 Found 678 hits with Last Name = 'murphy' and Initial = 'd'
Found 678 hits with Last Name = 'murphy' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(MOUSE) | BDBM86422

(CAS_61869-08-7 | NSC_43815 | PAROXETINE | US987368...)Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50004927

(4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...)Show InChI InChI=1S/C7H14NO5P/c9-7(10)6-3-5(1-2-8-6)4-14(11,12)13/h5-6,8H,1-4H2,(H,9,10)(H2,11,12,13)/t5-,6+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002360

((CPP)4-(3-Phosphono-propyl)-piperazine-2-carboxyli...)Show InChI InChI=1S/C8H17N2O5P/c11-8(12)7-6-10(4-2-9-7)3-1-5-16(13,14)15/h7,9H,1-6H2,(H,11,12)(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002360

((CPP)4-(3-Phosphono-propyl)-piperazine-2-carboxyli...)Show InChI InChI=1S/C8H17N2O5P/c11-8(12)7-6-10(4-2-9-7)3-1-5-16(13,14)15/h7,9H,1-6H2,(H,11,12)(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM81977

(CAS_79055-68-8 | CB3371309 | D-AP5 | D-APV)Show InChI InChI=1S/C5H12NO5P/c6-4(5(7)8)2-1-3-12(9,10)11/h4H,1-3,6H2,(H,7,8)(H2,9,10,11)/t4-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM81977

(CAS_79055-68-8 | CB3371309 | D-AP5 | D-APV)Show InChI InChI=1S/C5H12NO5P/c6-4(5(7)8)2-1-3-12(9,10)11/h4H,1-3,6H2,(H,7,8)(H2,9,10,11)/t4-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM31174
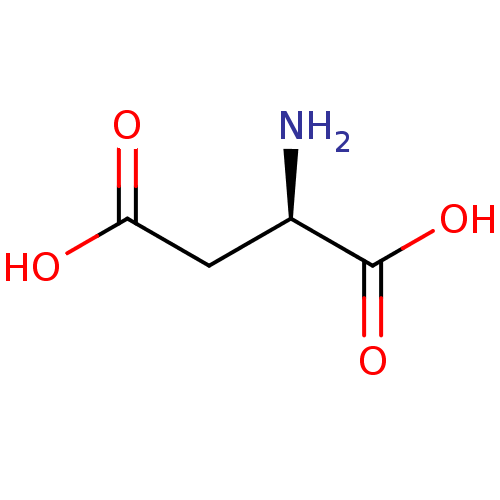
(CHEMBL29757 | D-Aspartate | D-Aspartic Acid)Show InChI InChI=1S/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)/t2-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM31174

(CHEMBL29757 | D-Aspartate | D-Aspartic Acid)Show InChI InChI=1S/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)/t2-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002363
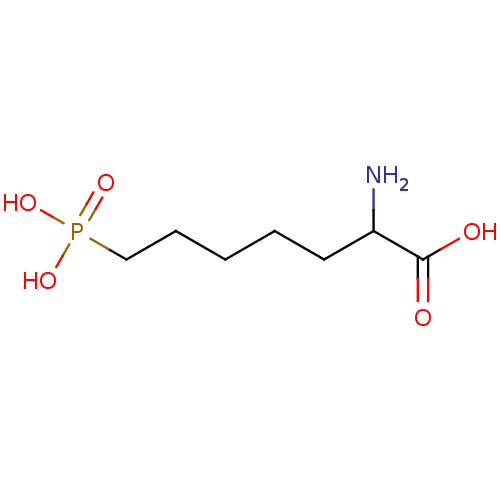
((APH)2-Amino-7-phosphono-heptanoic acid | 2-Amino-...)Show InChI InChI=1S/C7H16NO5P/c8-6(7(9)10)4-2-1-3-5-14(11,12)13/h6H,1-5,8H2,(H,9,10)(H2,11,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002363

