 Found 13475 hits with Last Name = 'no' and Initial = 'z'
Found 13475 hits with Last Name = 'no' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606735
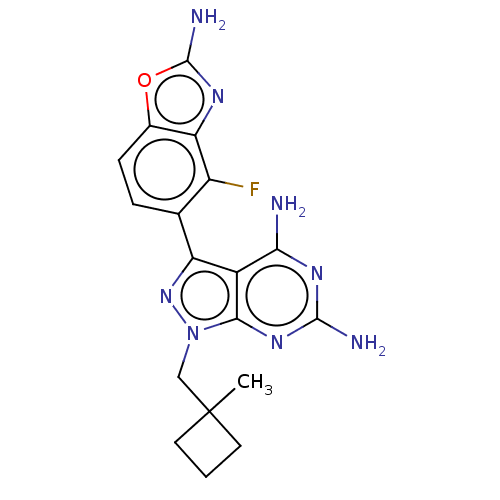
(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606737
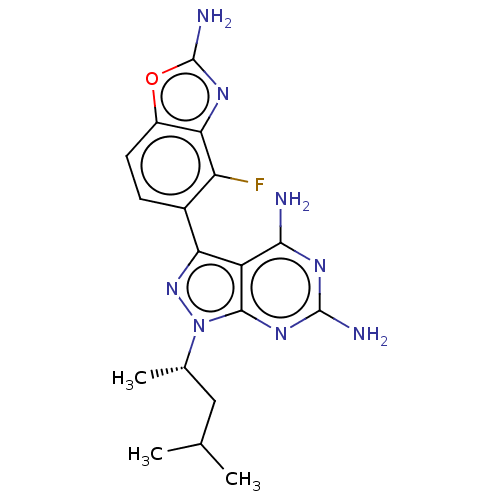
(CHEMBL5218727 | US11731973, Example 5)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606738
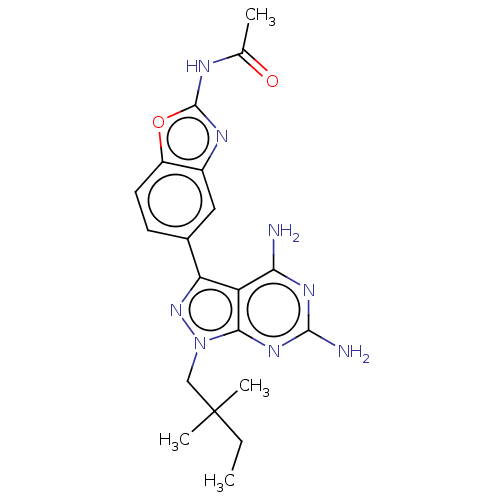
(CHEMBL5218590 | US11731973, Example 6)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(NC(C)=O)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606740
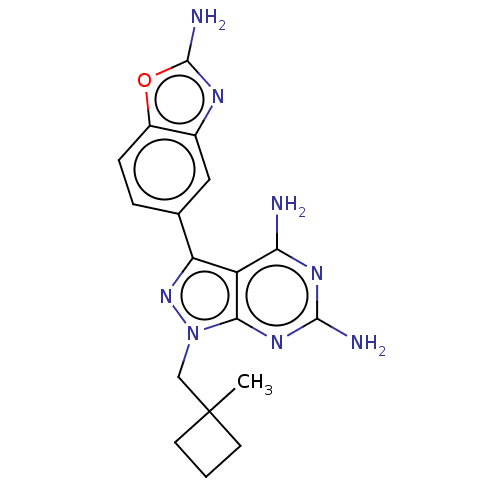
(CHEMBL5220536 | US11731973, Example 30)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606742
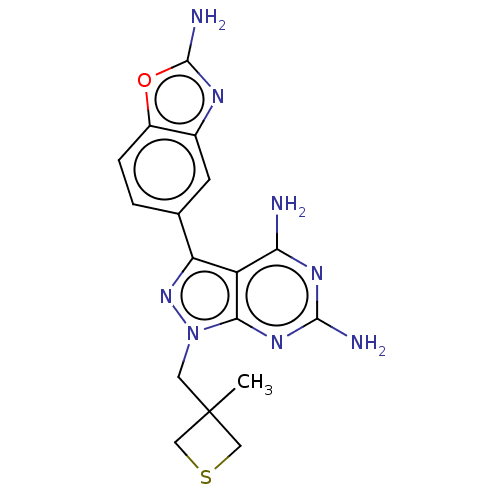
(CHEMBL5220948 | US11731973, Example 9)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CSC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606739
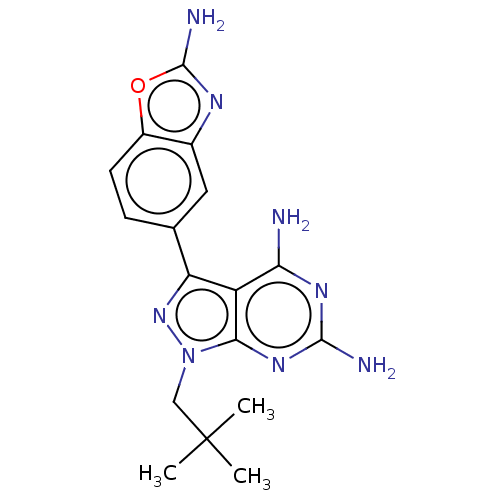
(CHEMBL5220152 | US11731973, Example 7)Show SMILES CC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606736
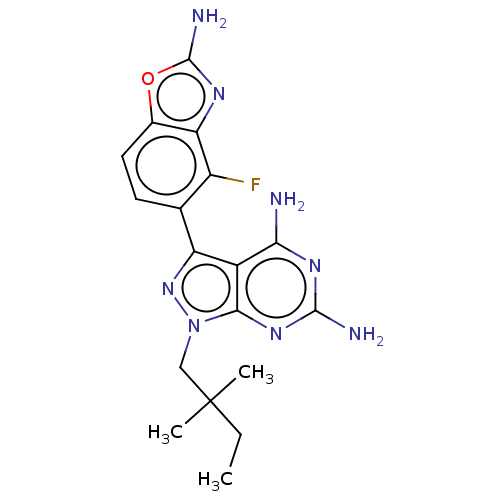
(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606741
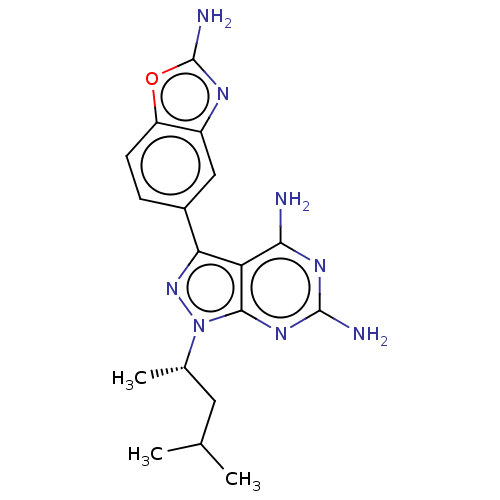
(CHEMBL5219710 | US11731973, Example 1)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606733

