 Found 232 hits with Last Name = 'ogawa' and Initial = 'n'
Found 232 hits with Last Name = 'ogawa' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557765

(CHEMBL4791414)Show SMILES Cn1cc(cn1)-c1nc2c(cnn2cc1OCC(C)(C)O)-c1ccnc(OC2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557761

(CHEMBL4758580)Show SMILES COc1cn2ncc(-c3ccnc(OC(C)C)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557764

(CHEMBL4787891)Show SMILES COc1cn2ncc(-c3ccnc(OC4CCC4)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557766

(CHEMBL4751832)Show SMILES Cn1cc(cn1)-c1nc2c(cnn2cc1OCCC(C)(C)O)-c1ccnc(OC2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557742

(CHEMBL4752199) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557753

(CHEMBL4748097) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557760

(CHEMBL4749011)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557756

(CHEMBL4779273) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557755

(CHEMBL4782729) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557744

(CHEMBL4741185) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557763

(CHEMBL4744618)Show SMILES COc1cn2ncc(-c3ccnc(OC4CC4)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557767

(CHEMBL4764221)Show SMILES Cn1cc(cn1)-c1nc2c(cnn2cc1OCCCC(C)(C)O)-c1ccnc(OC2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557759

(CHEMBL4763627)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)-c1cc(C)no1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557746
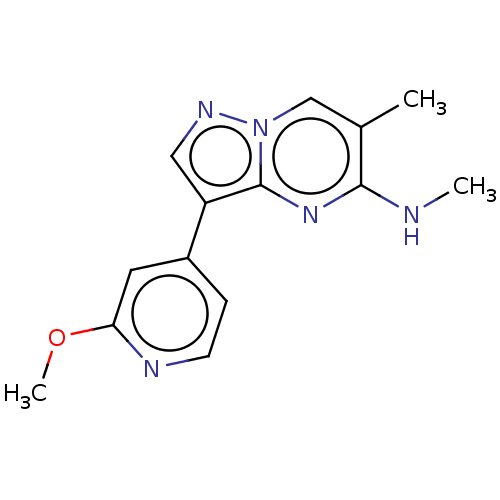
(CHEMBL4779579) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003815
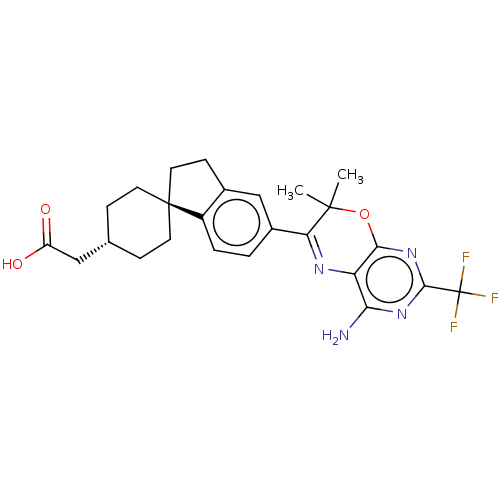
(CHEMBL3235321)Show SMILES CC1(C)Oc2nc(nc(N)c2N=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1)C(F)(F)F |r,wU:20.22,wD:23.26,c:12,(27.23,-21.22,;25.69,-21.22,;26.46,-22.55,;24.36,-22,;23.03,-21.23,;21.69,-22.01,;20.36,-21.24,;20.36,-19.69,;21.69,-18.92,;21.68,-17.38,;23.02,-19.69,;24.35,-18.91,;25.69,-19.67,;27.02,-18.9,;27,-17.37,;28.32,-16.58,;29.67,-17.35,;29.68,-18.88,;31.15,-19.35,;32.04,-18.1,;31.12,-16.86,;30.02,-15.78,;30.42,-14.29,;31.91,-13.88,;32.3,-12.39,;33.79,-11.99,;34.89,-13.08,;34.19,-10.5,;33,-14.97,;32.61,-16.45,;28.35,-19.66,;19.02,-22.01,;17.69,-21.24,;19.02,-23.55,;17.68,-22.77,)| Show InChI InChI=1S/C25H27F3N4O3/c1-23(2)19(30-18-20(29)31-22(25(26,27)28)32-21(18)35-23)15-3-4-16-14(12-15)7-10-24(16)8-5-13(6-9-24)11-17(33)34/h3-4,12-13H,5-11H2,1-2H3,(H,33,34)(H2,29,31,32)/t13-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557757

(CHEMBL4792464)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)[C@H]1COC(C)(C)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557745

