

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658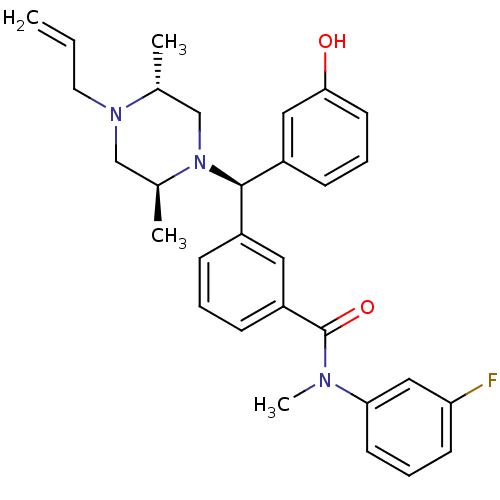 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240887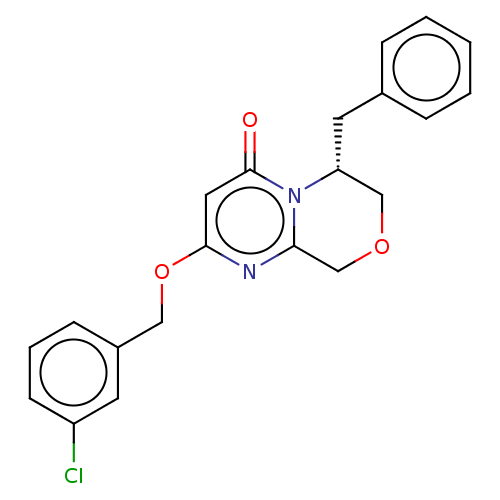 (CHEMBL4066731) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092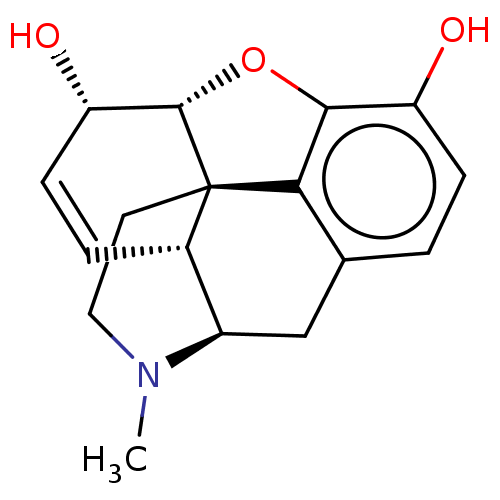 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123647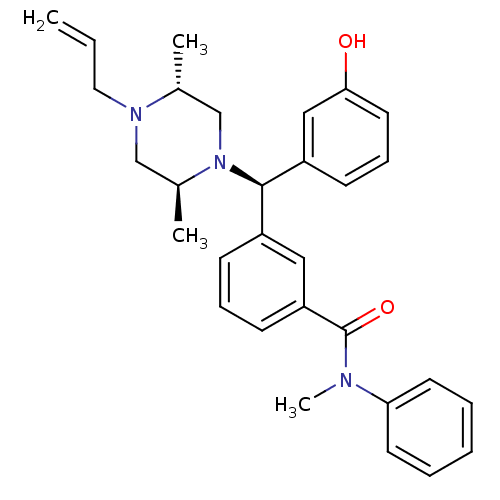 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123655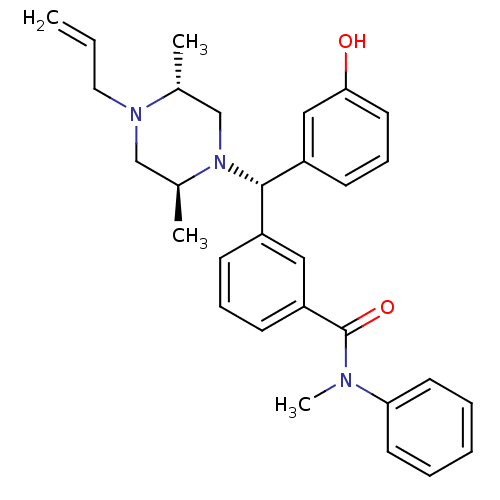 (3-[(S)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123654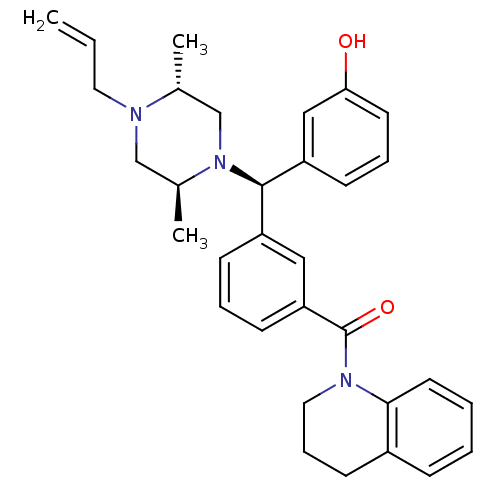 (CHEMBL157660 | {3-[(R)-((2S,5R)-4-Allyl-2,5-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123664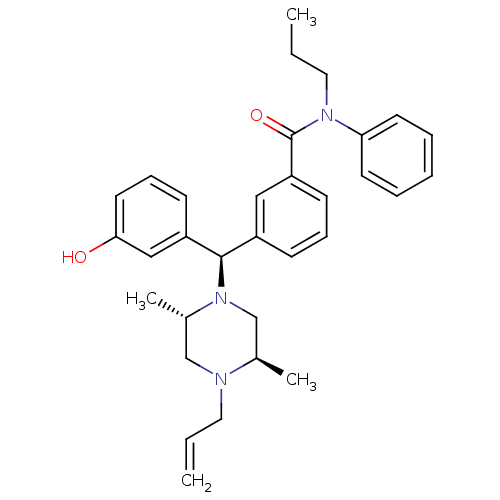 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123647 