(CHEMBL5218988) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM582479

(5-(2-aminobenzoxazol-5-yl)-7-methyl-7-methylsulfan...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606717
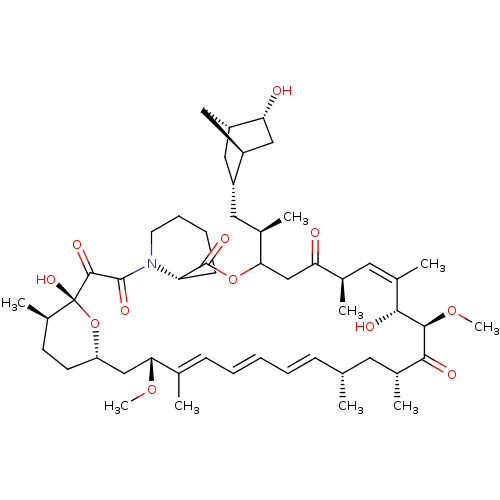
(CHEMBL5218737)Show SMILES [H][C@]12C[C@@H](C[C@@H](C)C3CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@]4([H])CC[C@@H](C)[C@@](O)(O4)C(=O)C(=O)N4CCCC[C@H]4C(=O)O3)OC)[C@]([H])(C[C@H]1O)C2 |r,c:13,32,t:28,30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606714
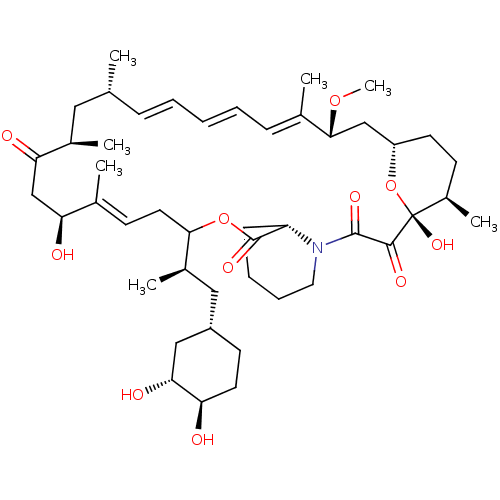
(CHEMBL5220794)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@H](O)C1 |r,c:26,43,t:39,41| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606713
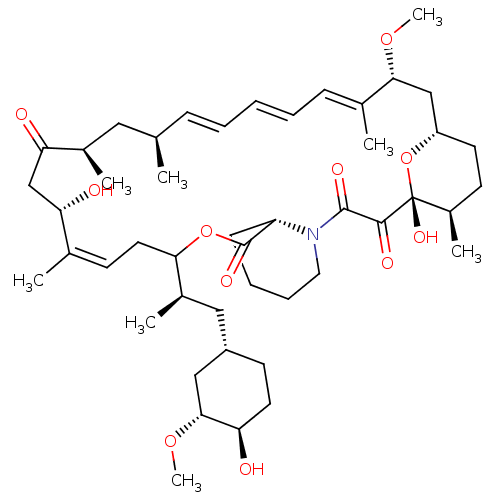
(CHEMBL5218786)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:26,43,t:39,41| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606715
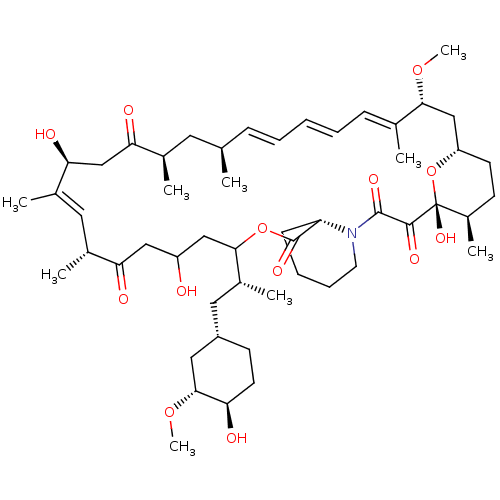
(CHEMBL5219513)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(CC(O)CC(=O)[C@H](C)\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:33,50,t:46,48| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606716