(CHEMBL4741913) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557754

(CHEMBL4764416) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557758
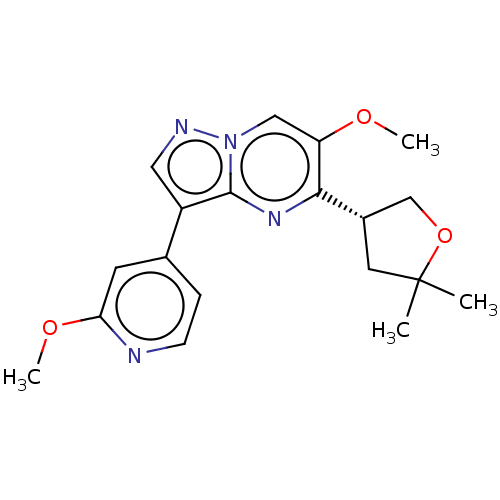
(CHEMBL4749061)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)[C@@H]1COC(C)(C)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557743
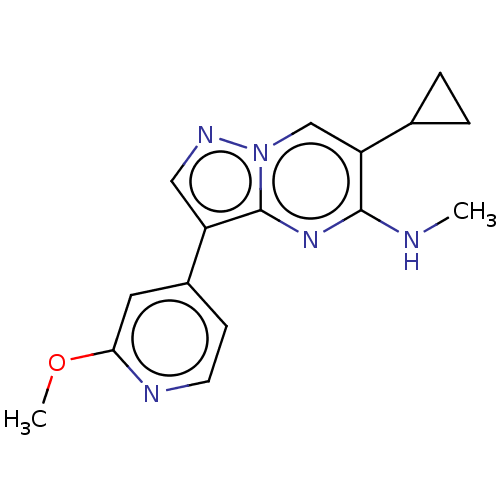
(CHEMBL4744988) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557747

(CHEMBL4795223) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003815

(CHEMBL3235321)Show SMILES CC1(C)Oc2nc(nc(N)c2N=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1)C(F)(F)F |r,wU:20.22,wD:23.26,c:12,(27.23,-21.22,;25.69,-21.22,;26.46,-22.55,;24.36,-22,;23.03,-21.23,;21.69,-22.01,;20.36,-21.24,;20.36,-19.69,;21.69,-18.92,;21.68,-17.38,;23.02,-19.69,;24.35,-18.91,;25.69,-19.67,;27.02,-18.9,;27,-17.37,;28.32,-16.58,;29.67,-17.35,;29.68,-18.88,;31.15,-19.35,;32.04,-18.1,;31.12,-16.86,;30.02,-15.78,;30.42,-14.29,;31.91,-13.88,;32.3,-12.39,;33.79,-11.99,;34.89,-13.08,;34.19,-10.5,;33,-14.97,;32.61,-16.45,;28.35,-19.66,;19.02,-22.01,;17.69,-21.24,;19.02,-23.55,;17.68,-22.77,)| Show InChI InChI=1S/C25H27F3N4O3/c1-23(2)19(30-18-20(29)31-22(25(26,27)28)32-21(18)35-23)15-3-4-16-14(12-15)7-10-24(16)8-5-13(6-9-24)11-17(33)34/h3-4,12-13H,5-11H2,1-2H3,(H,33,34)(H2,29,31,32)/t13-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50337114
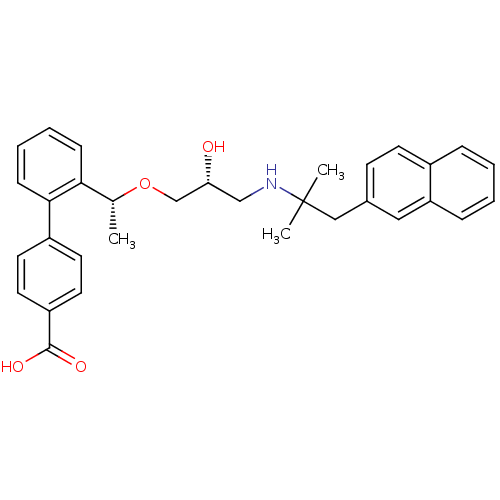
(2'-((1R)-1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...)Show SMILES C[C@@H](OC[C@H](O)CNC(C)(C)Cc1ccc2ccccc2c1)c1ccccc1-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C32H35NO4/c1-22(29-10-6-7-11-30(29)25-14-16-26(17-15-25)31(35)36)37-21-28(34)20-33-32(2,3)19-23-12-13-24-8-4-5-9-27(24)18-23/h4-18,22,28,33-34H,19-21H2,1-3H3,(H,35,36)/t22-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM27947
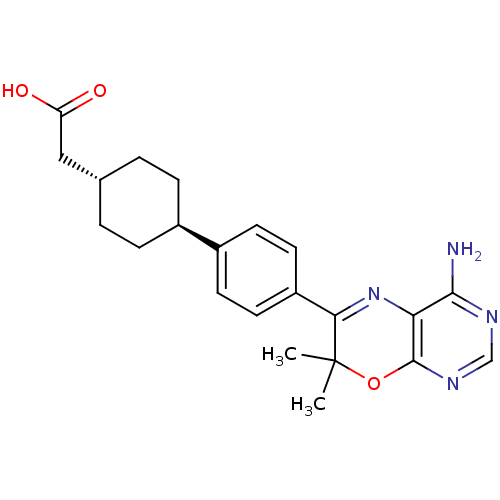
(2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(1.74,.99,;.97,2.32,;2.46,2.72,;-.37,1.55,;-1.7,2.32,;-3.03,1.55,;-4.37,2.32,;-4.37,3.86,;-3.03,4.63,;-3.03,6.17,;-1.7,3.86,;-.37,4.63,;.97,3.86,;2.3,4.63,;2.3,6.17,;3.63,6.94,;4.97,6.17,;4.97,4.63,;3.63,3.86,;6.19,6.75,;6.19,8.3,;7.53,9.07,;8.86,8.3,;10.19,9.07,;11.53,8.3,;12.86,9.07,;11.53,6.76,;8.86,6.75,;7.53,5.98,)| Show InChI InChI=1S/C22H26N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H,27,28)(H2,23,24,25)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50337116
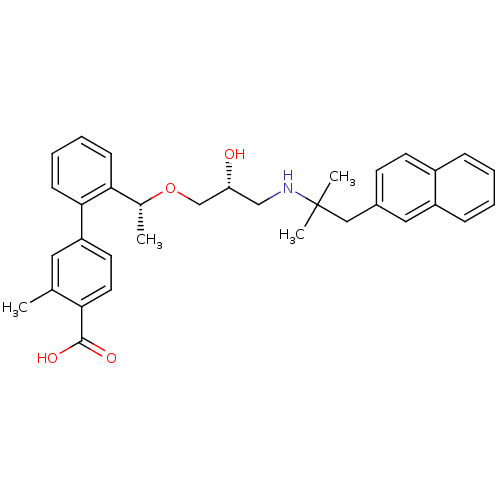
(2'-((1R)-1-{(2R)-3-[2-methyl-1-(naphthalen-2-yl)pr...)Show SMILES C[C@@H](OC[C@H](O)CNC(C)(C)Cc1ccc2ccccc2c1)c1ccccc1-c1ccc(C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C33H37NO4/c1-22-17-27(15-16-29(22)32(36)37)31-12-8-7-11-30(31)23(2)38-21-28(35)20-34-33(3,4)19-24-13-14-25-9-5-6-10-26(25)18-24/h5-18,23,28,34-35H,19-21H2,1-4H3,(H,36,37)/t23-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003860