((APH)2-Amino-7-phosphono-heptanoic acid | 2-Amino-...)Show InChI InChI=1S/C7H16NO5P/c8-6(7(9)10)4-2-1-3-5-14(11,12)13/h6H,1-5,8H2,(H,9,10)(H2,11,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM18125
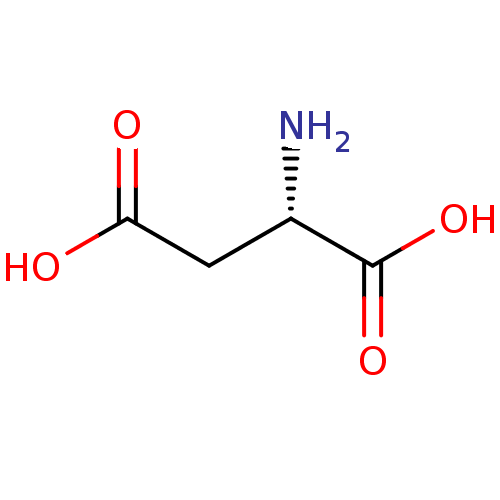
((2S)-2-aminobutanedioic acid | Aspartate | CHEMBL2...)Show InChI InChI=1S/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)/t2-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM18125

((2S)-2-aminobutanedioic acid | Aspartate | CHEMBL2...)Show InChI InChI=1S/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)/t2-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002343
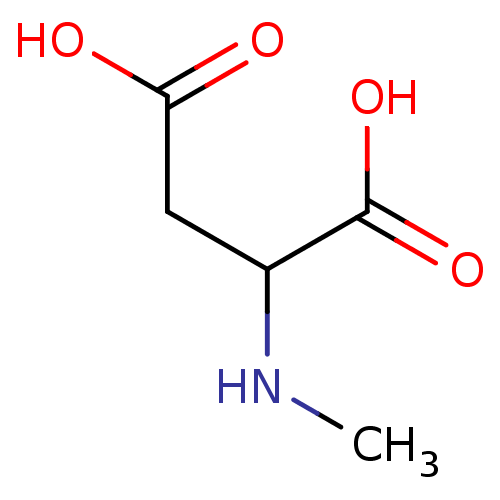
(2-Methylamino-succinic acid | CHEMBL275325 | NMDA)Show InChI InChI=1S/C5H9NO4/c1-6-3(5(9)10)2-4(7)8/h3,6H,2H2,1H3,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002343

(2-Methylamino-succinic acid | CHEMBL275325 | NMDA)Show InChI InChI=1S/C5H9NO4/c1-6-3(5(9)10)2-4(7)8/h3,6H,2H2,1H3,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(MOUSE) | BDBM22417
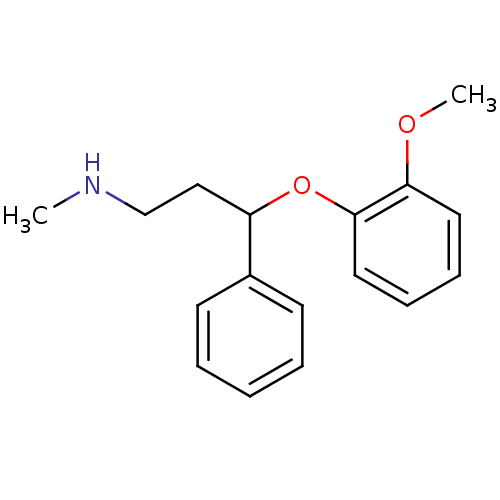
(3-(2-methoxyphenoxy)-N-methyl-3-phenylpropan-1-ami...)Show InChI InChI=1S/C17H21NO2/c1-18-13-12-15(14-8-4-3-5-9-14)20-17-11-7-6-10-16(17)19-2/h3-11,15,18H,12-13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(MOUSE) | BDBM84738
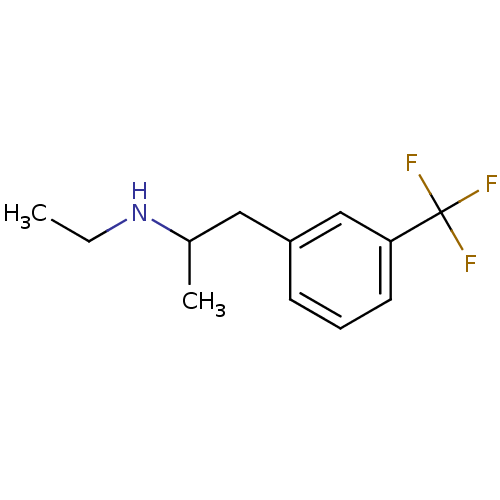
(CAS_16105-77-4 | Fenfluramine | Fenfluramine (+) |...)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM26431
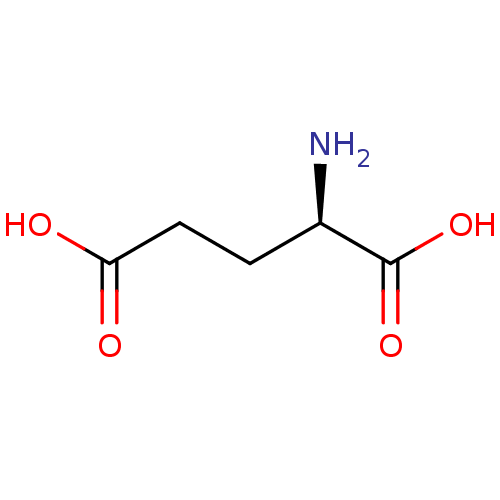
((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(MOUSE) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM26431