(CHEMBL5218757)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](O)C2)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:4.4,18.20,47.50,50.52,6.6,1.0,44.46,wD:53.56,27.29,32.34,35.37,c:26,43,t:39,41,(-3.43,-3.47,;-4.92,-3.86,;-6.38,-4.38,;-7.46,-3.39,;-7.18,-1.89,;-8.35,-.89,;-5.81,-1.37,;-6.9,-.28,;-4.64,-2.36,;-5.55,.05,;-6.64,1.03,;-4.09,.55,;-3.01,-.43,;-3.83,1.96,;-5,2.96,;-4.72,4.47,;-3.27,4.97,;-2.19,3.99,;-2.47,2.48,;-.98,2.88,;-.58,4.37,;.11,1.79,;1.59,2.19,;4.97,.24,;4.72,-1.11,;5.75,-2.25,;7.25,-1.94,;5.27,-3.72,;3.76,-4.04,;6.3,-4.86,;5.82,-6.33,;4.31,-6.65,;6.85,-7.47,;8.35,-7.16,;6.37,-8.94,;4.86,-9.26,;4.38,-10.72,;3.83,-8.11,;2.33,-8.43,;1.3,-7.28,;-.21,-7.6,;-1.24,-6.45,;-2.74,-6.77,;-3.22,-8.23,;-3.77,-5.62,;-4.54,-6.96,;-5.05,-5.05,;1.99,3.68,;3.48,4.08,;.9,4.77,;1.3,6.26,;.21,7.35,;.61,8.83,;2.1,9.23,;2.5,10.72,;3.19,8.14,;2.79,6.66,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606712
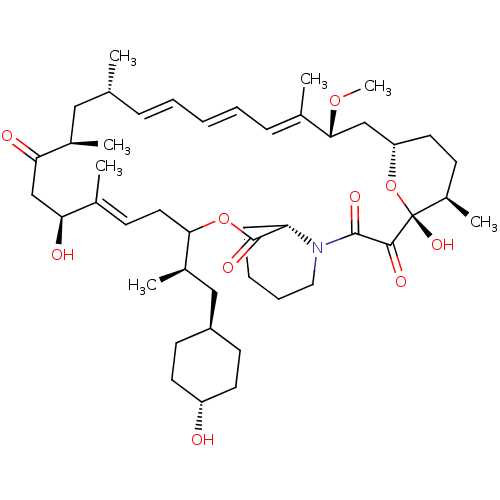
(CHEMBL5219200)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:4.4,18.20,48.51,51.53,6.6,1.0,44.48,wD:54.57,27.29,32.34,35.37,c:26,43,t:39,41,(-3.43,-3.47,;-4.92,-3.86,;-6.38,-4.38,;-7.46,-3.39,;-7.18,-1.89,;-8.35,-.89,;-5.81,-1.37,;-6.9,-.28,;-4.64,-2.36,;-5.55,.05,;-6.64,1.03,;-4.09,.55,;-3.01,-.42,;-3.83,1.96,;-5.01,2.96,;-4.72,4.47,;-3.27,4.97,;-2.19,3.99,;-2.47,2.48,;-.98,2.88,;-.58,4.37,;.11,1.79,;1.59,2.19,;5.2,.36,;4.72,-1.11,;5.75,-2.25,;7.25,-1.94,;5.27,-3.72,;3.76,-4.04,;6.3,-4.86,;5.82,-6.33,;4.31,-6.65,;6.85,-7.47,;8.35,-7.16,;6.37,-8.94,;4.86,-9.26,;4.38,-10.72,;3.83,-8.11,;2.33,-8.43,;1.3,-7.28,;-.21,-7.6,;-1.24,-6.45,;-2.74,-6.77,;-3.22,-8.23,;-3.77,-5.62,;-5.05,-5.05,;-4.54,-6.96,;-6.08,-6.96,;1.99,3.68,;3.48,4.08,;.9,4.77,;1.3,6.26,;.21,7.35,;.61,8.83,;2.1,9.23,;2.5,10.72,;3.19,8.14,;2.79,6.66,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM582478