(CHEMBL3235317)Show SMILES CC1(C)Oc2nc(nc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1)C(F)(F)F |r,wU:19.21,wD:22.25,c:12,(27.64,-8.52,;26.1,-8.52,;26.87,-9.85,;24.77,-9.3,;23.44,-8.53,;22.1,-9.31,;20.77,-8.54,;20.77,-6.99,;22.1,-6.22,;22.1,-4.68,;23.44,-6.99,;24.76,-6.21,;26.1,-6.97,;27.43,-6.19,;28.76,-6.96,;30.09,-6.19,;30.08,-4.64,;28.73,-3.88,;27.41,-4.66,;31.41,-3.86,;31.39,-2.32,;32.72,-1.55,;34.06,-2.31,;35.39,-1.54,;36.73,-2.3,;36.74,-3.84,;38.06,-1.53,;34.06,-3.85,;32.74,-4.63,;19.44,-9.31,;18.1,-8.53,;19.43,-10.85,;18.09,-10.06,)| Show InChI InChI=1S/C23H25F3N4O3/c1-22(2)18(28-17-19(27)29-21(23(24,25)26)30-20(17)33-22)15-9-7-14(8-10-15)13-5-3-12(4-6-13)11-16(31)32/h7-10,12-13H,3-6,11H2,1-2H3,(H,31,32)(H2,27,29,30)/t12-,13- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557739

