 Found 231 hits with Last Name = 'normandin' and Initial = 'd'
Found 231 hits with Last Name = 'normandin' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50282324

(2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2cccnc12)C1CC1 Show InChI InChI=1S/C25H18N6O3/c32-25(33)20-22-19(6-3-13-26-22)27-21(15-7-8-15)23(20)34-16-11-9-14(10-12-16)17-4-1-2-5-18(17)24-28-30-31-29-24/h1-6,9-13,15H,7-8H2,(H,32,33)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50049201

(2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ccccc12)C1CC1 Show InChI InChI=1S/C27H21N5O3/c33-27(34)23-21-7-3-4-8-22(21)28-24(18-13-14-18)25(23)35-15-16-9-11-17(12-10-16)19-5-1-2-6-20(19)26-29-31-32-30-26/h1-12,18H,13-15H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282318
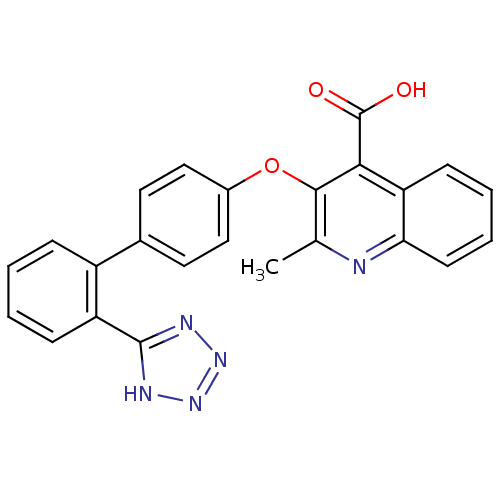
(2-Methyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...)Show SMILES Cc1nc2ccccc2c(C(O)=O)c1Oc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H17N5O3/c1-14-22(21(24(30)31)19-8-4-5-9-20(19)25-14)32-16-12-10-15(11-13-16)17-6-2-3-7-18(17)23-26-28-29-27-23/h2-13H,1H3,(H,30,31)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282322
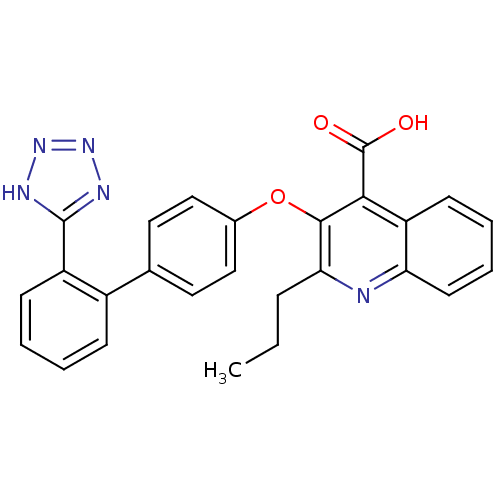
(2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...)Show SMILES CCCc1nc2ccccc2c(C(O)=O)c1Oc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H21N5O3/c1-2-7-22-24(23(26(32)33)20-10-5-6-11-21(20)27-22)34-17-14-12-16(13-15-17)18-8-3-4-9-19(18)25-28-30-31-29-25/h3-6,8-15H,2,7H2,1H3,(H,32,33)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282316
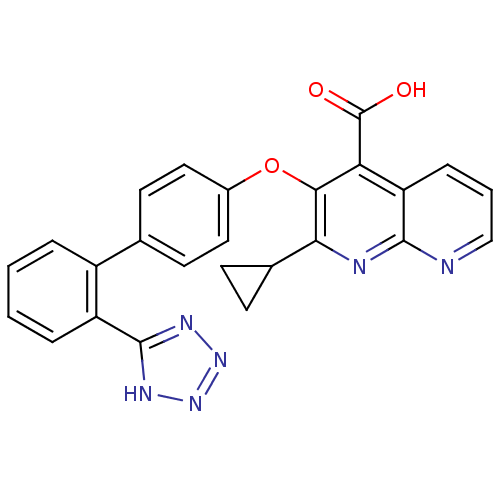
(2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ncccc12)C1CC1 Show InChI InChI=1S/C25H18N6O3/c32-25(33)20-19-6-3-13-26-23(19)27-21(15-7-8-15)22(20)34-16-11-9-14(10-12-16)17-4-1-2-5-18(17)24-28-30-31-29-24/h1-6,9-13,15H,7-8H2,(H,32,33)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282315
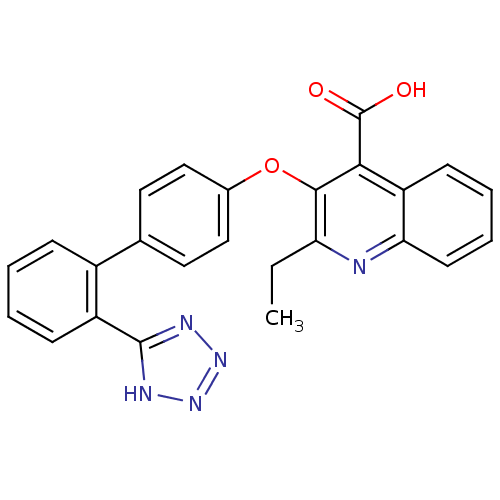
(2-Ethyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]...)Show SMILES CCc1nc2ccccc2c(C(O)=O)c1Oc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H19N5O3/c1-2-20-23(22(25(31)32)19-9-5-6-10-21(19)26-20)33-16-13-11-15(12-14-16)17-7-3-4-8-18(17)24-27-29-30-28-24/h3-14H,2H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282317