((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM17660
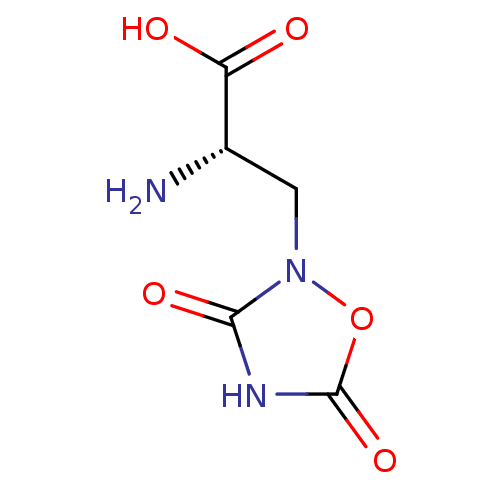
((2S)-2-amino-3-(3,5-dioxo-1,2,4-oxadiazolidin-2-yl...)Show InChI InChI=1S/C5H7N3O5/c6-2(3(9)10)1-8-4(11)7-5(12)13-8/h2H,1,6H2,(H,9,10)(H,7,11,12)/t2-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM17660

((2S)-2-amino-3-(3,5-dioxo-1,2,4-oxadiazolidin-2-yl...)Show InChI InChI=1S/C5H7N3O5/c6-2(3(9)10)1-8-4(11)7-5(12)13-8/h2H,1,6H2,(H,9,10)(H,7,11,12)/t2-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM81975
(4-hydroxyquinoline-2-carboxylic acid | CAS_492-27-...)Show InChI InChI=1S/C10H7NO3/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9/h1-5H,(H,11,12)(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(MOUSE) | BDBM22167
(1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)pip...)Show SMILES C(CN1CCN(CCOC(c2ccccc2)c2ccccc2)CC1)Cc1ccccc1 Show InChI InChI=1S/C28H34N2O/c1-4-11-25(12-5-1)13-10-18-29-19-21-30(22-20-29)23-24-31-28(26-14-6-2-7-15-26)27-16-8-3-9-17-27/h1-9,11-12,14-17,28H,10,13,18-24H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Mus musculus (Mouse)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Mus musculus (Mouse)) | BDBM31005
(2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyraz...)Show InChI InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Mus musculus (Mouse)) | BDBM85098

(N-[5-[5-(2,4-Dioxo-1,3,8-triazaspiro[4.5]decan-8-y...)Show SMILES [#6]-[#8]-c1cc(-[#8]-[#6])c(cc1-[#7][S;v6](=O)(=O)c1ccc(cc1)C(F)(F)F)-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]C2([#6]-[#6]-1)[#7]-[#6](=O)-[#7]-[#6]2=O Show InChI InChI=1S/C27H31F3N4O7S/c1-40-22-16-23(41-2)20(33-42(38,39)18-8-6-17(7-9-18)27(28,29)30)15-19(22)21(35)5-3-4-12-34-13-10-26(11-14-34)24(36)31-25(37)32-26/h6-9,15-16,33H,3-5,10-14H2,1-2H3,(H2,31,32,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 308: 481-6 (2004)
Article DOI: 10.1124/jpet.103.058636
BindingDB Entry DOI: 10.7270/Q2QZ28J1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM81975