(CHEMBL4798505) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM27947

(2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(1.74,.99,;.97,2.32,;2.46,2.72,;-.37,1.55,;-1.7,2.32,;-3.03,1.55,;-4.37,2.32,;-4.37,3.86,;-3.03,4.63,;-3.03,6.17,;-1.7,3.86,;-.37,4.63,;.97,3.86,;2.3,4.63,;2.3,6.17,;3.63,6.94,;4.97,6.17,;4.97,4.63,;3.63,3.86,;6.19,6.75,;6.19,8.3,;7.53,9.07,;8.86,8.3,;10.19,9.07,;11.53,8.3,;12.86,9.07,;11.53,6.76,;8.86,6.75,;7.53,5.98,)| Show InChI InChI=1S/C22H26N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H,27,28)(H2,23,24,25)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003857
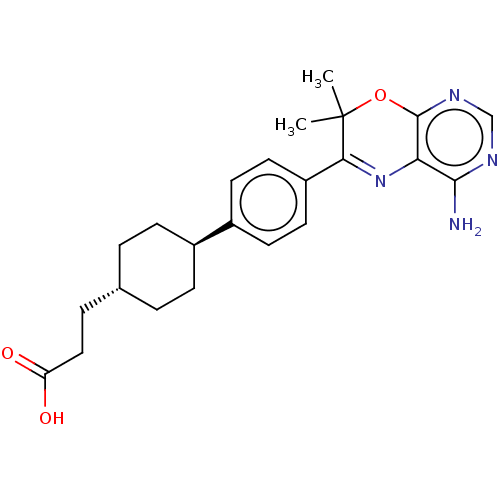
(CHEMBL3235314)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CCC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(25.63,-58.39,;24.09,-58.39,;24.86,-59.72,;22.76,-59.17,;21.42,-58.4,;20.09,-59.18,;18.75,-58.41,;18.76,-56.86,;20.09,-56.09,;20.08,-54.55,;21.42,-56.86,;22.75,-56.08,;24.08,-56.84,;25.41,-56.06,;26.75,-56.83,;28.07,-56.06,;28.07,-54.51,;26.72,-53.75,;25.4,-54.53,;29.39,-53.73,;29.37,-52.2,;30.71,-51.42,;32.05,-52.18,;33.38,-51.41,;34.71,-52.17,;34.72,-53.71,;33.39,-54.49,;36.06,-54.48,;32.05,-53.72,;30.73,-54.5,)| Show InChI InChI=1S/C23H28N4O3/c1-23(2)20(27-19-21(24)25-13-26-22(19)30-23)17-10-8-16(9-11-17)15-6-3-14(4-7-15)5-12-18(28)29/h8-11,13-15H,3-7,12H2,1-2H3,(H,28,29)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003858
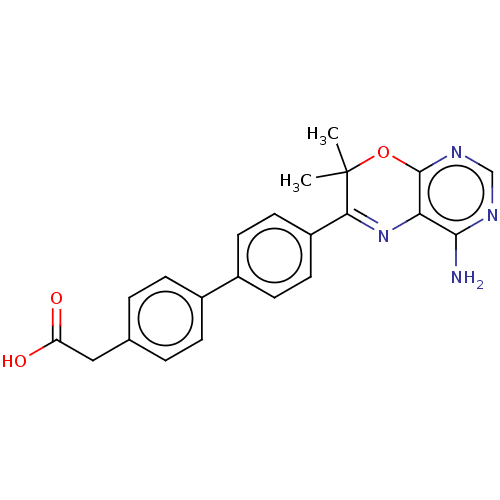
(CHEMBL3235315)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)-c1ccc(CC(O)=O)cc1 |c:12| Show InChI InChI=1S/C22H20N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h3-10,12H,11H2,1-2H3,(H,27,28)(H2,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557762
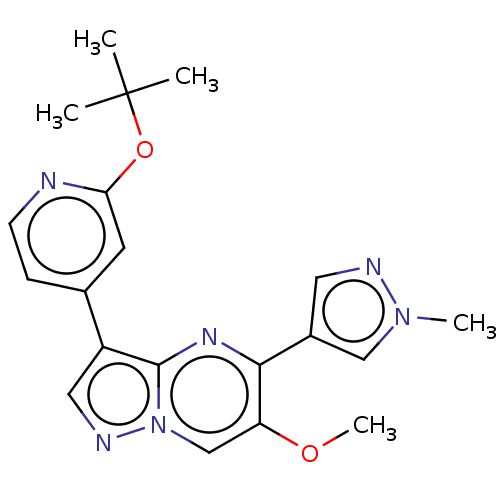
(CHEMBL4747422)Show SMILES COc1cn2ncc(-c3ccnc(OC(C)(C)C)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003862

(CHEMBL3235319)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](Cc2nnn[nH]2)CC1 |r,wU:19.21,wD:22.25,c:12,(58.66,-9.38,;57.12,-9.38,;57.89,-10.71,;55.79,-10.16,;54.46,-9.39,;53.12,-10.17,;51.79,-9.4,;51.79,-7.86,;53.12,-7.08,;53.11,-5.54,;54.45,-7.85,;55.78,-7.08,;57.12,-7.84,;58.45,-7.06,;59.78,-7.83,;61.11,-7.05,;61.1,-5.51,;59.75,-4.75,;58.43,-5.53,;62.43,-4.73,;62.4,-3.19,;63.74,-2.41,;65.08,-3.18,;66.41,-2.4,;67.75,-3.17,;69.14,-2.53,;70.18,-3.67,;69.41,-5.01,;67.9,-4.7,;65.08,-4.72,;63.76,-5.49,)| Show InChI InChI=1S/C22H26N8O/c1-22(2)19(26-18-20(23)24-12-25-21(18)31-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17-27-29-30-28-17/h7-10,12-14H,3-6,11H2,1-2H3,(H2,23,24,25)(H,27,28,29,30)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557740

(CHEMBL4777071) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003859
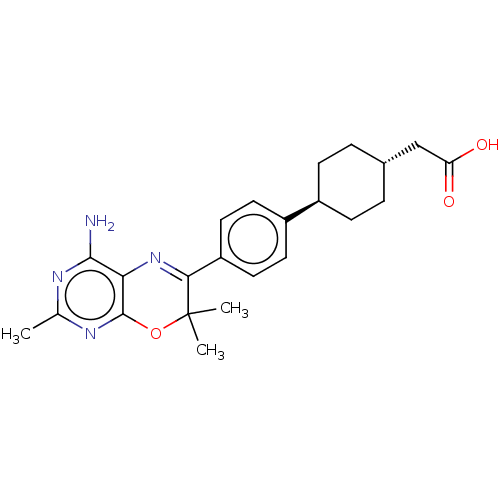
(CHEMBL3235316)Show SMILES Cc1nc(N)c2N=C(c3ccc(cc3)[C@H]3CC[C@H](CC(O)=O)CC3)C(C)(C)Oc2n1 |r,wU:14.14,wD:17.18,t:6,(.98,-9.8,;2.31,-9.03,;2.32,-7.49,;3.64,-6.72,;3.64,-5.18,;4.98,-7.48,;6.31,-6.71,;7.64,-7.47,;8.97,-6.69,;10.3,-7.46,;11.63,-6.68,;11.62,-5.14,;10.28,-4.38,;8.95,-5.16,;12.95,-4.36,;12.93,-2.82,;14.26,-2.05,;15.6,-2.81,;16.93,-2.04,;18.27,-2.8,;18.28,-4.34,;19.61,-2.02,;15.61,-4.35,;14.28,-5.12,;7.65,-9.01,;9.19,-9.01,;8.42,-10.35,;6.32,-9.79,;4.98,-9.03,;3.65,-9.8,)| Show InChI InChI=1S/C23H28N4O3/c1-13-25-21(24)19-22(26-13)30-23(2,3)20(27-19)17-10-8-16(9-11-17)15-6-4-14(5-7-15)12-18(28)29/h8-11,14-15H,4-7,12H2,1-3H3,(H,28,29)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003864