(2-Cyclopropyl-5-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES Cc1cccc2nc(C3CC3)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(C(O)=O)c12 Show InChI InChI=1S/C27H21N5O3/c1-15-5-4-8-21-22(15)23(27(33)34)25(24(28-21)17-9-10-17)35-18-13-11-16(12-14-18)19-6-2-3-7-20(19)26-29-31-32-30-26/h2-8,11-14,17H,9-10H2,1H3,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282319

(2-Cyclopropyl-6-fluoro-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ccc(F)cc12)C1CC1 Show InChI InChI=1S/C26H18FN5O3/c27-16-9-12-21-20(13-16)22(26(33)34)24(23(28-21)15-5-6-15)35-17-10-7-14(8-11-17)18-3-1-2-4-19(18)25-29-31-32-30-25/h1-4,7-13,15H,5-6H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062397
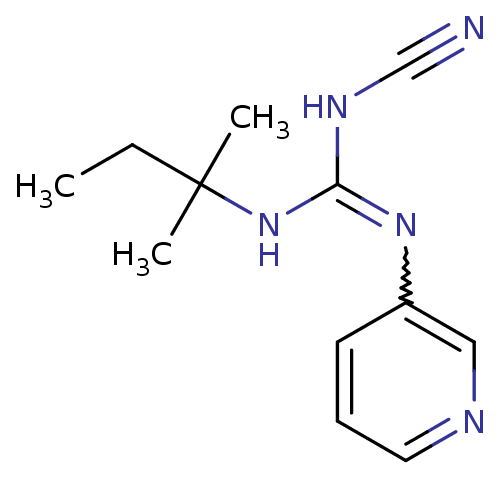
(3N-cyanoimino(tert-pentylamino)methyl-3-pyridinami...)Show InChI InChI=1S/C12H17N5/c1-4-12(2,3)17-11(15-9-13)16-10-6-5-7-14-8-10/h5-8H,4H2,1-3H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282321
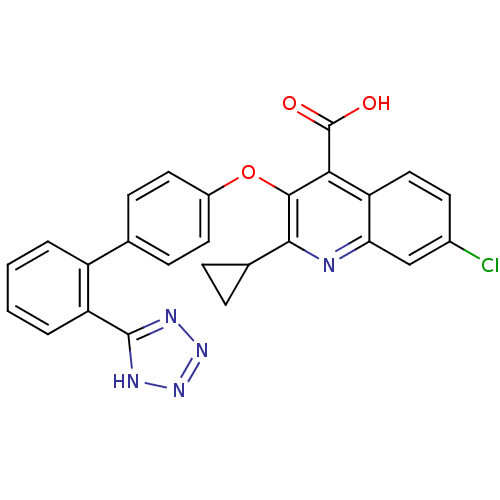
(7-Chloro-2-cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2cc(Cl)ccc12)C1CC1 Show InChI InChI=1S/C26H18ClN5O3/c27-16-9-12-20-21(13-16)28-23(15-5-6-15)24(22(20)26(33)34)35-17-10-7-14(8-11-17)18-3-1-2-4-19(18)25-29-31-32-30-25/h1-4,7-13,15H,5-6H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282313

(2-Cyclopropyl-6-methoxy-3-[2'-(2H-tetrazol-5-yl)-b...)Show SMILES COc1ccc2nc(C3CC3)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(C(O)=O)c2c1 Show InChI InChI=1S/C27H21N5O4/c1-35-18-12-13-22-21(14-18)23(27(33)34)25(24(28-22)16-6-7-16)36-17-10-8-15(9-11-17)19-4-2-3-5-20(19)26-29-31-32-30-26/h2-5,8-14,16H,6-7H2,1H3,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062402