(5-(2-aminobenzoxazol-5-yl)-7-isobutyl-6, 7-dihydro...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606734

(CHEMBL5221072) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
S-methylmethionine--homocysteine S-methyltransferase BHMT2
(Homo sapiens (Human)) | BDBM50395382
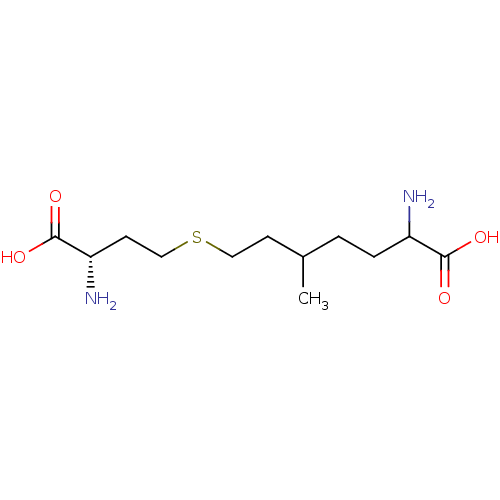
(CHEMBL2164724)Show InChI InChI=1S/C12H24N2O4S/c1-8(2-3-9(13)11(15)16)4-6-19-7-5-10(14)12(17)18/h8-10H,2-7,13-14H2,1H3,(H,15,16)(H,17,18)/t8?,9?,10-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BHMT2 expressed in Escherichia coli BL31(DE3) by Dixon plot method in presence of D,L-homocysteine |
J Med Chem 55: 6822-31 (2012)
Article DOI: 10.1021/jm300571h
BindingDB Entry DOI: 10.7270/Q2BV7HSH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609
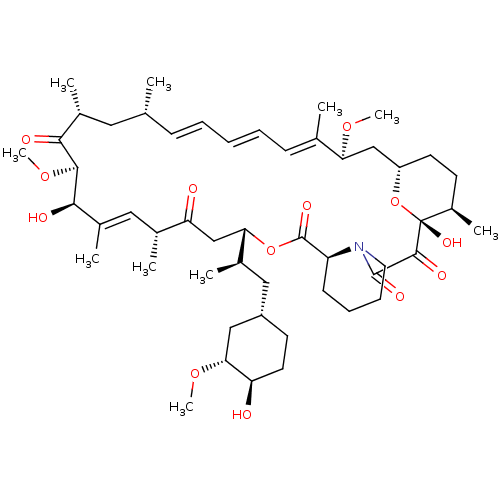
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50088378
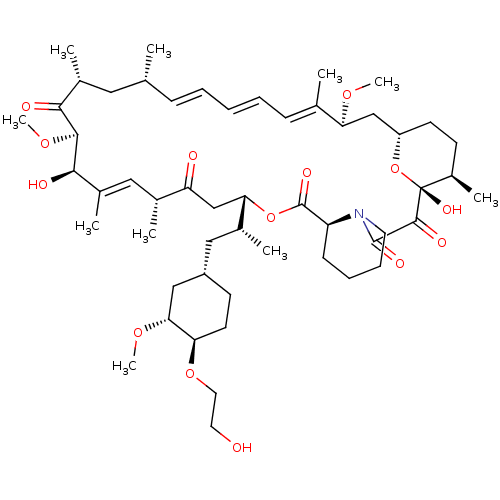
(Afinitor | Afinitor Disperz | CHEBI:68478 | Everol...)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCCO)[C@@H](C1)OC |r,c:32,51,t:47,49| Show InChI InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM36609