(CHEMBL3235322)Show SMILES CC1(C)Oc2ncnc(N)c2C=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(42.97,-20.87,;41.43,-20.87,;42.2,-22.2,;40.1,-21.65,;38.77,-20.88,;37.43,-21.66,;36.1,-20.89,;36.1,-19.34,;37.43,-18.57,;37.43,-17.03,;38.77,-19.34,;40.09,-18.56,;41.43,-19.32,;42.76,-18.55,;44.09,-19.31,;45.42,-18.54,;45.41,-17,;44.06,-16.23,;42.74,-17.02,;46.74,-16.22,;46.72,-14.68,;48.05,-13.9,;49.39,-14.66,;50.72,-13.89,;52.06,-14.65,;52.07,-16.19,;53.39,-13.88,;49.39,-16.2,;48.07,-16.98,)| Show InChI InChI=1S/C23H27N3O3/c1-23(2)19(12-18-21(24)25-13-26-22(18)29-23)17-9-7-16(8-10-17)15-5-3-14(4-6-15)11-20(27)28/h7-10,12-15H,3-6,11H2,1-2H3,(H,27,28)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003713
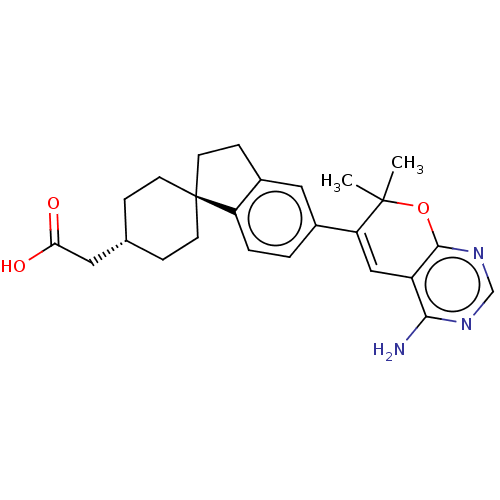
(CHEMBL3235323)Show SMILES CC1(C)Oc2ncnc(N)c2C=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1 |r,wU:20.22,wD:23.26,c:12,(61.12,-21.75,;59.58,-21.75,;60.35,-23.09,;58.25,-22.53,;56.91,-21.76,;55.58,-22.54,;54.24,-21.77,;54.25,-20.23,;55.57,-19.46,;55.57,-17.92,;56.91,-20.22,;58.24,-19.45,;59.57,-20.21,;60.9,-19.43,;60.88,-17.9,;62.21,-17.12,;63.56,-17.88,;63.57,-19.42,;65.03,-19.88,;65.93,-18.63,;65.01,-17.39,;63.91,-16.31,;64.31,-14.82,;65.79,-14.42,;66.19,-12.93,;67.68,-12.52,;68.77,-13.61,;68.08,-11.03,;66.88,-15.5,;66.49,-16.98,;62.24,-20.2,)| Show InChI InChI=1S/C25H29N3O3/c1-24(2)20(13-18-22(26)27-14-28-23(18)31-24)16-3-4-19-17(12-16)7-10-25(19)8-5-15(6-9-25)11-21(29)30/h3-4,12-15H,5-11H2,1-2H3,(H,29,30)(H2,26,27,28)/t15-,25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003856
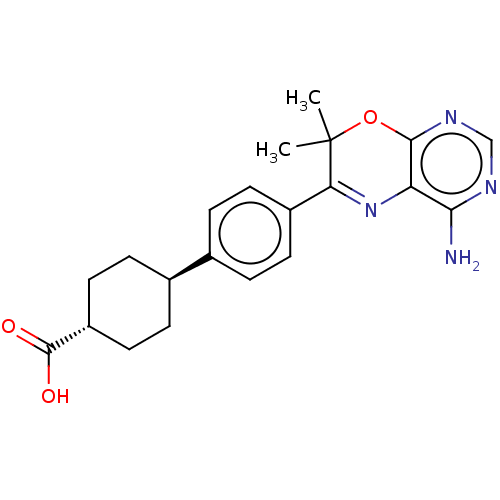
(CHEMBL3235313)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:19.21,wD:22.28,c:12,(6.26,-59.02,;4.72,-59.02,;5.49,-60.36,;3.39,-59.8,;2.06,-59.04,;.72,-59.82,;-.62,-59.04,;-.62,-57.5,;.72,-56.73,;.71,-55.19,;2.05,-57.49,;3.38,-56.72,;4.72,-57.48,;6.05,-56.7,;7.38,-57.47,;8.71,-56.69,;8.7,-55.15,;7.35,-54.39,;6.03,-55.17,;10.03,-54.37,;10,-52.83,;11.34,-52.06,;12.68,-52.82,;12.68,-54.36,;11.36,-55.13,;14.01,-52.05,;15.35,-52.81,;14.01,-50.51,)| Show InChI InChI=1S/C21H24N4O3/c1-21(2)17(25-16-18(22)23-11-24-19(16)28-21)14-7-3-12(4-8-14)13-5-9-15(10-6-13)20(26)27/h3-4,7-8,11,13,15H,5-6,9-10H2,1-2H3,(H,26,27)(H2,22,23,24)/t13-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50337113
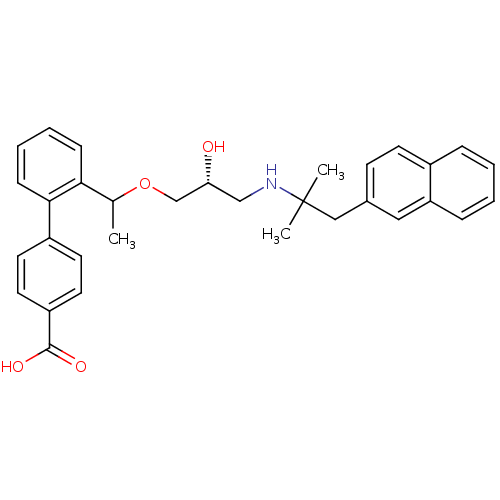
((RS)-2'-(1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...)Show SMILES CC(OC[C@H](O)CNC(C)(C)Cc1ccc2ccccc2c1)c1ccccc1-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C32H35NO4/c1-22(29-10-6-7-11-30(29)25-14-16-26(17-15-25)31(35)36)37-21-28(34)20-33-32(2,3)19-23-12-13-24-8-4-5-9-27(24)18-23/h4-18,22,28,33-34H,19-21H2,1-3H3,(H,35,36)/t22?,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50337103
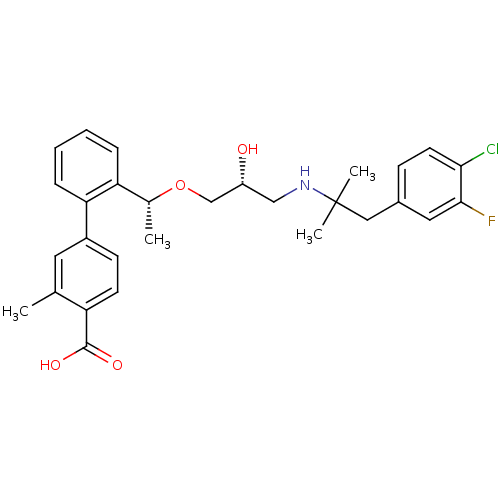
(2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...)Show SMILES C[C@@H](OC[C@H](O)CNC(C)(C)Cc1ccc(Cl)c(F)c1)c1ccccc1-c1ccc(C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C29H33ClFNO4/c1-18-13-21(10-11-23(18)28(34)35)25-8-6-5-7-24(25)19(2)36-17-22(33)16-32-29(3,4)15-20-9-12-26(30)27(31)14-20/h5-14,19,22,32-33H,15-17H2,1-4H3,(H,34,35)/t19-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003713