(1N-cyanoimino(tert-pentylamino)methyl-3-azido-4-io...)Show SMILES CCC(C)(C)NC(Nc1ccc(I)c(c1)N=[N+]=[N-])=NC#N |w:18.19| Show InChI InChI=1S/C13H16IN7/c1-4-13(2,3)19-12(17-8-15)18-9-5-6-10(14)11(7-9)20-21-16/h5-7H,4H2,1-3H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062394
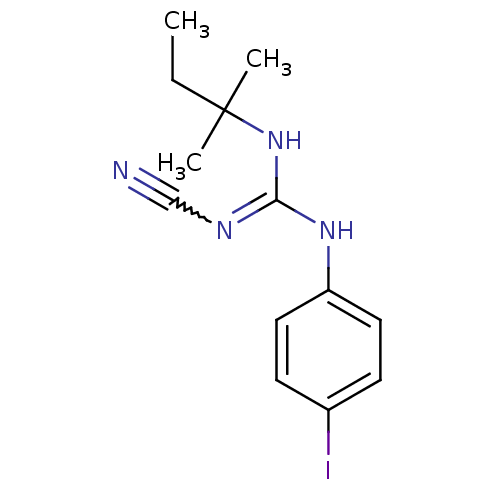
(1N-cyanoimino(tert-pentylamino)methyl-4-iodoanilin...)Show InChI InChI=1S/C13H17IN4/c1-4-13(2,3)18-12(16-9-15)17-11-7-5-10(14)6-8-11/h5-8H,4H2,1-3H3,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062396
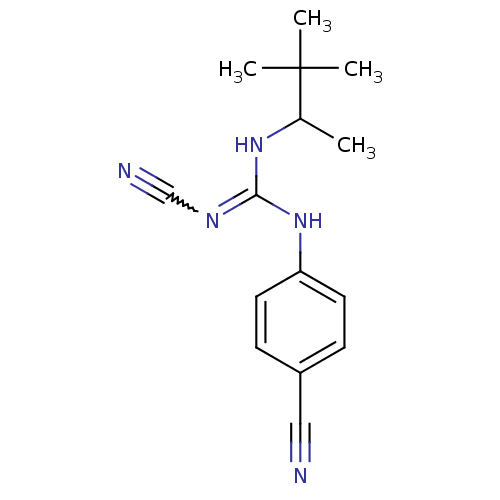
(4-cyanoimino(1,2,2-trimethylpropylamino)methylamin...)Show SMILES CC(NC(Nc1ccc(cc1)C#N)=NC#N)C(C)(C)C |w:13.14| Show InChI InChI=1S/C15H19N5/c1-11(15(2,3)4)19-14(18-10-17)20-13-7-5-12(9-16)6-8-13/h5-8,11H,1-4H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282323
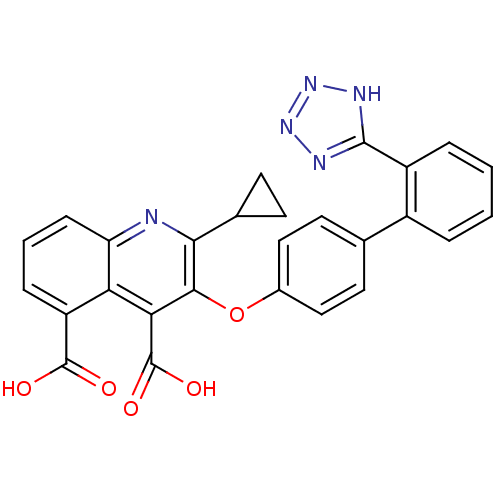
(2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1cccc2nc(C3CC3)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(C(O)=O)c12 Show InChI InChI=1S/C27H19N5O5/c33-26(34)19-6-3-7-20-21(19)22(27(35)36)24(23(28-20)15-8-9-15)37-16-12-10-14(11-13-16)17-4-1-2-5-18(17)25-29-31-32-30-25/h1-7,10-13,15H,8-9H2,(H,33,34)(H,35,36)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50012957
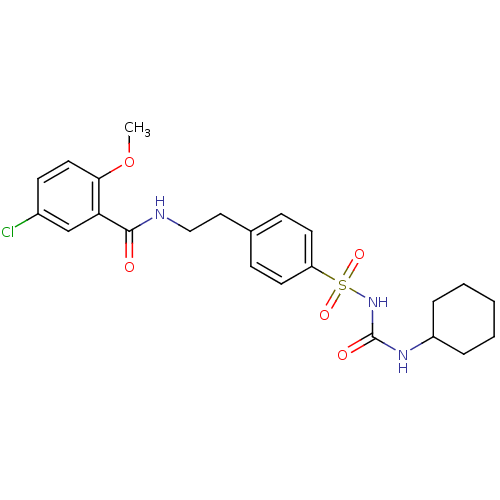
(1-((p-(2-(5-chloro-o-anisamido)ethyl)phenyl)sulfon...)Show SMILES COc1ccc(Cl)cc1C(=O)NCCc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062398

(4-cyanoimino(tert-pentylamino)methylaminobenzonitr...)Show InChI InChI=1S/C14H17N5/c1-4-14(2,3)19-13(17-10-16)18-12-7-5-11(9-15)6-8-12/h5-8H,4H2,1-3H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50240750

(()-N-Cyano-N'-4-pyridinyl-N''-(1,2,2-trimethylprop...)Show InChI InChI=1S/C13H19N5/c1-10(13(2,3)4)17-12(16-9-14)18-11-5-7-15-8-6-11/h5-8,10H,1-4H3,(H2,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062393
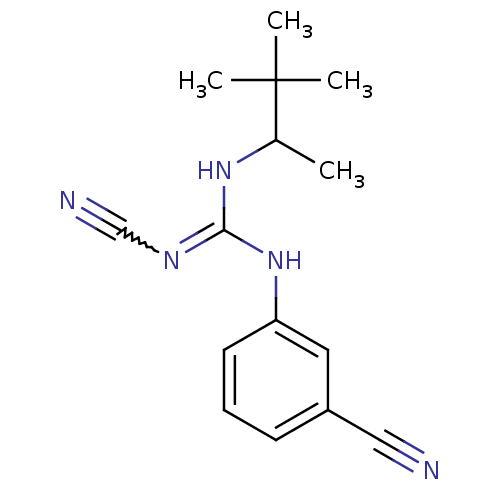
(3-cyanoimino(1,2,2-trimethylpropylamino)methylamin...)Show SMILES CC(NC(Nc1cccc(c1)C#N)=NC#N)C(C)(C)C |w:13.14| Show InChI InChI=1S/C15H19N5/c1-11(15(2,3)4)19-14(18-10-17)20-13-7-5-6-12(8-13)9-16/h5-8,11H,1-4H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062399