(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50468173
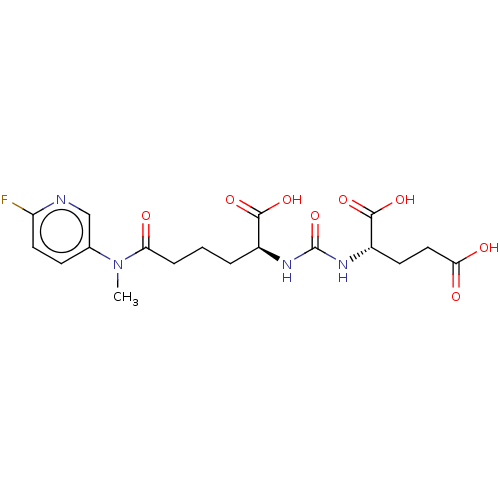
(CHEMBL4284995)Show SMILES CN(C(=O)CCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)c1ccc(F)nc1 |r| Show InChI InChI=1S/C18H23FN4O8/c1-23(10-5-7-13(19)20-9-10)14(24)4-2-3-11(16(27)28)21-18(31)22-12(17(29)30)6-8-15(25)26/h5,7,9,11-12H,2-4,6,8H2,1H3,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606717

(CHEMBL5218737)Show SMILES [H][C@]12C[C@@H](C[C@@H](C)C3CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@]4([H])CC[C@@H](C)[C@@](O)(O4)C(=O)C(=O)N4CCCC[C@H]4C(=O)O3)OC)[C@]([H])(C[C@H]1O)C2 |r,c:13,32,t:28,30| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606703
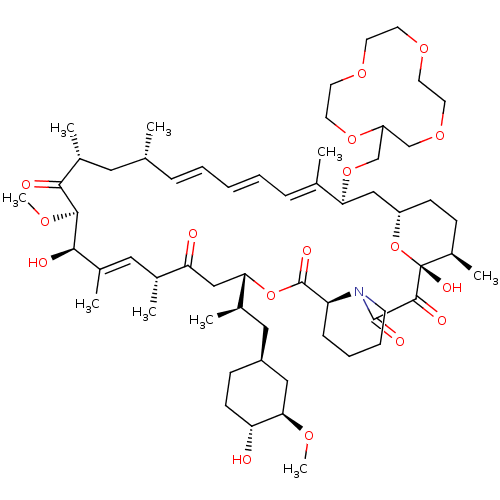
(CHEMBL5219040)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OCC1COCCOCCOCCO1)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606702
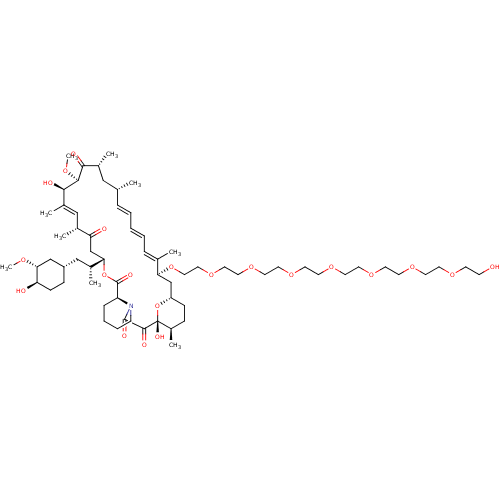
(CHEMBL5219001)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OCCOCCOCCOCCOCCOCCOCCOCCO)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606701
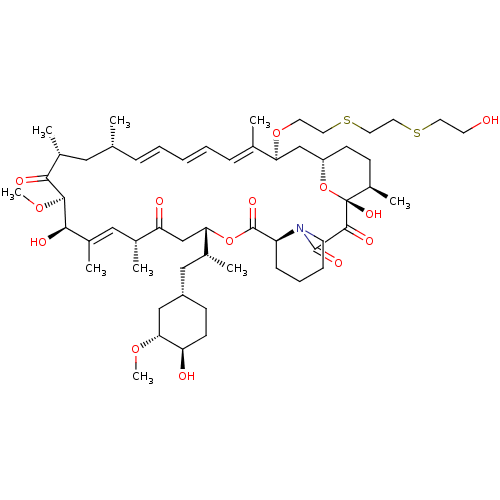
(CHEMBL5219477)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OCCSCCSCCO)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606700
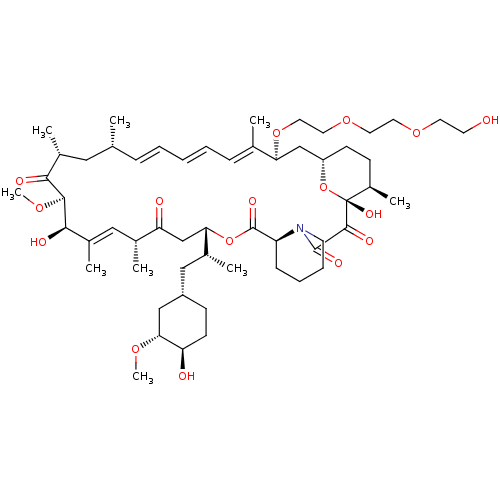
(CHEMBL5218941)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OCCOCCOCCO)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606699