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123649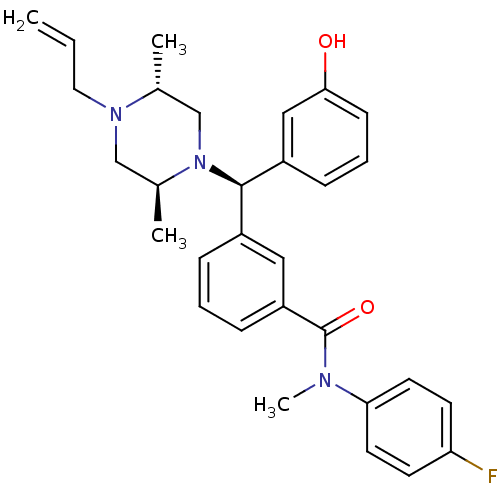 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123649 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240900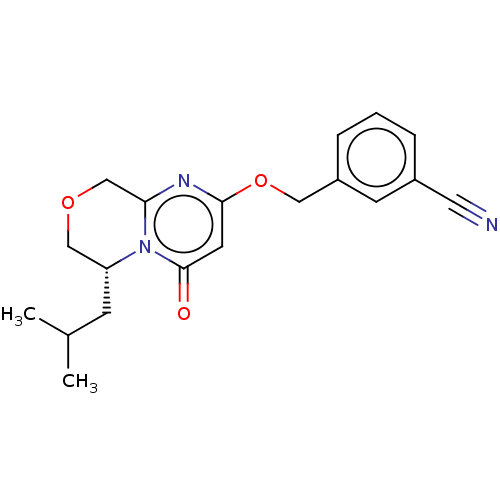 (CHEMBL4091620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008984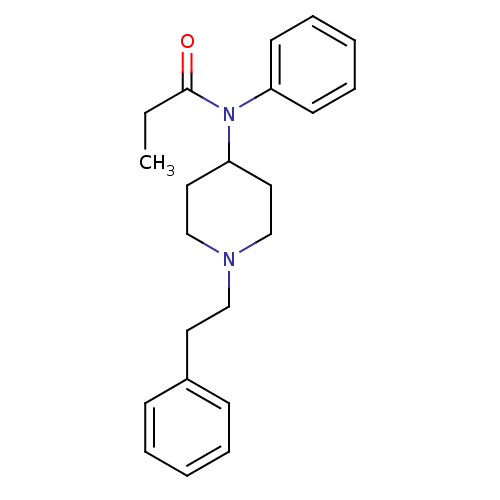 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123655 (3-[(S)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123665 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123665 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123662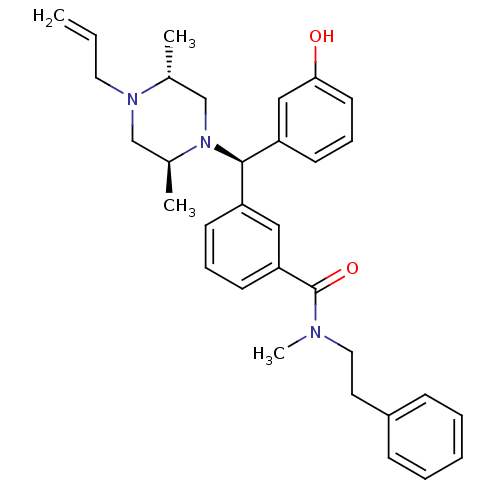 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039026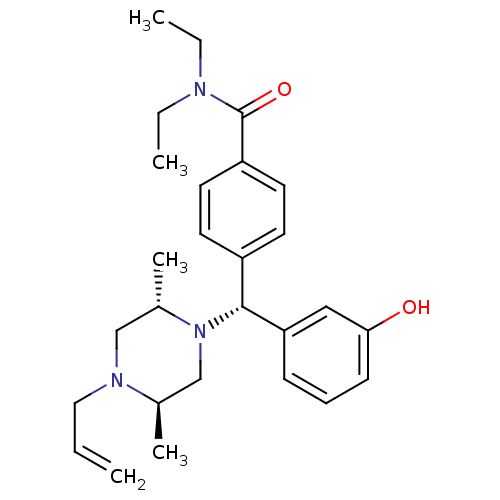 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123664 