(4-[tert-butylamino(cyanoimino)methylamino]benzonit...)Show InChI InChI=1S/C13H15N5/c1-13(2,3)18-12(16-9-15)17-11-6-4-10(8-14)5-7-11/h4-7H,1-3H3,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062400

(1N-cyanoimino(1,2,2-trimethylpropylamino)methylani...)Show InChI InChI=1S/C14H20N4/c1-11(14(2,3)4)17-13(16-10-15)18-12-8-6-5-7-9-12/h5-9,11H,1-4H3,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282320
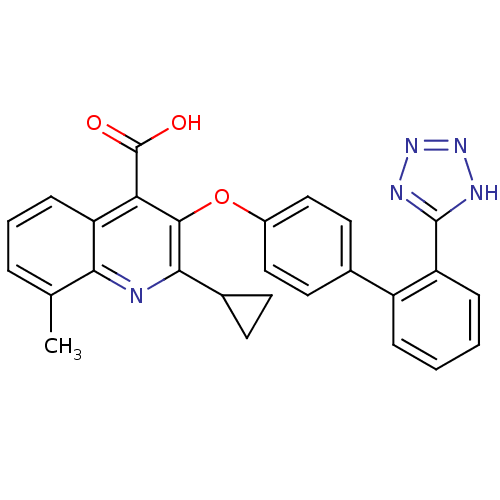
(2-Cyclopropyl-8-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES Cc1cccc2c(C(O)=O)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(nc12)C1CC1 Show InChI InChI=1S/C27H21N5O3/c1-15-5-4-8-21-22(27(33)34)25(24(17-9-10-17)28-23(15)21)35-18-13-11-16(12-14-18)19-6-2-3-7-20(19)26-29-31-32-30-26/h2-8,11-14,17H,9-10H2,1H3,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062392
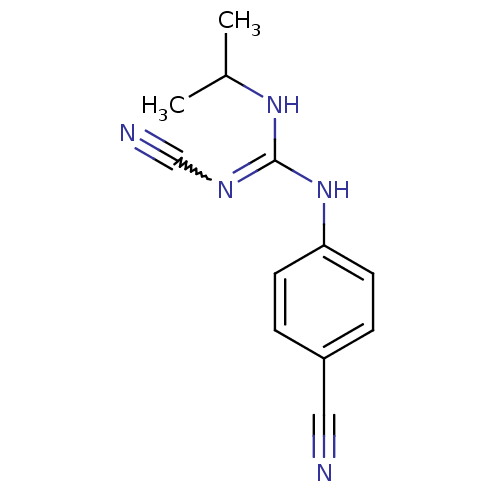
(4-isopropylamino(cyanoimino)methylaminobenzonitril...)Show InChI InChI=1S/C12H13N5/c1-9(2)16-12(15-8-14)17-11-5-3-10(7-13)4-6-11/h3-6,9H,1-2H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062395
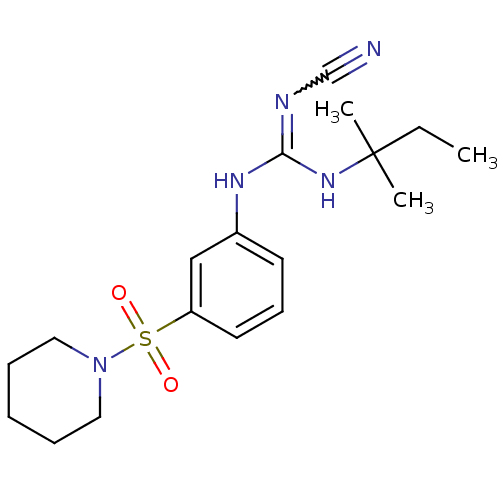
(1N-cyanoimino(tert-pentylamino)methyl-3-hexahydro-...)Show SMILES CCC(C)(C)NC(Nc1cccc(c1)S(=O)(=O)N1CCCCC1)=NC#N |w:23.25| Show InChI InChI=1S/C18H27N5O2S/c1-4-18(2,3)22-17(20-14-19)21-15-9-8-10-16(13-15)26(24,25)23-11-6-5-7-12-23/h8-10,13H,4-7,11-12H2,1-3H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062391
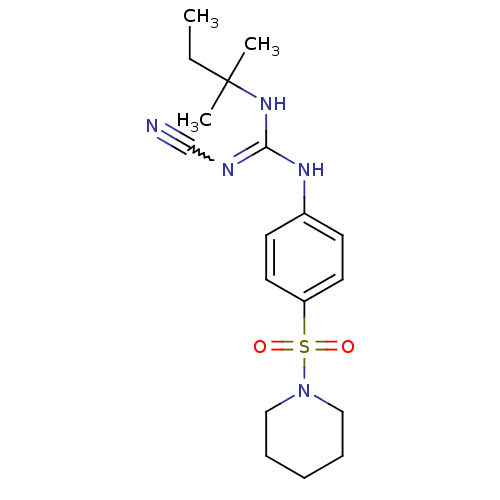
(1N-cyanoimino(tert-pentylamino)methyl-4-hexahydro-...)Show SMILES CCC(C)(C)NC(Nc1ccc(cc1)S(=O)(=O)N1CCCCC1)=NC#N |w:23.25| Show InChI InChI=1S/C18H27N5O2S/c1-4-18(2,3)22-17(20-14-19)21-15-8-10-16(11-9-15)26(24,25)23-12-6-5-7-13-23/h8-11H,4-7,12-13H2,1-3H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50062401

