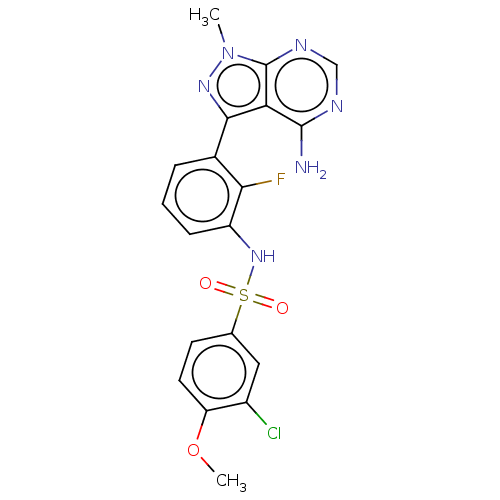 (N-[3-(4-Amino-1-methyl-1H-pyrazolo[3,4-d]pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482196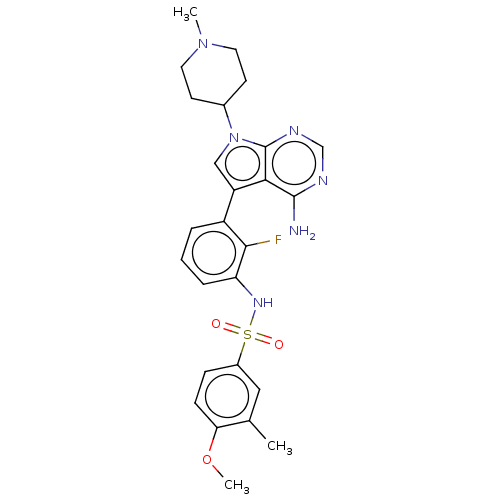 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482200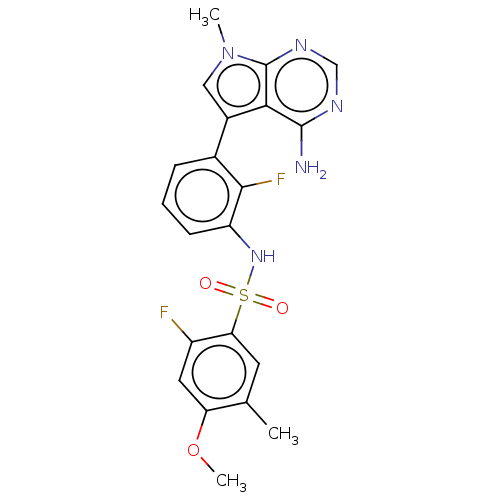 (N-[3-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482201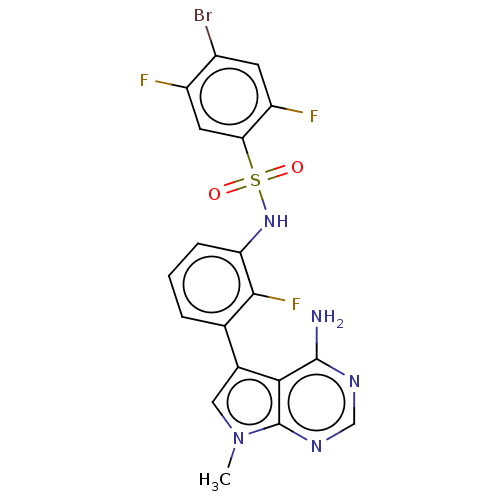 (N-[3-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482207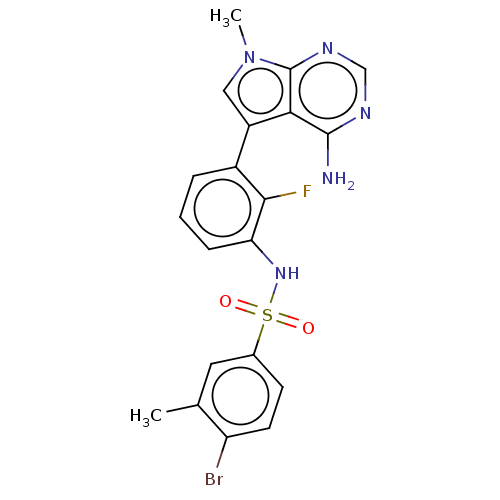 (N-[3-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482217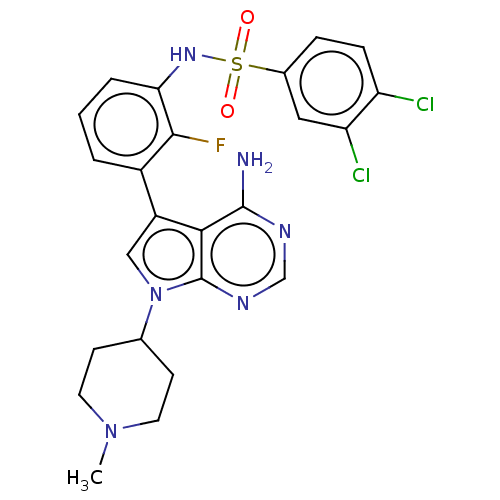 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482219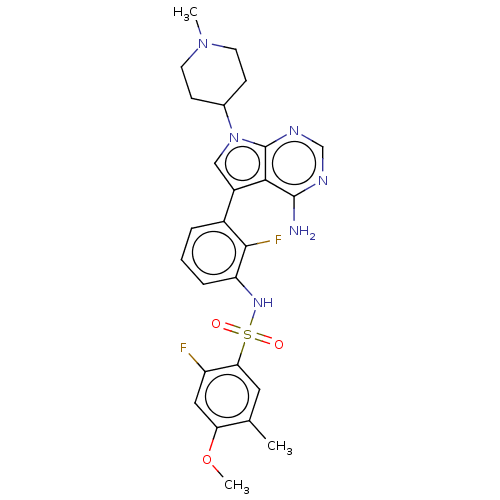 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 [540-1115] (Homo sapiens (Human)) | BDBM482219 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C2518M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 [540-1115] (Homo sapiens (Human)) | BDBM482217 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C2518M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 [540-1115] (Homo sapiens (Human)) | BDBM482207 (N-[3-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C2518M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 409 total ) | Next | Last >> |