(CHEMBL5220295)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OCCOCCO)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50468167
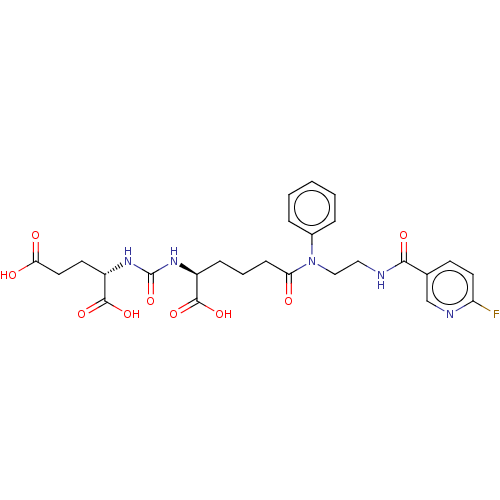
(CHEMBL4280968)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCC(=O)N(CCNC(=O)c1ccc(F)nc1)c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C26H30FN5O9/c27-20-11-9-16(15-29-20)23(36)28-13-14-32(17-5-2-1-3-6-17)21(33)8-4-7-18(24(37)38)30-26(41)31-19(25(39)40)10-12-22(34)35/h1-3,5-6,9,11,15,18-19H,4,7-8,10,12-14H2,(H,28,36)(H,34,35)(H,37,38)(H,39,40)(H2,30,31,41)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50468163
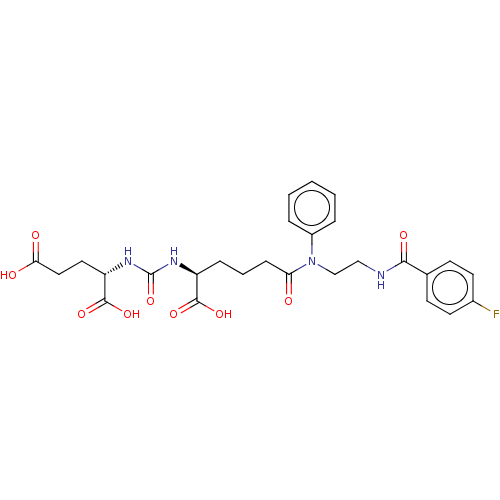
(CHEMBL4286872)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCC(=O)N(CCNC(=O)c1ccc(F)cc1)c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C27H31FN4O9/c28-18-11-9-17(10-12-18)24(36)29-15-16-32(19-5-2-1-3-6-19)22(33)8-4-7-20(25(37)38)30-27(41)31-21(26(39)40)13-14-23(34)35/h1-3,5-6,9-12,20-21H,4,7-8,13-16H2,(H,29,36)(H,34,35)(H,37,38)(H,39,40)(H2,30,31,41)/t20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606736

