

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonstructural protein 3 (Zika virus) | BDBM50212337 (CHEMBL5267698) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128629 BindingDB Entry DOI: 10.7270/Q2S186JS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50212337 (CHEMBL5267698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50587014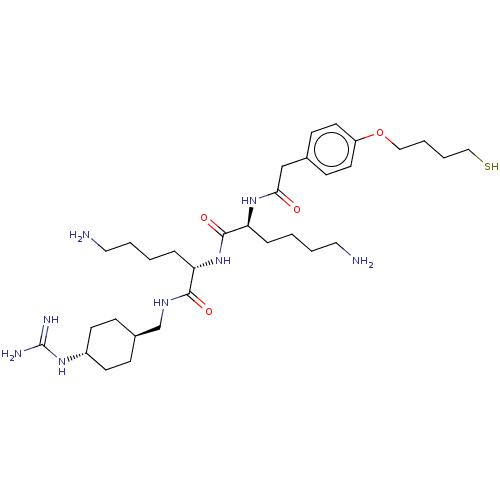 (CHEMBL5094815) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal His-tagged ZIKV NSB2 (52 to 96 amino acids)-NS3 (1 to 184 amino acids) protease expressed in Escherichia coli BL... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00515 BindingDB Entry DOI: 10.7270/Q21N8511 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50030459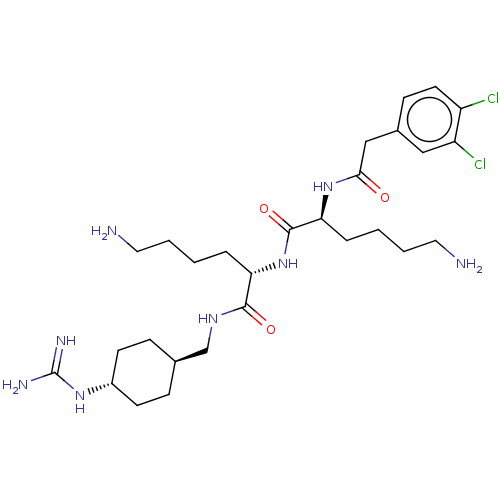 (CHEMBL3344321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM13142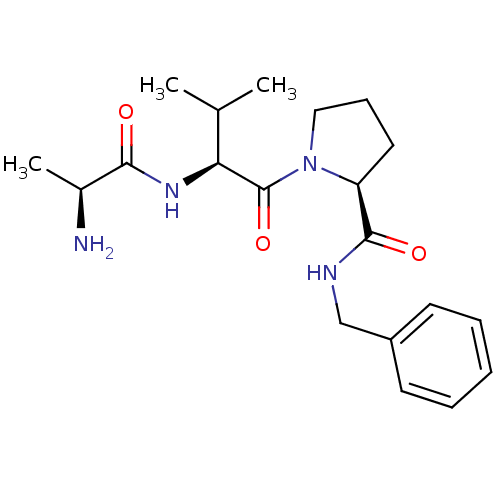 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Inhibition of Zika virus NS2B-NS3 protease | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126965 BindingDB Entry DOI: 10.7270/Q27M0CGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus) | BDBM50470562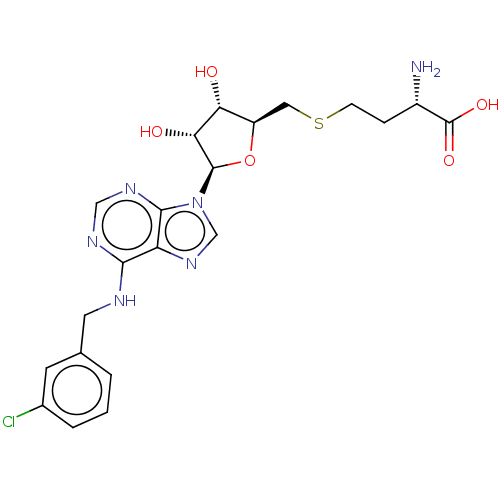 (CHEMBL1230055) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of N-terminal GST tagged recombinant Dengue virus 3 2'-O methyltransferase expressed in Escherichia coli BL21 cells by scintillation count... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50587014 (CHEMBL5094815) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal His-tagged WNV NSB2 (52 to 96 amino acids)-NS3 (1 to 184 amino acids) protease expressed in Escherichia coli BL2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00515 BindingDB Entry DOI: 10.7270/Q21N8511 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50212336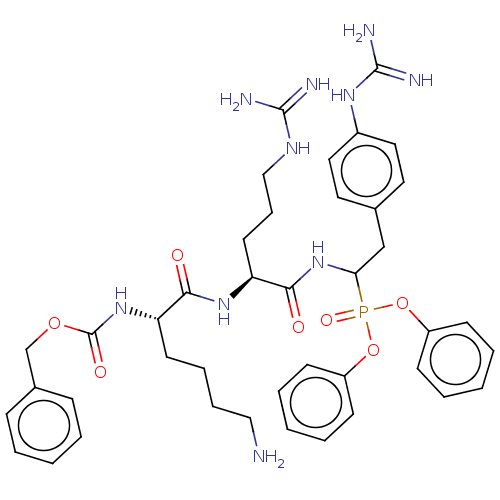 (CHEMBL5282745) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM32580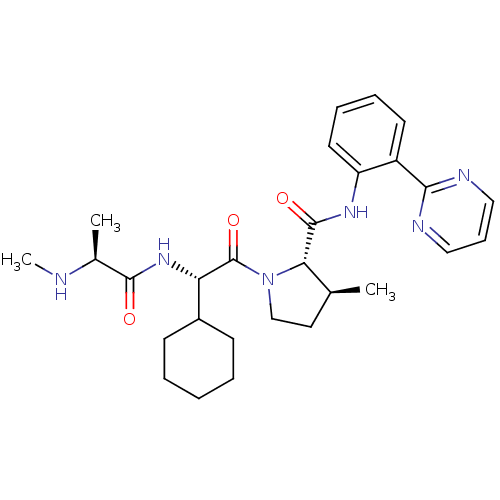 (CS-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Inhibition