(4-methylamino(cyanoimino)methylaminobenzonitrile |...)Show InChI InChI=1S/C10H9N5/c1-13-10(14-7-12)15-9-4-2-8(6-11)3-5-9/h2-5H,1H3,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50044253

((3R,4R)-3-Hydroxy-2,2-dimethyl-4-(2-oxo-pyrrolidin...)Show SMILES CC1(C)Oc2ccc(cc2[C@@H]([C@H]1O)N1CCCC1=O)C#N |r| Show InChI InChI=1S/C16H18N2O3/c1-16(2)15(20)14(18-7-3-4-13(18)19)11-8-10(9-17)5-6-12(11)21-16/h5-6,8,14-15,20H,3-4,7H2,1-2H3/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11
(Homo sapiens (Human)) | BDBM50044253

((3R,4R)-3-Hydroxy-2,2-dimethyl-4-(2-oxo-pyrrolidin...)Show SMILES CC1(C)Oc2ccc(cc2[C@@H]([C@H]1O)N1CCCC1=O)C#N |r| Show InChI InChI=1S/C16H18N2O3/c1-16(2)15(20)14(18-7-3-4-13(18)19)11-8-10(9-17)5-6-12(11)21-16/h5-6,8,14-15,20H,3-4,7H2,1-2H3/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes |
J Med Chem 41: 271-5 (1998)
Article DOI: 10.1021/jm970762d
BindingDB Entry DOI: 10.7270/Q2FN159P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123474
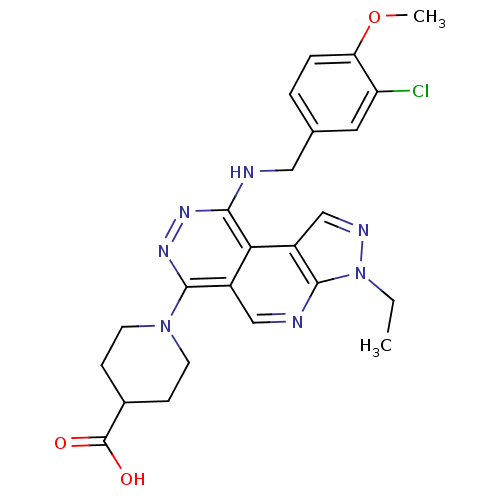
(1-[9-(3-Chloro-4-methoxy-benzylamino)-3-ethyl-3H-2...)Show SMILES CCn1ncc2c1ncc1c(nnc(NCc3ccc(OC)c(Cl)c3)c21)N1CCC(CC1)C(O)=O Show InChI InChI=1S/C24H26ClN7O3/c1-3-32-22-17(13-28-32)20-16(12-27-22)23(31-8-6-15(7-9-31)24(33)34)30-29-21(20)26-11-14-4-5-19(35-2)18(25)10-14/h4-5,10,12-13,15H,3,6-9,11H2,1-2H3,(H,26,29)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141580
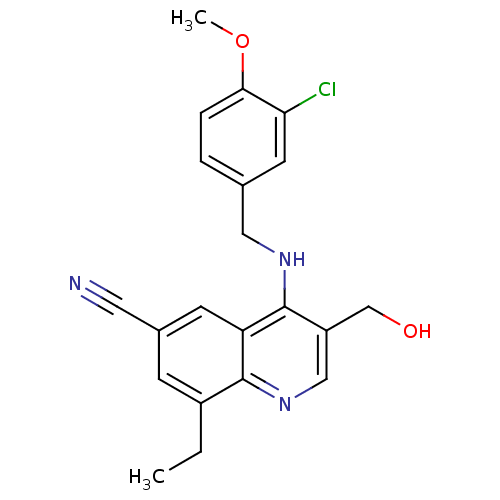
(4-(3-Chloro-4-methoxy-benzylamino)-8-ethyl-3-hydro...)Show SMILES CCc1cc(cc2c(NCc3ccc(OC)c(Cl)c3)c(CO)cnc12)C#N Show InChI InChI=1S/C21H20ClN3O2/c1-3-15-6-14(9-23)7-17-20(15)25-11-16(12-26)21(17)24-10-13-4-5-19(27-2)18(22)8-13/h4-8,11,26H,3,10,12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123477