(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606735

(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50468164
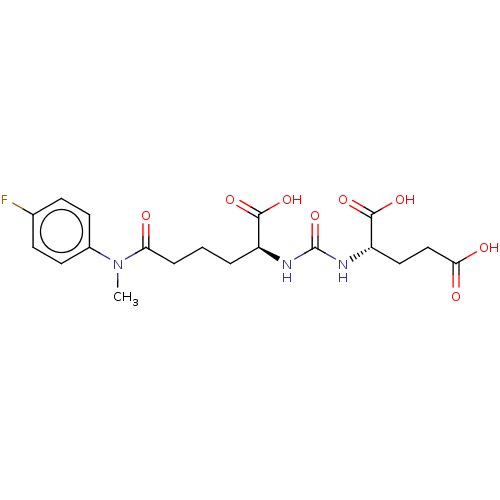
(CHEMBL4288368)Show SMILES CN(C(=O)CCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H24FN3O8/c1-23(12-7-5-11(20)6-8-12)15(24)4-2-3-13(17(27)28)21-19(31)22-14(18(29)30)9-10-16(25)26/h5-8,13-14H,2-4,9-10H2,1H3,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM132084
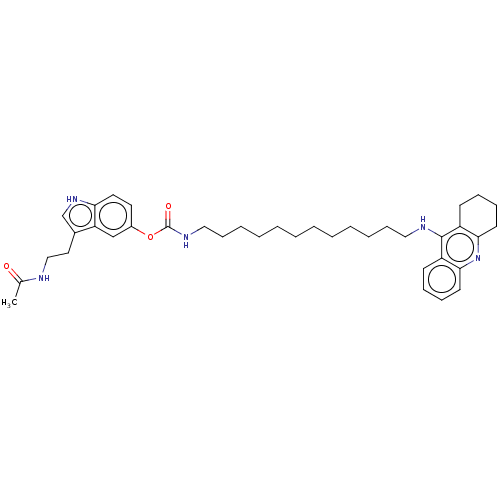
(US8841453, 15)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)cc12 Show InChI InChI=1S/C38H51N5O3/c1-28(44)39-25-22-29-27-42-34-21-20-30(26-33(29)34)46-38(45)41-24-15-9-7-5-3-2-4-6-8-14-23-40-37-31-16-10-12-18-35(31)43-36-19-13-11-17-32(36)37/h10,12,16,18,20-21,26-27,42H,2-9,11,13-15,17,19,22-25H2,1H3,(H,39,44)(H,40,43)(H,41,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM132071
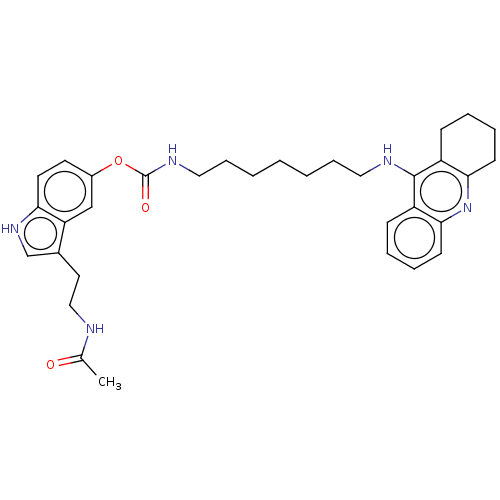
(US8841453, 1)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCNc3c4CCCCc4nc4ccccc34)cc12 Show InChI InChI=1S/C33H41N5O3/c1-23(39)34-20-17-24-22-37-29-16-15-25(21-28(24)29)41-33(40)36-19-10-4-2-3-9-18-35-32-26-11-5-7-13-30(26)38-31-14-8-6-12-27(31)32/h5,7,11,13,15-16,21-22,37H,2-4,6,8-10,12,14,17-20H2,1H3,(H,34,39)(H,35,38)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM132080

(US8841453, 11)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCCNc3c4CCCCc4nc4ccccc34)cc12 Show InChI InChI=1S/C34H43N5O3/c1-24(40)35-21-18-25-23-38-30-17-16-26(22-29(25)30)42-34(41)37-20-11-5-3-2-4-10-19-36-33-27-12-6-8-14-31(27)39-32-15-9-7-13-28(32)33/h6,8,12,14,16-17,22-23,38H,2-5,7,9-11,13,15,18-21H2,1H3,(H,35,40)(H,36,39)(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM132083

(US8841453, 14)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)cc12 Show InChI InChI=1S/C36H47N5O3/c1-26(42)37-23-20-27-25-40-32-19-18-28(24-31(27)32)44-36(43)39-22-13-7-5-3-2-4-6-12-21-38-35-29-14-8-10-16-33(29)41-34-17-11-9-15-30(34)35/h8,10,14,16,18-19,24-25,40H,2-7,9,11-13,15,17,20-23H2,1H3,(H,37,42)(H,38,41)(H,39,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50468174
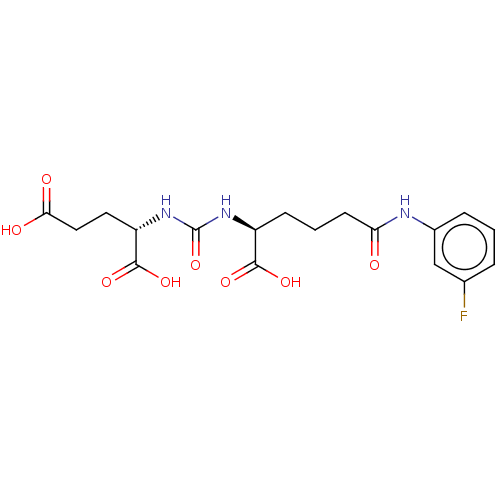
(CHEMBL4277729)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCC(=O)Nc1cccc(F)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H22FN3O8/c19-10-3-1-4-11(9-10)20-14(23)6-2-5-12(16(26)27)21-18(30)22-13(17(28)29)7-8-15(24)25/h1,3-4,9,12-13H,2,5-8H2,(H,20,23)(H,24,25)(H,26,27)(H,28,29)(H2,21,22,30)/t12-,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606711