of Zika virus NS2B-NS3 protease | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126965 BindingDB Entry DOI: 10.7270/Q27M0CGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50508772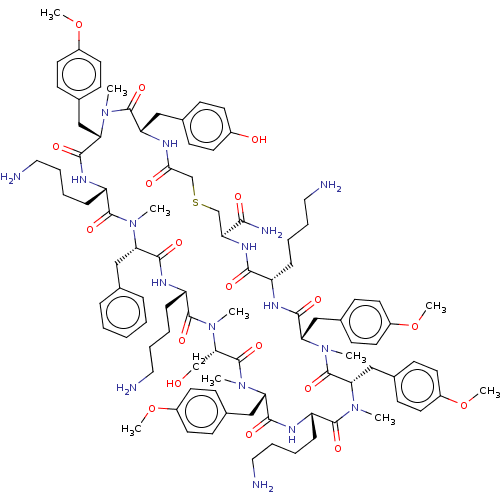 (CHEMBL4452943) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Non-competitive inhibition of C-terminal His6-tagged linked Zika virus NS2B-NS3 protease domain expressed in Escherichia coli Bl21(DE3) using Bz-Nle-... | ACS Med Chem Lett 10: 168-174 (2019) Article DOI: 10.1021/acsmedchemlett.8b00535 BindingDB Entry DOI: 10.7270/Q2N019TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50179360 (CHEMBL3040216) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Non-competitive inhibition of Dengue virus 4 NS3 helicase | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50179360 (CHEMBL3040216) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Non-competitive inhibition of Dengue virus 3 NS3 helicase | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50508769 (CHEMBL4483351) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Non-competitive inhibition of C-terminal His6-tagged linked Zika virus NS2B-NS3 protease domain expressed in Escherichia coli Bl21(DE3) using Bz-Nle-... | ACS Med Chem Lett 10: 168-174 (2019) Article DOI: 10.1021/acsmedchemlett.8b00535 BindingDB Entry DOI: 10.7270/Q2N019TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50450046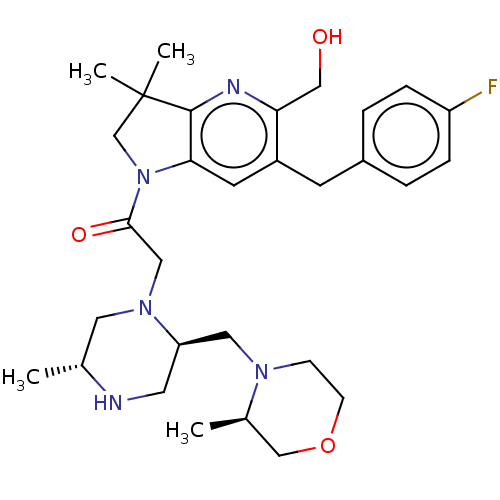 (CHEMBL4173974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Inhibition of Zika virus NS2B-NS3 protease | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126965 BindingDB Entry DOI: 10.7270/Q27M0CGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109138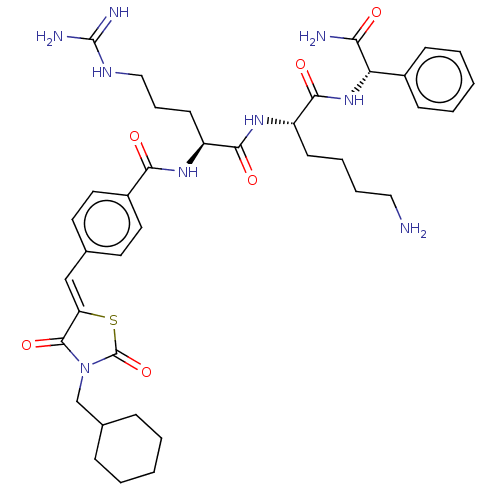 (CHEMBL3601351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109138 (CHEMBL3601351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Competitive inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50508771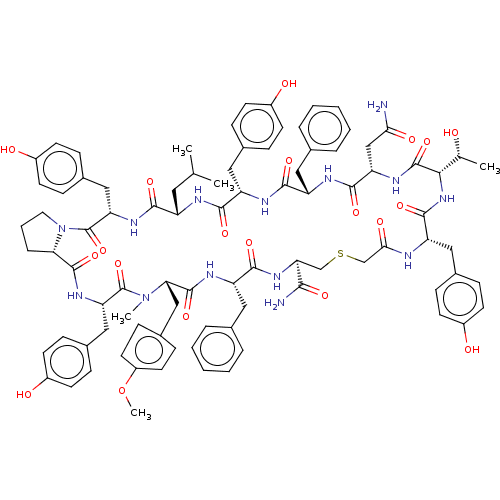 (CHEMBL4555617) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Non-competitive inhibition of C-terminal His6-tagged linked Zika virus NS2B-NS3 protease domain expressed in Escherichia coli Bl21(DE3) using Bz-Nle-... | ACS Med Chem Lett 10: 168-174 (2019) Article DOI: 10.1021/acsmedchemlett.8b00535 BindingDB Entry DOI: 10.7270/Q2N019TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50538477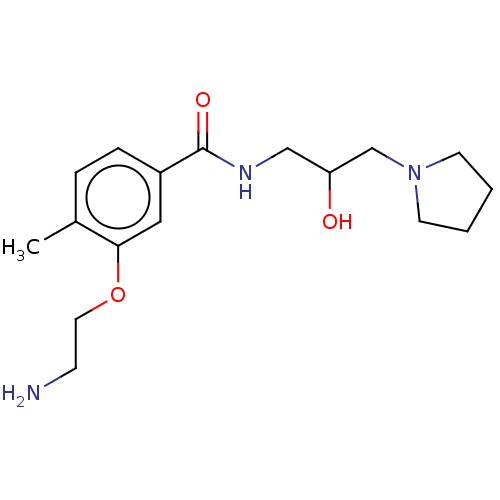 (CHEMBL4633138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of Zika virus NS2B-NS3 protease using Bz-Nle-Lys-Lys-Arg-AMC as substrate preincubated for 10 mins followed by substrate addition and meas... | ACS Med Chem Lett 11: 514-520 (2020) Article DOI: 10.1021/acsmedchemlett.9b00629 BindingDB Entry DOI: 10.7270/Q2TT4VGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50212339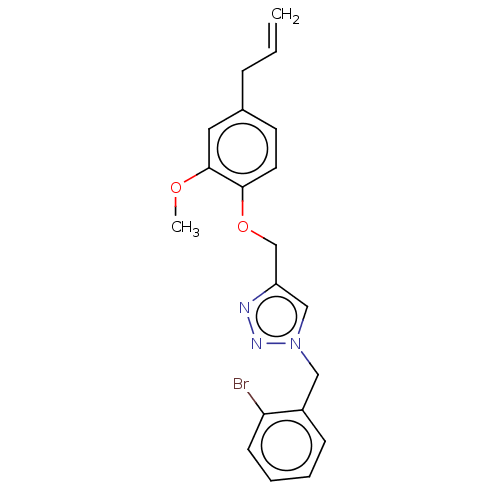 (CHEMBL4173093) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50508774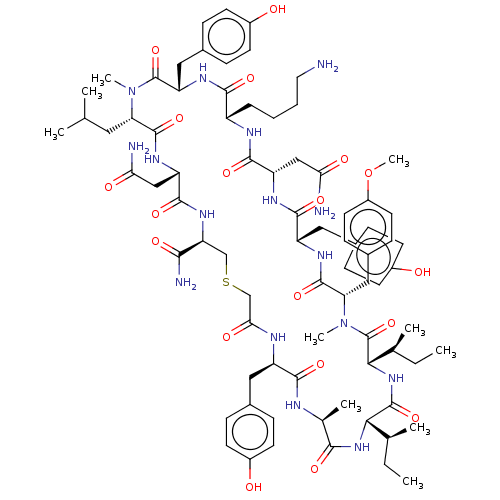 (CHEMBL4538618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Non-competitive inhibition of C-terminal His6-tagged linked Zika virus NS2B-NS3 protease domain expressed in Escherichia coli Bl21(DE3) using Bz-Nle-... | ACS Med Chem Lett 10: 168-174 (2019) Article DOI: 10.1021/acsmedchemlett.8b00535 BindingDB Entry DOI: 10.7270/Q2N019TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50030464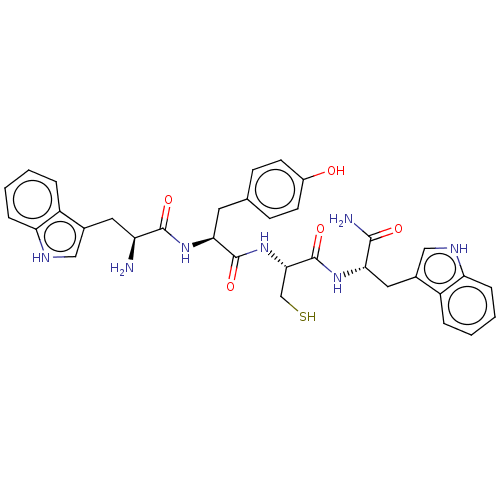 (CHEMBL3344310) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of Dengue virus 1 NS2B-NS3 protease expressed in Escherichia coli using Abz-RRRRSAG-nY-NH2 as substrate preincubated for 20 mins followed ... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50212340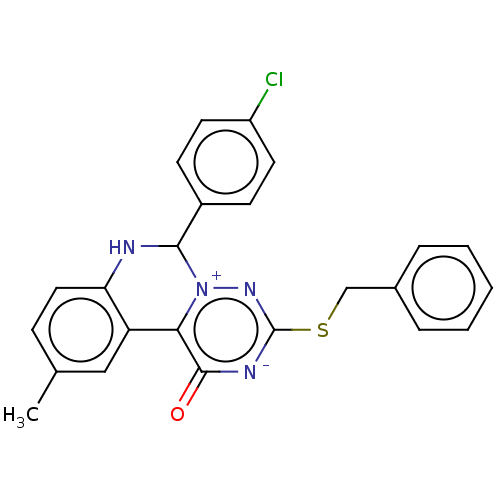 (CHEMBL5276951) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50030464 (CHEMBL3344310) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of Dengue virus 2 NS2B-NS3 protease expressed in Escherichia coli using Abz-RRRRSAG-nY-NH2 as substrate preincubated for 20 mins followed ... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50026808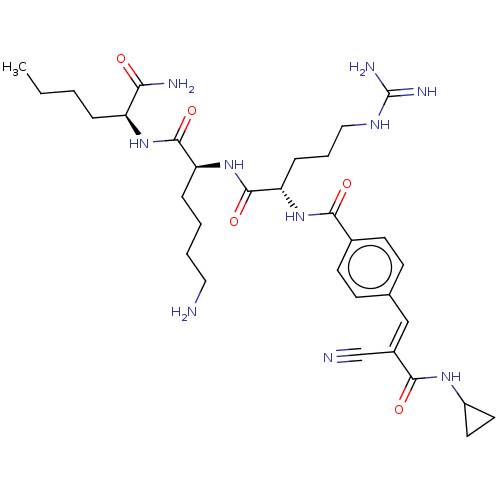 (CHEMBL2440341) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of Dengue virus 2 NS2B-NS3 protease using Abz-Nle-Lys-Arg-Arg-Ser-3-(NO2)Tyr as substrate preincubated for 15 mins followed by addition of... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50534351 (CHEMBL4531266) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Mixed noncompetitive inhibition of the Dengue virus 2 NS2B-NS3 protease expressed in Escherichia coli using Boc-GRR-AMC as substrate assessed as enzy... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50538478 (CHEMBL4634219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged West Nile virus NS2B (52 to 96 residues)-G4SG4-NS3 (1 to 184 residues) expressed in Escherichia coli BL21(DE3) c... | ACS Med Chem Lett 11: 514-520 (2020) Article DOI: 10.1021/acsmedchemlett.9b00629 BindingDB Entry DOI: 10.7270/Q2TT4VGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50030464 (CHEMBL3344310) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of Dengue virus 4 NS2B-NS3 protease expressed in Escherichia coli using Abz-RRRRSAG-nY-NH2 as substrate preincubated for 20 mins followed ... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50538478 (CHEMBL4634219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of Zika virus NS2B-NS3 protease using Bz-Nle-Lys-Lys-Arg-AMC as substrate preincubated for 10 mins followed by substrate addition and meas... | ACS Med Chem Lett 11: 514-520 (2020) Article DOI: 10.1021/acsmedchemlett.9b00629 BindingDB Entry DOI: 10.7270/Q2TT4VGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50513887 (CHEMBL4458555) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of recombinant Dengue virus type 2 NS2B-NS3 protease expressed in Escherichia coli C41 cells using GRR-AMC as substrate after 15 mins by f... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50587014 (CHEMBL5094815) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of recombinant N-terminal His-tagged DENV NS2B-NS3 protease expressed in Escherichia coli BL21(DE3) using Boc-GRR-AMC as subst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00515 BindingDB Entry DOI: 10.7270/Q21N8511 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50393505 (CHEMBL2158051) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Zika virus NS2B-NS3 protease (1 to 187 residues) using Boc-Gly-Arg-Arg-AMC as substrate | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126965 BindingDB Entry DOI: 10.7270/Q27M0CGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50534351 (CHEMBL4531266) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Mixed noncompetitive inhibition of the Dengue virus 2 NS2B-NS3 protease expressed in Escherichia coli using Boc-GRR-AMC as substrate assessed as enzy... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50026784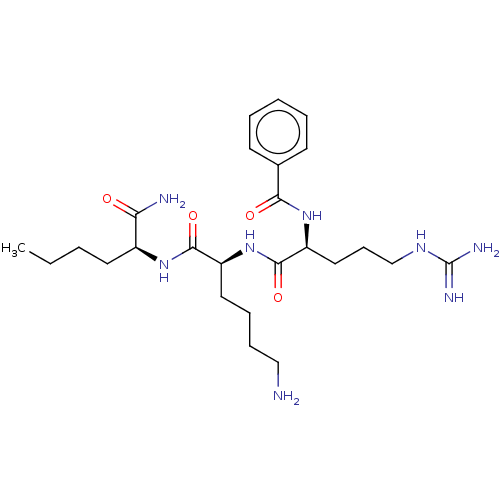 (CHEMBL2440339) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of Dengue virus 2 NS2B-NS3 protease using Abz-Nle-Lys-Arg-Arg-Ser-3-(NO2)Tyr as substrate preincubated for 15 mins followed by addition of... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50508770 (CHEMBL4454475) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Non-competitive inhibition of C-terminal His6-tagged linked Zika virus NS2B-NS3 protease domain expressed in Escherichia coli Bl21(DE3) using Bz-Nle-... | ACS Med Chem Lett 10: 168-174 (2019) Article DOI: 10.1021/acsmedchemlett.8b00535 BindingDB Entry DOI: 10.7270/Q2N019TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50359734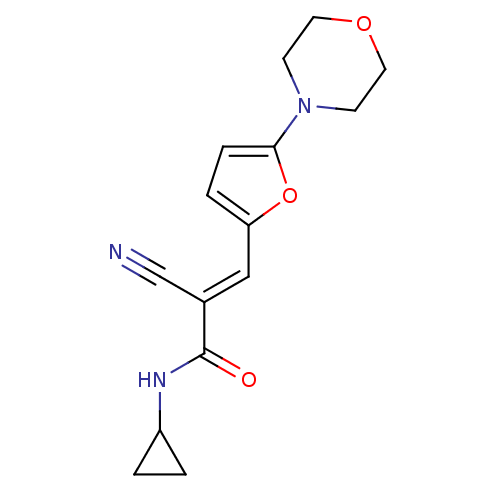 (CHEMBL1927522) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Heidelberg Curated by ChEMBL | Assay Description Inhibition of human thrombin using Boc-VPR-AMC as substrate compound preincubated for 15 mins before substrate addition measured up to 10 mins by spe... | Bioorg Med Chem 19: 7318-37 (2011) Article DOI: 10.1016/j.bmc.2011.10.061 BindingDB Entry DOI: 10.7270/Q2474B8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50030464 (CHEMBL3344310) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of Dengue virus 3 NS2B-NS3 protease expressed in Escherichia coli using Abz-RRRRSAG-nY-NH2 as substrate preincubated for 20 mins followed ... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50538477 (CHEMBL4633138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged West Nile virus NS2B (52 to 96 residues)-G4SG4-NS3 (1 to 184 residues) expressed in Escherichia coli BL21(DE3) c... | ACS Med Chem Lett 11: 514-520 (2020) Article DOI: 10.1021/acsmedchemlett.9b00629 BindingDB Entry DOI: 10.7270/Q2TT4VGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50026765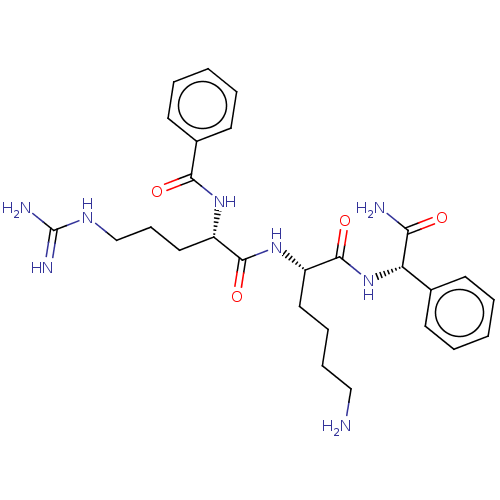 (CHEMBL3335494) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of West Nile virus serotype 2 NS2B-NS3 protease by homogeneous fluorimetric assay | ACS Med Chem Lett 5: 1037-42 (2014) Article DOI: 10.1021/ml500245v BindingDB Entry DOI: 10.7270/Q2028T42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50567201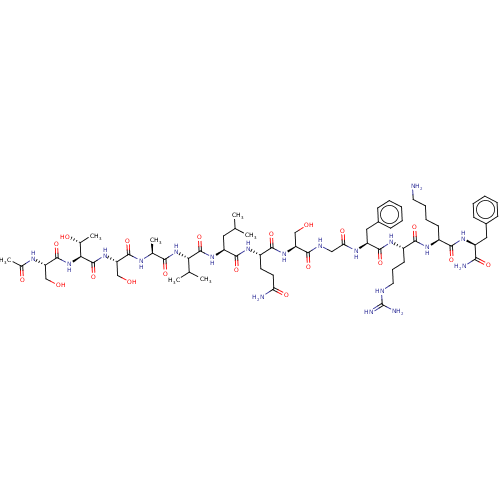 (CHEMBL4875501) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of SARS CoV-2 main protease using varying concentrations of DABCYL-KTSAVLQ1SGFRKM-E(EDANS)-NH2 as substrate by Dixon plot anal... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128333 BindingDB Entry DOI: 10.7270/Q29W0K7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50467780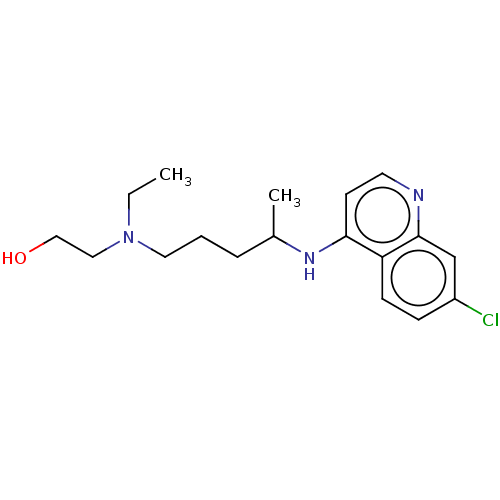 (CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 9.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Inhibition of Zika virus NS2B-NS3 protease | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126965 BindingDB Entry DOI: 10.7270/Q27M0CGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50026808 (CHEMBL2440341) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 3 (Zika virus) | BDBM50508773 (CHEMBL4589938) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Non-competitive inhibition of C-terminal His6-tagged linked Zika virus NS2B-NS3 protease domain expressed