(4-hydroxyquinoline-2-carboxylic acid | CAS_492-27-...)Show InChI InChI=1S/C10H7NO3/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9/h1-5H,(H,11,12)(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM26116
(CHEMBL284104 | Dipicolinate | pyridine carboxylate...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-5(8-4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM26116

(CHEMBL284104 | Dipicolinate | pyridine carboxylate...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-5(8-4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM26115
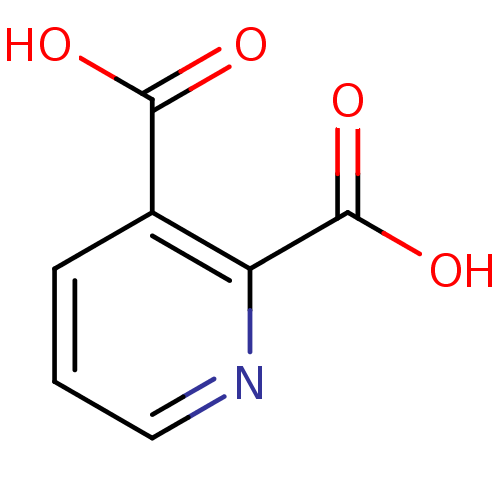
(CHEMBL286204 | Quinolinate | Quinolinic acid | pyr...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM81976
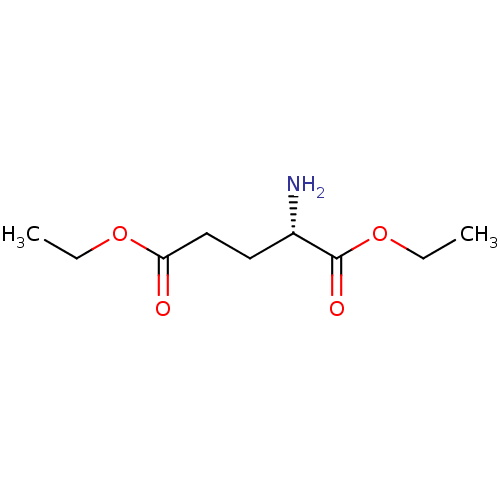
(CAS_16450-41-2 | L-GDEE | glutamic acid diethyl es...)Show InChI InChI=1S/C9H17NO4/c1-3-13-8(11)6-5-7(10)9(12)14-4-2/h7H,3-6,10H2,1-2H3/t7-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM81976

(CAS_16450-41-2 | L-GDEE | glutamic acid diethyl es...)Show InChI InChI=1S/C9H17NO4/c1-3-13-8(11)6-5-7(10)9(12)14-4-2/h7H,3-6,10H2,1-2H3/t7-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM26115