(CHEMBL5220287)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\C(C2)OCCO)[C@H](C)C[C@@H]1CC[C@@H](OCCCN2C[C@H](C)O[C@H](C)C2)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606709
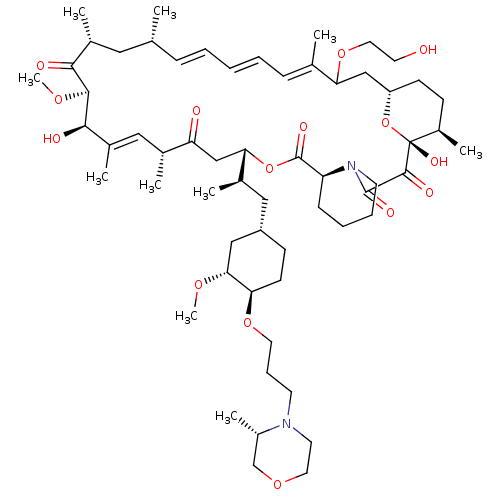
(CHEMBL5219729)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\C(C2)OCCO)[C@H](C)C[C@@H]1CC[C@@H](OCCCN2CCOC[C@@H]2C)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606708

(CHEMBL5220300)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\C(C2)OCCO)[C@H](C)C[C@@H]1CC[C@@H](OCCCN2CCOC[C@H]2C)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606707

(CHEMBL5220505)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\C(C2)OCCO)[C@H](C)C[C@@H]1CC[C@@H](OC(=O)NCCc2ccccc2)[C@@H](C1)OC |r,c:32,51,t:47,49| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50606710

(CHEMBL5219271)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\C(C2)OCCO)[C@H](C)C[C@@H]1CC[C@@H](OCCCN2CCOC(C)(C)C2)[C@@H](C1)OC |r,c:31,50,t:46,48| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50468175
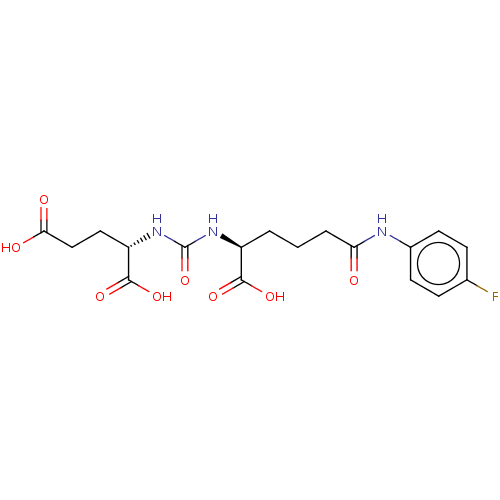
(CHEMBL4291782)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCC(=O)Nc1ccc(F)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H22FN3O8/c19-10-4-6-11(7-5-10)20-14(23)3-1-2-12(16(26)27)21-18(30)22-13(17(28)29)8-9-15(24)25/h4-7,12-13H,1-3,8-9H2,(H,20,23)(H,24,25)(H,26,27)(H,28,29)(H2,21,22,30)/t12-,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM132074
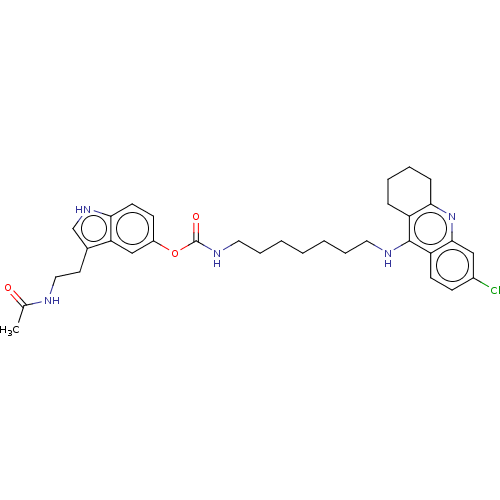
(US8841453, 4)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)cc12 Show InChI InChI=1S/C33H40ClN5O3/c1-22(40)35-18-15-23-21-38-29-14-12-25(20-28(23)29)42-33(41)37-17-8-4-2-3-7-16-36-32-26-9-5-6-10-30(26)39-31-19-24(34)11-13-27(31)32/h11-14,19-21,38H,2-10,15-18H2,1H3,(H,35,40)(H,36,39)(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50468177

(CHEMBL4291245)Show SMILES CN(Cc1cccc(F)c1)C(=O)CCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H26FN3O8/c1-24(11-12-4-2-5-13(21)10-12)16(25)7-3-6-14(18(28)29)22-20(32)23-15(19(30)31)8-9-17(26)27/h2,4-5,10,14-15H,3,6-9,11H2,1H3,(H,26,27)(H,28,29)(H,30,31)(H2,22,23,32)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PSMA using [3H]NAAG as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by li... |
ACS Med Chem Lett 9: 1099-1104 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00318
BindingDB Entry DOI: 10.7270/Q2Q81GSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50606726

(CHEMBL5220911)Show SMILES CC(=O)N1CCN(CC1)c1cnc2ccc(cc2n1)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50606725

(CHEMBL5220248)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(nc2c1)N1CCNCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data