(CHEMBL3235323)Show SMILES CC1(C)Oc2ncnc(N)c2C=C1c1ccc2c(CC[C@@]22CC[C@H](CC(O)=O)CC2)c1 |r,wU:20.22,wD:23.26,c:12,(61.12,-21.75,;59.58,-21.75,;60.35,-23.09,;58.25,-22.53,;56.91,-21.76,;55.58,-22.54,;54.24,-21.77,;54.25,-20.23,;55.57,-19.46,;55.57,-17.92,;56.91,-20.22,;58.24,-19.45,;59.57,-20.21,;60.9,-19.43,;60.88,-17.9,;62.21,-17.12,;63.56,-17.88,;63.57,-19.42,;65.03,-19.88,;65.93,-18.63,;65.01,-17.39,;63.91,-16.31,;64.31,-14.82,;65.79,-14.42,;66.19,-12.93,;67.68,-12.52,;68.77,-13.61,;68.08,-11.03,;66.88,-15.5,;66.49,-16.98,;62.24,-20.2,)| Show InChI InChI=1S/C25H29N3O3/c1-24(2)20(13-18-22(26)27-14-28-23(18)31-24)16-3-4-19-17(12-16)7-10-25(19)8-5-15(6-9-25)11-21(29)30/h3-4,12-15H,5-11H2,1-2H3,(H,29,30)(H2,26,27,28)/t15-,25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557741