(CHEMBL286204 | Quinolinate | Quinolinic acid | pyr...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by PDSP Ki Database
| |
Br J Pharmacol 95: 932-8 (1988)
Article DOI: 10.1111/j.1476-5381.1988.tb11723.x
BindingDB Entry DOI: 10.7270/Q2Z036NF |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1/2
(Homo sapiens (Human)) | BDBM50263270
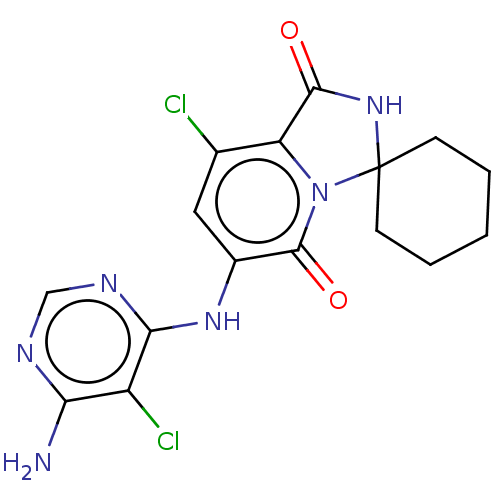
(CHEMBL4086747)Show SMILES Nc1ncnc(Nc2cc(Cl)c3C(=O)NC4(CCCCC4)n3c2=O)c1Cl Show InChI InChI=1S/C16H16Cl2N6O2/c17-8-6-9(22-13-10(18)12(19)20-7-21-13)15(26)24-11(8)14(25)23-16(24)4-2-1-3-5-16/h6-7H,1-5H2,(H,23,25)(H3,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of MNK1/2 in human HCT116 cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by HTRF assay |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50263254
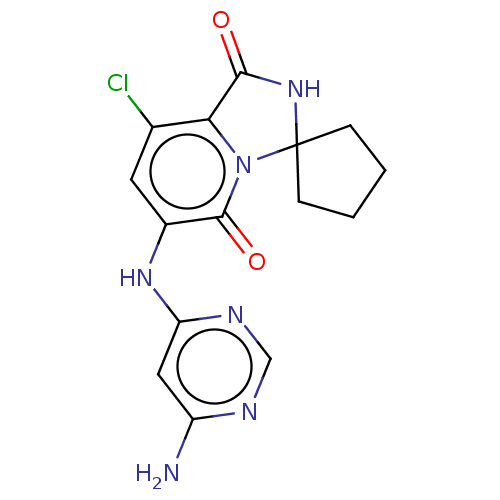
(CHEMBL4094975)Show SMILES Nc1cc(Nc2cc(Cl)c3C(=O)NC4(CCCC4)n3c2=O)ncn1 Show InChI InChI=1S/C15H15ClN6O2/c16-8-5-9(20-11-6-10(17)18-7-19-11)14(24)22-12(8)13(23)21-15(22)3-1-2-4-15/h5-7H,1-4H2,(H,21,23)(H3,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal GST-tagged recombinant human MNK2 expressed in baculovirus expression system using Ac-TATKSGSTTKNR-NH2 as substr... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50263253
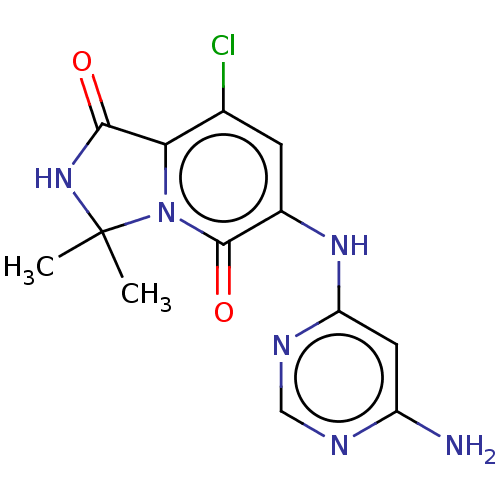
(CHEMBL4067557)Show SMILES CC1(C)NC(=O)c2c(Cl)cc(Nc3cc(N)ncn3)c(=O)n12 Show InChI InChI=1S/C13H13ClN6O2/c1-13(2)19-11(21)10-6(14)3-7(12(22)20(10)13)18-9-4-8(15)16-5-17-9/h3-5H,1-2H3,(H,19,21)(H3,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal GST-tagged recombinant human MNK2 expressed in baculovirus expression system using Ac-TATKSGSTTKNR-NH2 as substr... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1/2
(Homo sapiens (Human)) | BDBM168214
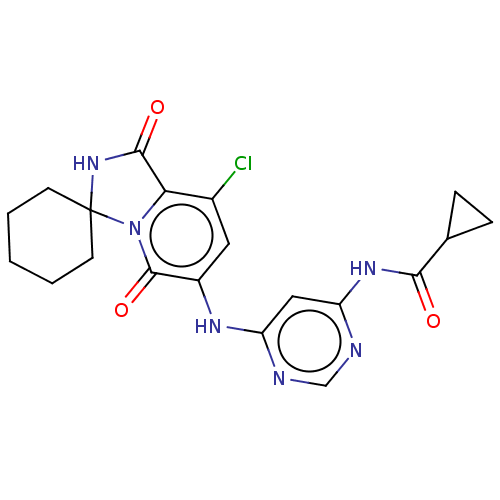
(US9669031, 207 6′-((6-aminopyrimidin-4-yl)am...)Show SMILES Clc1cc(Nc2cc(NC(=O)C3CC3)ncn2)c(=O)n2c1C(=O)NC21CCCCC1 Show InChI InChI=1S/C20H21ClN6O3/c21-12-8-13(24-14-9-15(23-10-22-14)25-17(28)11-4-5-11)19(30)27-16(12)18(29)26-20(27)6-2-1-3-7-20/h8-11H,1-7H2,(H,26,29)(H2,22,23,24,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of MNK1/2 in human HCT116 cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by HTRF assay |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50263270