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123657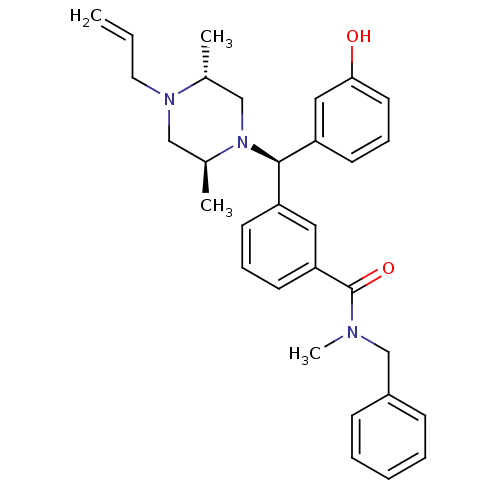 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123660 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123656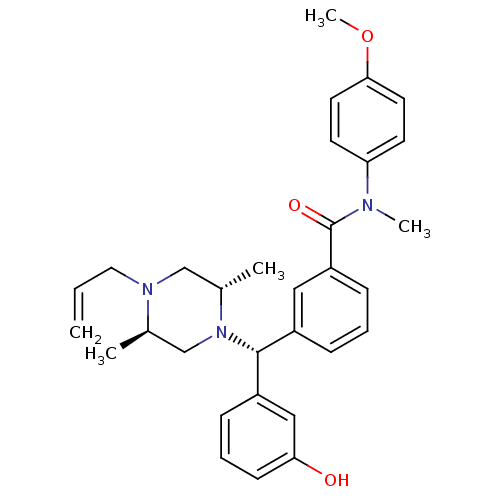 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123652 (3-[(S)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123657 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123650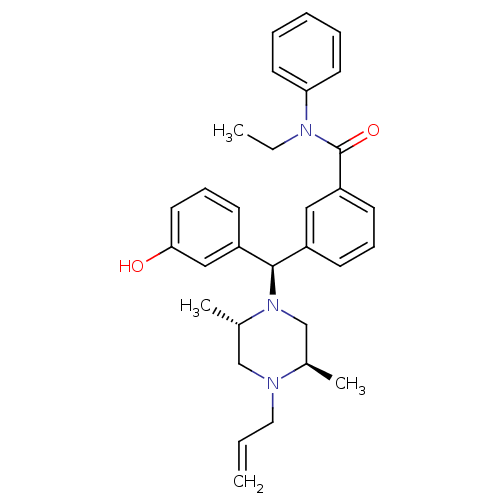 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123662 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123648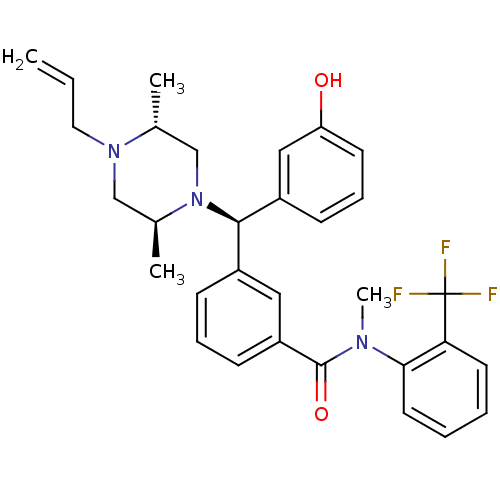 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123656 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123660 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123653 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123654 (CHEMBL157660 | {3-[(R)-((2S,5R)-4-Allyl-2,5-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123646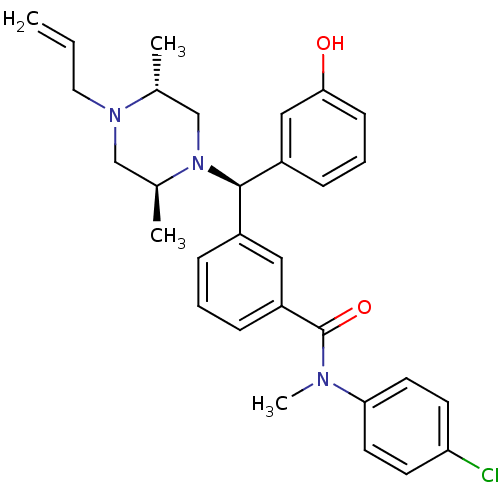 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123648 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123651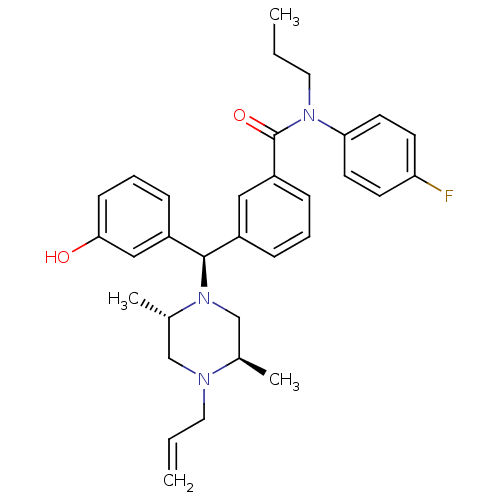 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123653 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240888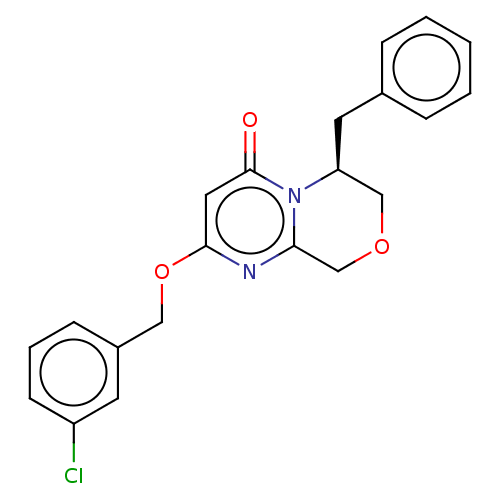 (CHEMBL4094256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123652 (3-[(S)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240881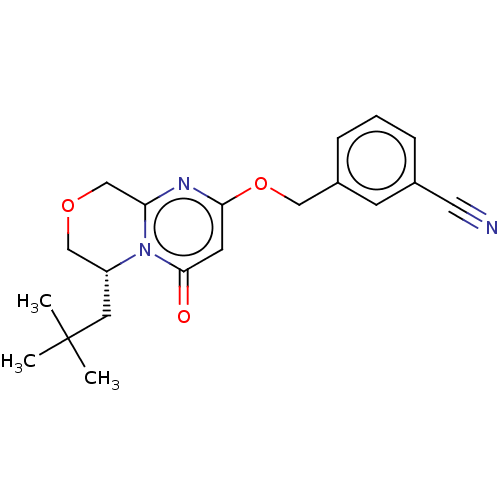 (CHEMBL4090712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123650 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123646 