(CHEMBL344018 | N*9*-(3-Chloro-4-methoxy-benzyl)-3-...)Show SMILES CCn1ncc2c1ncc1c(NCc3ccncc3)nnc(NCc3ccc(OC)c(Cl)c3)c21 Show InChI InChI=1S/C24H23ClN8O/c1-3-33-24-18(14-30-33)21-17(13-29-24)22(27-11-15-6-8-26-9-7-15)31-32-23(21)28-12-16-4-5-20(34-2)19(25)10-16/h4-10,13-14H,3,11-12H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50023921

(CHEMBL2112876)Show SMILES CCCOc1ccc(cc1-c1nc2cc3ncn(Cc4ccc(F)cc4)c3cc2c(=O)[nH]1)S(=O)(=O)N1CC[C@H](C1)N(C)C |r| Show InChI InChI=1S/C31H33FN6O4S/c1-4-13-42-29-10-9-23(43(40,41)38-12-11-22(18-38)36(2)3)14-25(29)30-34-26-16-27-28(15-24(26)31(39)35-30)37(19-33-27)17-20-5-7-21(32)8-6-20/h5-10,14-16,19,22H,4,11-13,17-18H2,1-3H3,(H,34,35,39)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human platelet Phosphodiesterase 5 was determined |
J Med Chem 43: 5037-43 (2001)
BindingDB Entry DOI: 10.7270/Q2862FP8 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123473
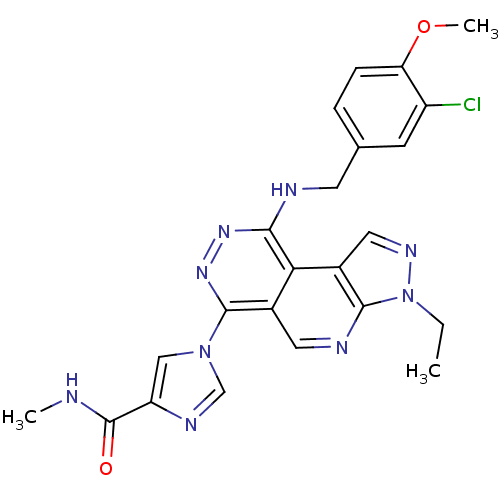
(1-[9-(3-Chloro-4-methoxy-benzylamino)-3-ethyl-3H-2...)Show SMILES CCn1ncc2c1ncc1c(nnc(NCc3ccc(OC)c(Cl)c3)c21)-n1cnc(c1)C(=O)NC Show InChI InChI=1S/C23H22ClN9O2/c1-4-33-21-15(10-29-33)19-14(9-27-21)22(32-11-17(28-12-32)23(34)25-2)31-30-20(19)26-8-13-5-6-18(35-3)16(24)7-13/h5-7,9-12H,4,8H2,1-3H3,(H,25,34)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50104208
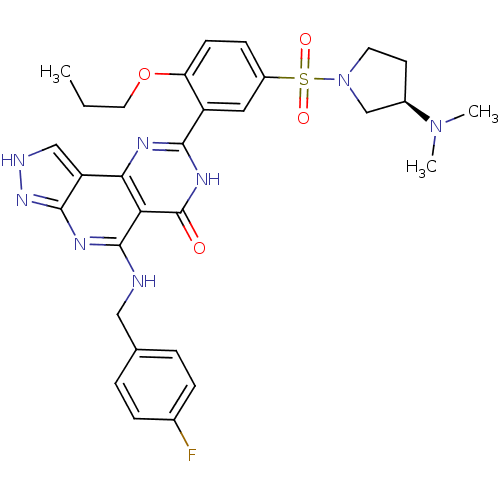
(8-[5-((R)-3-Dimethylamino-pyrrolidine-1-sulfonyl)-...)Show SMILES CCCOc1ccc(cc1-c1nc2c3c[nH]nc3nc(NCc3ccc(F)cc3)c2c(=O)[nH]1)S(=O)(=O)N1CC[C@H](C1)N(C)C Show InChI InChI=1S/C30H33FN8O4S/c1-4-13-43-24-10-9-21(44(41,42)39-12-11-20(17-39)38(2)3)14-22(24)27-34-26-23-16-33-37-28(23)35-29(25(26)30(40)36-27)32-15-18-5-7-19(31)8-6-18/h5-10,14,16,20H,4,11-13,15,17H2,1-3H3,(H,34,36,40)(H2,32,33,35,37)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from human platelets |
Bioorg Med Chem Lett 11: 2461-4 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BM5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141584
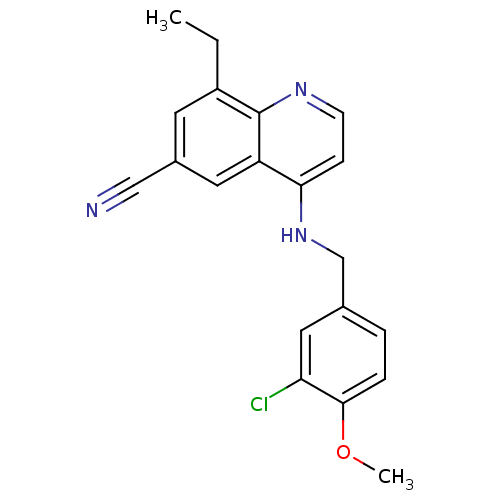
(4-(3-Chloro-4-methoxy-benzylamino)-8-ethyl-quinoli...)Show InChI InChI=1S/C20H18ClN3O/c1-3-15-8-14(11-22)9-16-18(6-7-23-20(15)16)24-12-13-4-5-19(25-2)17(21)10-13/h4-10H,3,12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50086873