(CHEMBL4086747)Show SMILES Nc1ncnc(Nc2cc(Cl)c3C(=O)NC4(CCCCC4)n3c2=O)c1Cl Show InChI InChI=1S/C16H16Cl2N6O2/c17-8-6-9(22-13-10(18)12(19)20-7-21-13)15(26)24-11(8)14(25)23-16(24)4-2-1-3-5-16/h6-7H,1-5H2,(H,23,25)(H3,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal GST-tagged recombinant human MNK2 expressed in baculovirus expression system using Ac-TATKSGSTTKNR-NH2 as substr... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Rattus norvegicus) | BDBM50122102
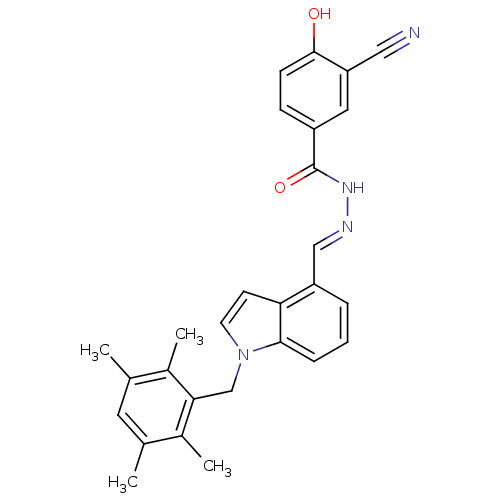
(3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...)Show SMILES Cc1cc(C)c(C)c(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(c4)C#N)cccc23)c1C Show InChI InChI=1S/C28H26N4O2/c1-17-12-18(2)20(4)25(19(17)3)16-32-11-10-24-22(6-5-7-26(24)32)15-30-31-28(34)21-8-9-27(33)23(13-21)14-29/h5-13,15,33H,16H2,1-4H3,(H,31,34)/b30-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound towards rat glucagon receptor |
J Med Chem 45: 5755-75 (2002)
BindingDB Entry DOI: 10.7270/Q26W99FD |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM168214

(US9669031, 207 6′-((6-aminopyrimidin-4-yl)am...)Show SMILES Clc1cc(Nc2cc(NC(=O)C3CC3)ncn2)c(=O)n2c1C(=O)NC21CCCCC1 Show InChI InChI=1S/C20H21ClN6O3/c21-12-8-13(24-14-9-15(23-10-22-14)25-17(28)11-4-5-11)19(30)27-16(12)18(29)26-20(27)6-2-1-3-7-20/h8-11H,1-7H2,(H,26,29)(H2,22,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged recombinant human MNK1 expressed in insect cells using Ac-TATKSGSTTKNR-NH2 as substrate preincubated for 5 mins ... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1/2
(Homo sapiens (Human)) | BDBM50263254

(CHEMBL4094975)Show SMILES Nc1cc(Nc2cc(Cl)c3C(=O)NC4(CCCC4)n3c2=O)ncn1 Show InChI InChI=1S/C15H15ClN6O2/c16-8-5-9(20-11-6-10(17)18-7-19-11)14(24)22-12(8)13(23)21-15(22)3-1-2-4-15/h5-7H,1-4H2,(H,21,23)(H3,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of MNK1/2 in human HCT116 cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by HTRF assay |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50263269
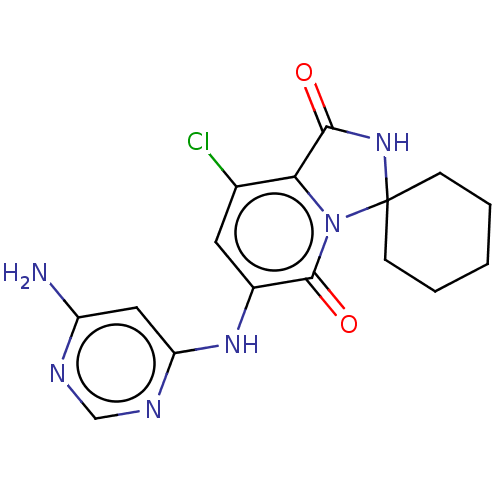
(CHEMBL4076439)Show SMILES Nc1cc(Nc2cc(Cl)c3C(=O)NC4(CCCCC4)n3c2=O)ncn1 Show InChI InChI=1S/C16H17ClN6O2/c17-9-6-10(21-12-7-11(18)19-8-20-12)15(25)23-13(9)14(24)22-16(23)4-2-1-3-5-16/h6-8H,1-5H2,(H,22,24)(H3,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged recombinant human MNK1 expressed in insect cells using Ac-TATKSGSTTKNR-NH2 as substrate preincubated for 5 mins ... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50263269