in Escherichia coli Bl21(DE3) using Bz-Nle-... | ACS Med Chem Lett 10: 168-174 (2019) Article DOI: 10.1021/acsmedchemlett.8b00535 BindingDB Entry DOI: 10.7270/Q2N019TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM4355 ((2E)-2-cyano-3-(4-hydroxyphenyl)prop-2-enamide | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Heidelberg Curated by ChEMBL | Assay Description Inhibition of human thrombin using Boc-VPR-AMC as substrate compound preincubated for 15 mins before substrate addition measured up to 10 mins by spe... | Bioorg Med Chem 19: 7318-37 (2011) Article DOI: 10.1016/j.bmc.2011.10.061 BindingDB Entry DOI: 10.7270/Q2474B8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50359735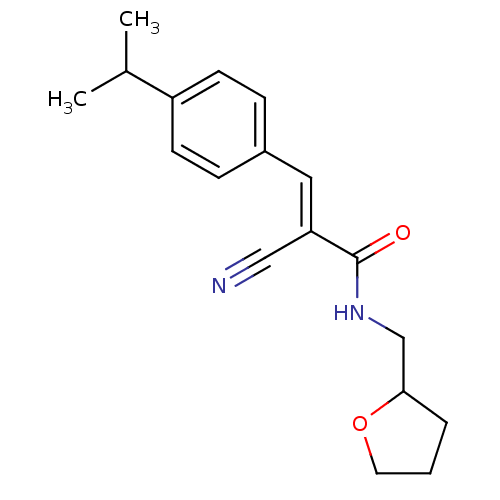 (CHEMBL1927348) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Heidelberg Curated by ChEMBL | Assay Description Inhibition of human thrombin using Boc-VPR-AMC as substrate compound preincubated for 15 mins before substrate addition measured up to 10 mins by spe... | Bioorg Med Chem 19: 7318-37 (2011) Article DOI: 10.1016/j.bmc.2011.10.061 BindingDB Entry DOI: 10.7270/Q2474B8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50030459 (CHEMBL3344321) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50212341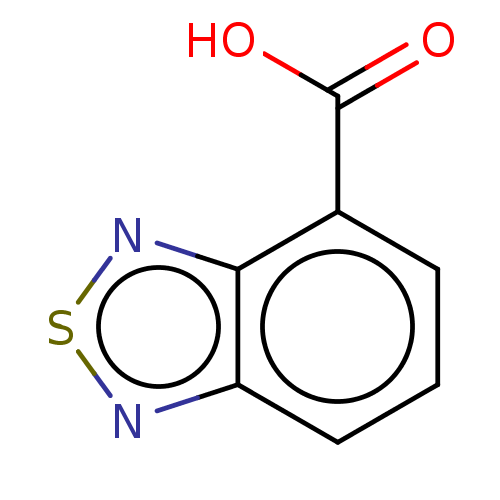 (CHEMBL5287718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50030459 (CHEMBL3344321) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128629 BindingDB Entry DOI: 10.7270/Q2S186JS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus) | BDBM50378739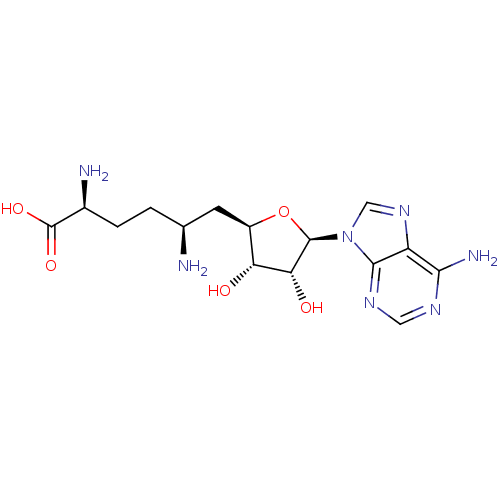 (SINEFUNGIN | jm2c00120, Sinefungin) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of Dengue virus ribose 2'-O methyltransferase using RNA substrate after 20 mins in presence of [methyl-3H]-AdoMet by microbeta counting an... | J Med Chem 59: 5622-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01653 BindingDB Entry DOI: 10.7270/Q2FX7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 210 total ) | Next | Last >> |