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240885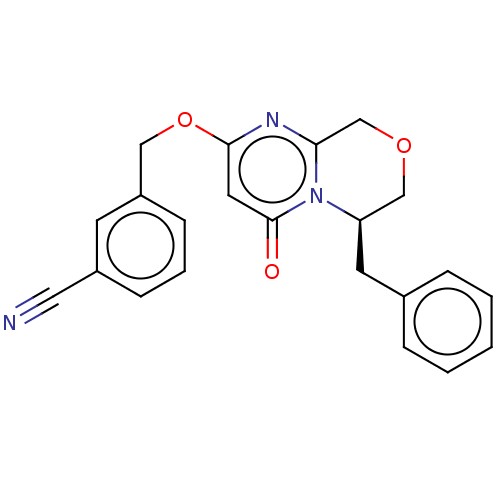 (CHEMBL4064010) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240890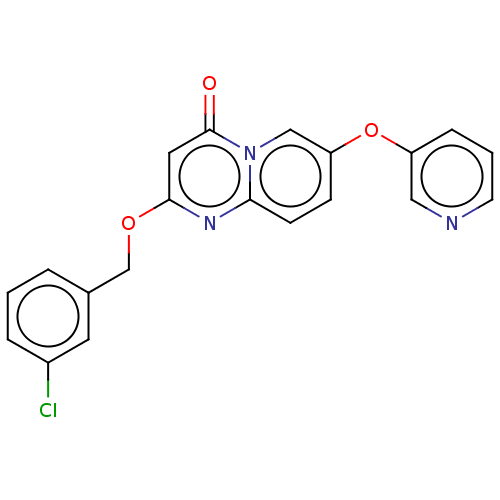 (CHEMBL4078647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240884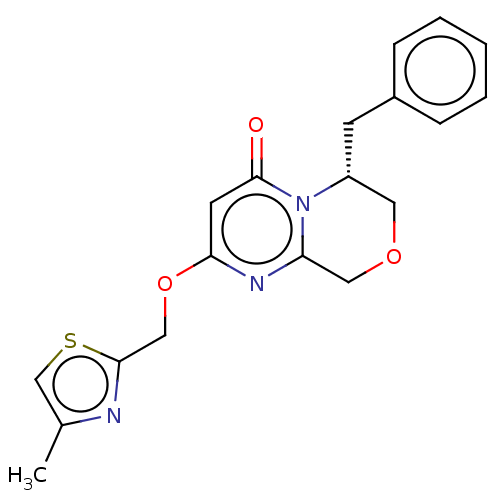 (CHEMBL4085572) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240882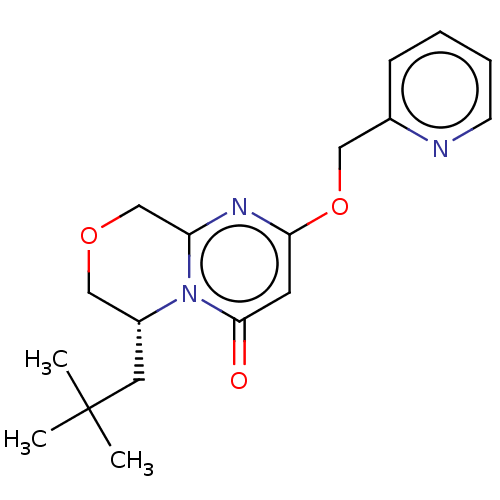 (CHEMBL4063837) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123659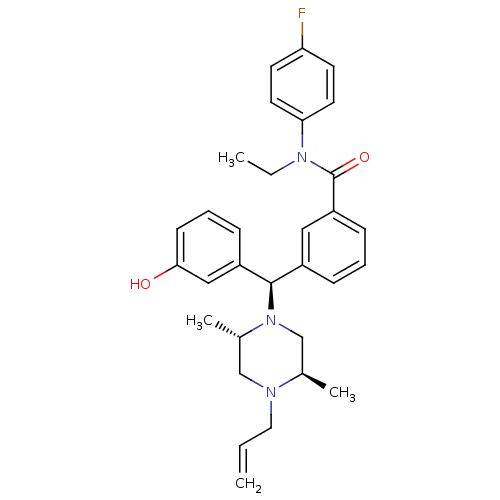 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240879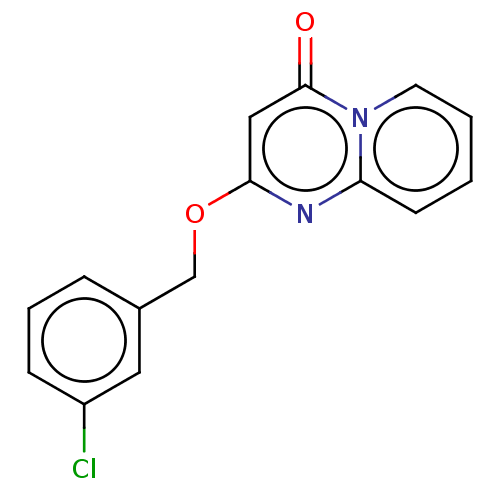 (CHEMBL4089073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123651 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123659 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 506 total ) | Next | Last >> |