(CHEMBL4076439)Show SMILES Nc1cc(Nc2cc(Cl)c3C(=O)NC4(CCCCC4)n3c2=O)ncn1 Show InChI InChI=1S/C16H17ClN6O2/c17-9-6-10(21-12-7-11(18)19-8-20-12)15(25)23-13(9)14(24)22-16(23)4-2-1-3-5-16/h6-8H,1-5H2,(H,22,24)(H3,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal GST-tagged recombinant human MNK2 expressed in baculovirus expression system using Ac-TATKSGSTTKNR-NH2 as substr... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1/2
(Homo sapiens (Human)) | BDBM50263269

(CHEMBL4076439)Show SMILES Nc1cc(Nc2cc(Cl)c3C(=O)NC4(CCCCC4)n3c2=O)ncn1 Show InChI InChI=1S/C16H17ClN6O2/c17-9-6-10(21-12-7-11(18)19-8-20-12)15(25)23-13(9)14(24)22-16(23)4-2-1-3-5-16/h6-8H,1-5H2,(H,22,24)(H3,18,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of MNK1/2 in human HCT116 cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by HTRF assay |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50263254

(CHEMBL4094975)Show SMILES Nc1cc(Nc2cc(Cl)c3C(=O)NC4(CCCC4)n3c2=O)ncn1 Show InChI InChI=1S/C15H15ClN6O2/c16-8-5-9(20-11-6-10(17)18-7-19-11)14(24)22-12(8)13(23)21-15(22)3-1-2-4-15/h5-7H,1-4H2,(H,21,23)(H3,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged recombinant human MNK1 expressed in insect cells using Ac-TATKSGSTTKNR-NH2 as substrate preincubated for 5 mins ... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Rattus norvegicus) | BDBM50110054

(3-Cyano-4-hydroxy-benzoic acid [1-[4-(4-isopropyl-...)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(c2)C#N)cc(OC)c1OCc1ccc(cc1)C(C)C Show InChI InChI=1S/C27H27N3O5/c1-17(2)20-7-5-18(6-8-20)16-35-26-24(33-3)11-19(12-25(26)34-4)15-29-30-27(32)21-9-10-23(31)22(13-21)14-28/h5-13,15,17,31H,16H2,1-4H3,(H,30,32)/b29-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity against rat glucagon receptor using [127I]-labeled glucagon |
Bioorg Med Chem Lett 12: 663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2J67G77 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50263263

(CHEMBL4073443)Show SMILES Cc1cc(Nc2cc(N)ncn2)c(=O)n2c1C(=O)NC21CCCCC1 Show InChI InChI=1S/C17H20N6O2/c1-10-7-11(21-13-8-12(18)19-9-20-13)16(25)23-14(10)15(24)22-17(23)5-3-2-4-6-17/h7-9H,2-6H2,1H3,(H,22,24)(H3,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal GST-tagged recombinant human MNK2 expressed in baculovirus expression system using Ac-TATKSGSTTKNR-NH2 as substr... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50263255

(CHEMBL4065802)Show SMILES Cc1cc(Nc2cc(N)ncn2)c(=O)n2c1C(=O)NC21CCC(F)(F)CC1 Show InChI InChI=1S/C17H18F2N6O2/c1-9-6-10(23-12-7-11(20)21-8-22-12)15(27)25-13(9)14(26)24-17(25)4-2-16(18,19)3-5-17/h6-8H,2-5H2,1H3,(H,24,26)(H3,20,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
eFFECTOR Therapeutics , 11180 Roselle Street , San Diego , California 92121 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal GST-tagged recombinant human MNK2 expressed in baculovirus expression system using Ac-TATKSGSTTKNR-NH2 as substr... |
J Med Chem 61: 3516-3540 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01795
BindingDB Entry DOI: 10.7270/Q2NC63PS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data