(CHEMBL4750258) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003861
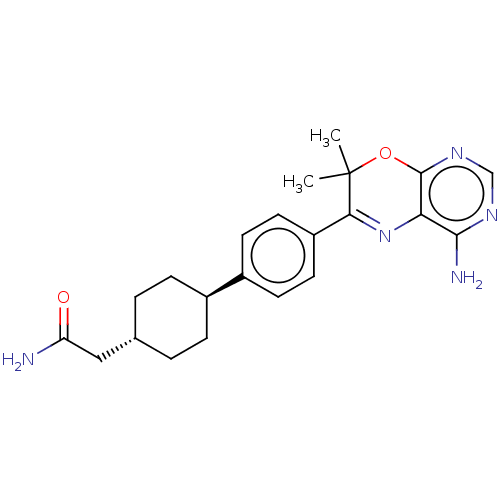
(CHEMBL3235318)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(N)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(42.45,-9.21,;40.91,-9.21,;41.68,-10.54,;39.58,-9.99,;38.25,-9.22,;36.91,-10,;35.58,-9.23,;35.58,-7.68,;36.91,-6.91,;36.9,-5.37,;38.24,-7.68,;39.57,-6.9,;40.91,-7.66,;42.24,-6.89,;43.57,-7.65,;44.9,-6.88,;44.89,-5.34,;43.54,-4.58,;42.22,-5.36,;46.22,-4.56,;46.19,-3.02,;47.53,-2.24,;48.87,-3,;50.2,-2.23,;51.54,-2.99,;51.55,-4.53,;52.87,-2.22,;48.87,-4.55,;47.55,-5.32,)| Show InChI InChI=1S/C22H27N5O2/c1-22(2)19(27-18-20(24)25-12-26-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(23)28/h7-10,12-14H,3-6,11H2,1-2H3,(H2,23,28)(H2,24,25,26)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50557768
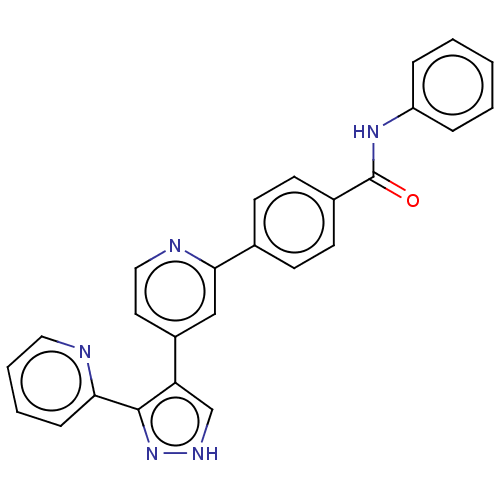
(CHEMBL4752410)Show SMILES O=C(Nc1ccccc1)c1ccc(cc1)-c1cc(ccn1)-c1c[nH]nc1-c1ccccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ALK5 (unknown origin) using biotin-labelled KKKVLTQMGSPSIRCSpSVS substrate in presence of [gamma33P] ATP measured after 40 min |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50337112
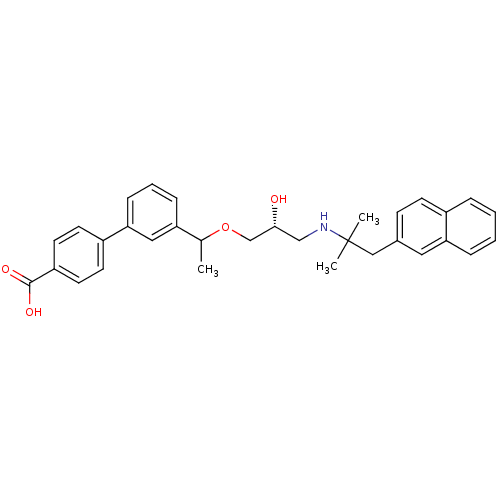
((RS)-3'-(1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...)Show SMILES CC(OC[C@H](O)CNC(C)(C)Cc1ccc2ccccc2c1)c1cccc(c1)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C32H35NO4/c1-22(27-9-6-10-29(18-27)25-13-15-26(16-14-25)31(35)36)37-21-30(34)20-33-32(2,3)19-23-11-12-24-7-4-5-8-28(24)17-23/h4-18,22,30,33-34H,19-21H2,1-3H3,(H,35,36)/t22?,30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50337105
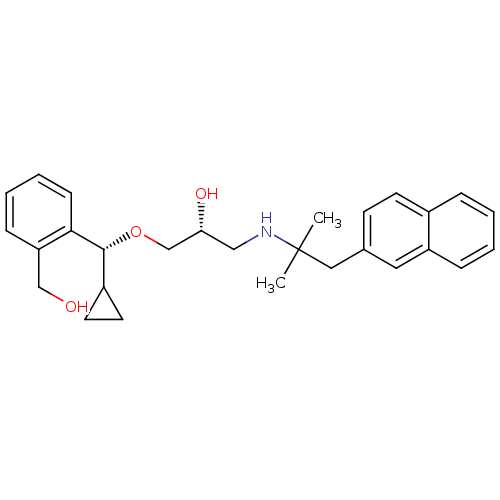
((R)-1-((R)-cyclopropyl(2-(hydroxymethyl)phenyl)met...)Show SMILES CC(C)(Cc1ccc2ccccc2c1)NC[C@@H](O)CO[C@H](C1CC1)c1ccccc1CO |r| Show InChI InChI=1S/C28H35NO3/c1-28(2,16-20-11-12-21-7-3-4-8-23(21)15-20)29-17-25(31)19-32-27(22-13-14-22)26-10-6-5-9-24(26)18-30/h3-12,15,22,25,27,29-31H,13-14,16-19H2,1-2H3/t25-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003863