(3-[1-(4-Fluoro-benzyl)-8-oxo-7,8-dihydro-1H-imidaz...)Show SMILES CCCOc1ccc(cc1-c1nc2cc3ncn(Cc4ccc(F)cc4)c3cc2c(=O)[nH]1)C(N)=O Show InChI InChI=1S/C26H22FN5O3/c1-2-9-35-23-8-5-16(24(28)33)10-19(23)25-30-20-12-21-22(11-18(20)26(34)31-25)32(14-29-21)13-15-3-6-17(27)7-4-15/h3-8,10-12,14H,2,9,13H2,1H3,(H2,28,33)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human platelet Phosphodiesterase 5 was determined |
J Med Chem 43: 5037-43 (2001)
BindingDB Entry DOI: 10.7270/Q2862FP8 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141588
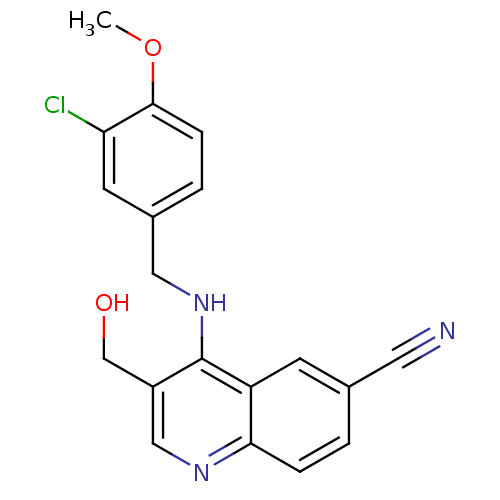
(4-(3-Chloro-4-methoxy-benzylamino)-3-hydroxymethyl...)Show InChI InChI=1S/C19H16ClN3O2/c1-25-18-5-3-13(7-16(18)20)9-23-19-14(11-24)10-22-17-4-2-12(8-21)6-15(17)19/h2-7,10,24H,9,11H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50086873

(3-[1-(4-Fluoro-benzyl)-8-oxo-7,8-dihydro-1H-imidaz...)Show SMILES CCCOc1ccc(cc1-c1nc2cc3ncn(Cc4ccc(F)cc4)c3cc2c(=O)[nH]1)C(N)=O Show InChI InChI=1S/C26H22FN5O3/c1-2-9-35-23-8-5-16(24(28)33)10-19(23)25-30-20-12-21-22(11-18(20)26(34)31-25)32(14-29-21)13-15-3-6-17(27)7-4-15/h3-8,10-12,14H,2,9,13H2,1H3,(H2,28,33)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase type 5 (PDE5) of human platelets |
J Med Chem 43: 1257-63 (2001)
BindingDB Entry DOI: 10.7270/Q2125RW8 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50023922

(CHEMBL2112881)Show SMILES CCCOc1ccc(cc1-c1nc(O)c2cc3n(Cc4ccc(Cl)cc4)cnc3cc2n1)S(=O)(=O)N1CC[C@H](C1)N(C)C |r| Show InChI InChI=1S/C31H33ClN6O4S/c1-4-13-42-29-10-9-23(43(40,41)38-12-11-22(18-38)36(2)3)14-25(29)30-34-26-16-27-28(15-24(26)31(39)35-30)37(19-33-27)17-20-5-7-21(32)8-6-20/h5-10,14-16,19,22H,4,11-13,17-18H2,1-3H3,(H,34,35,39)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human platelet Phosphodiesterase 5 was determined |
J Med Chem 43: 5037-43 (2001)
BindingDB Entry DOI: 10.7270/Q2862FP8 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141586
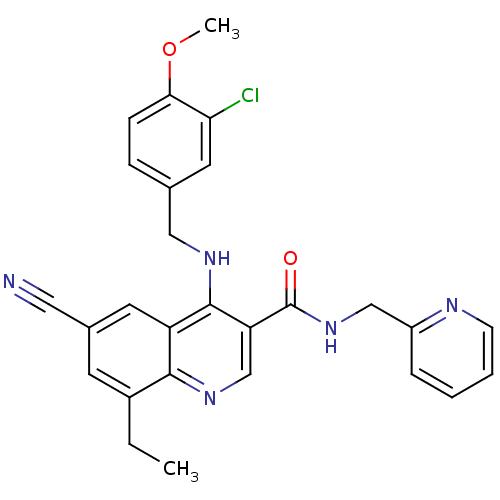
(4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-8-ethyl...)Show SMILES CCc1cc(cc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCc1ccccn1)C#N Show InChI InChI=1S/C27H24ClN5O2/c1-3-19-10-18(13-29)11-21-25(19)32-16-22(27(34)33-15-20-6-4-5-9-30-20)26(21)31-14-17-7-8-24(35-2)23(28)12-17/h4-12,16H,3,14-15H2,1-2H3,(H,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089814
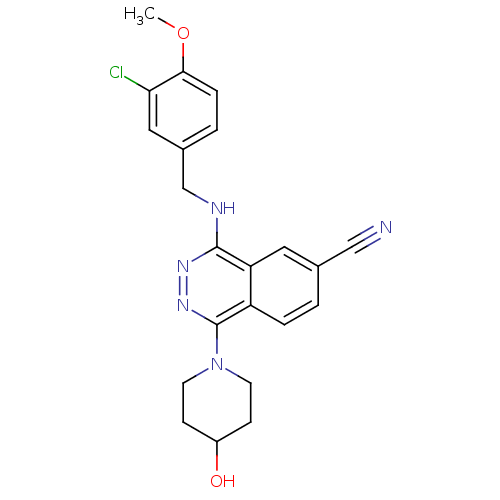
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H22ClN5O2/c1-30-20-5-3-15(11-19(20)23)13-25-21-18-10-14(12-24)2-4-17(18)22(27-26-21)28-8-6-16(29)7-9-28/h2-5,10-11,16,29H,6-9,13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089814

(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H22ClN5O2/c1-30-20-5-3-15(11-19(20)23)13-25-21-18-10-14(12-24)2-4-17(18)22(27-26-21)28-8-6-16(29)7-9-28/h2-5,10-11,16,29H,6-9,13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 of human platelets |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50141583
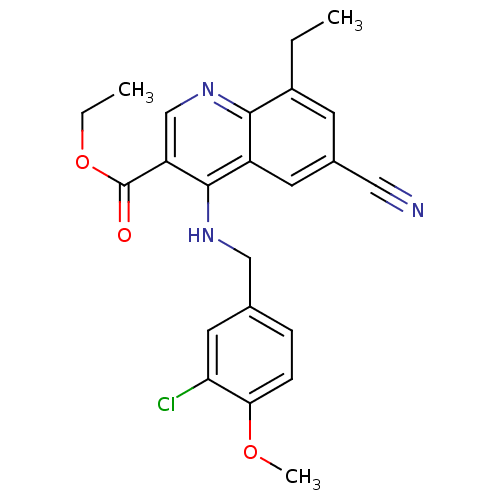
(4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-8-ethyl...)Show SMILES CCOC(=O)c1cnc2c(CC)cc(cc2c1NCc1ccc(OC)c(Cl)c1)C#N Show InChI InChI=1S/C23H22ClN3O3/c1-4-16-8-15(11-25)9-17-21(16)27-13-18(23(28)30-5-2)22(17)26-12-14-6-7-20(29-3)19(24)10-14/h6-10,13H,4-5,12H2,1-3H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 from human platelets |
Bioorg Med Chem Lett 14: 1577-80 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.090
BindingDB Entry DOI: 10.7270/Q2G1607Q |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50095727
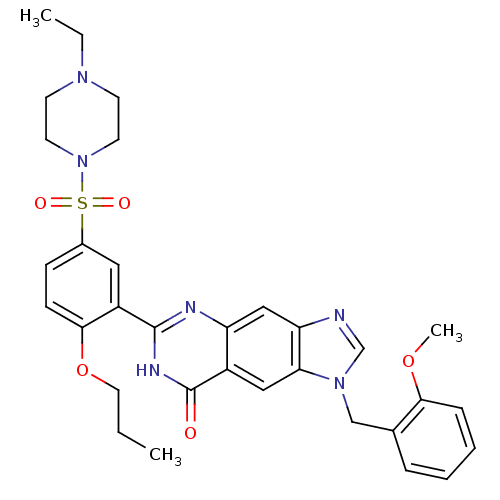
(6-[5-(4-Ethyl-piperazine-1-sulfonyl)-2-propoxy-phe...)Show SMILES CCCOc1ccc(cc1-c1nc2cc3ncn(Cc4ccccc4OC)c3cc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C32H36N6O5S/c1-4-16-43-30-11-10-23(44(40,41)38-14-12-36(5-2)13-15-38)17-25(30)31-34-26-19-27-28(18-24(26)32(39)35-31)37(21-33-27)20-22-8-6-7-9-29(22)42-3/h6-11,17-19,21H,4-5,12-16,20H2,1-3H3,(H,34,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human platelet Phosphodiesterase 5 was determined |
J Med Chem 43: 5037-43 (2001)
BindingDB Entry DOI: 10.7270/Q2862FP8 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50123475

(1-[9-(3-Chloro-4-methoxy-benzylamino)-3-ethyl-3H-2...)Show SMILES CCn1ncc2c1ncc1c(nnc(NCc3ccc(OC)c(Cl)c3)c21)N1CCC(O)CC1 Show InChI InChI=1S/C23H26ClN7O2/c1-3-31-22-17(13-27-31)20-16(12-26-22)23(30-8-6-15(32)7-9-30)29-28-21(20)25-11-14-4-5-19(33-2)18(24)10-14/h4-5,10,12-13,15,32H,3,6-9,11H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14776

(2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...)Show SMILES CCCc1nc(C)c2n1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 5 from human platelets |
J Med Chem 46: 457-60 (2003)
Article DOI: 10.1021/jm0256068
BindingDB Entry DOI: 10.7270/Q2125S1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data