(CHEMBL3235320)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CS(N)(=O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(8.17,-20.46,;6.63,-20.46,;7.4,-21.8,;5.3,-21.24,;3.97,-20.48,;2.64,-21.25,;1.3,-20.48,;1.3,-18.94,;2.63,-18.17,;2.63,-16.63,;3.97,-18.93,;5.29,-18.16,;6.63,-18.92,;7.96,-18.14,;9.29,-18.91,;10.62,-18.13,;10.61,-16.59,;9.26,-15.83,;7.94,-16.61,;11.94,-15.81,;11.92,-14.27,;13.25,-13.5,;14.59,-14.26,;15.92,-13.48,;17.26,-14.25,;17.27,-15.79,;18.02,-12.91,;18.8,-14.24,;14.6,-15.8,;13.27,-16.57,)| Show InChI InChI=1S/C21H27N5O3S/c1-21(2)18(26-17-19(22)24-12-25-20(17)29-21)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-30(23,27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H2,22,24,25)(H2,23,27,28)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver flag-tagged DGAT1 expressed in baculovirus infected insect cells after 2 hrs by SPA |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50320009
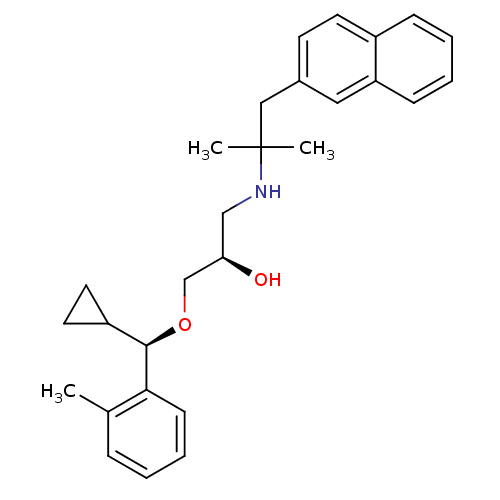
((R)-1-((R)-cyclopropyl(o-tolyl)methoxy)-3-(2-methy...)Show SMILES Cc1ccccc1[C@H](OC[C@H](O)CNC(C)(C)Cc1ccc2ccccc2c1)C1CC1 |r| Show InChI InChI=1S/C28H35NO2/c1-20-8-4-7-11-26(20)27(23-14-15-23)31-19-25(30)18-29-28(2,3)17-21-12-13-22-9-5-6-10-24(22)16-21/h4-13,16,23,25,27,29-30H,14-15,17-19H2,1-3H3/t25-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
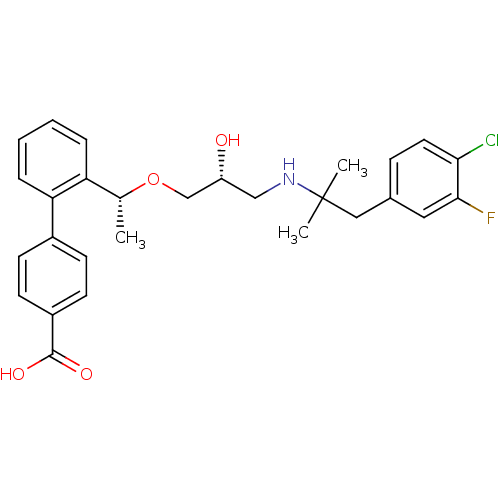
(Homo sapiens (Human)) | BDBM50337115
(2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...)Show SMILES C[C@@H](OC[C@H](O)CNC(C)(C)Cc1ccc(Cl)c(F)c1)c1ccccc1-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C28H31ClFNO4/c1-18(23-6-4-5-7-24(23)20-9-11-21(12-10-20)27(33)34)35-17-22(32)16-31-28(2,3)15-19-8-13-25(29)26(30)14-19/h4-14,18,22,31-32H,15-17H2,1-3H3,(H,33,34)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50337104

(3-[2-((1R)-1-{(2R)-2-Hydroxy-3-[2-methyl-1-(naphth...)Show SMILES C[C@@H](OC[C@H](O)CNC(C)(C)Cc1ccc2ccccc2c1)c1ccccc1CCC(O)=O |r| Show InChI InChI=1S/C28H35NO4/c1-20(26-11-7-6-9-23(26)14-15-27(31)32)33-19-25(30)18-29-28(2,3)17-21-12-13-22-8-4-5-10-24(22)16-21/h4-13,16,20,25,29-30H,14-15,17-19H2,1-3H3,(H,31,32)/t20-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay |
ACS Med Chem Lett 2: 238-242 (2011)
Article DOI: 10.1021/ml100268k
BindingDB Entry DOI: 10.7270/Q21R6QT7 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50003859

(CHEMBL3235316)Show SMILES Cc1nc(N)c2N=C(c3ccc(cc3)[C@H]3CC[C@H](CC(O)=O)CC3)C(C)(C)Oc2n1 |r,wU:14.14,wD:17.18,t:6,(.98,-9.8,;2.31,-9.03,;2.32,-7.49,;3.64,-6.72,;3.64,-5.18,;4.98,-7.48,;6.31,-6.71,;7.64,-7.47,;8.97,-6.69,;10.3,-7.46,;11.63,-6.68,;11.62,-5.14,;10.28,-4.38,;8.95,-5.16,;12.95,-4.36,;12.93,-2.82,;14.26,-2.05,;15.6,-2.81,;16.93,-2.04,;18.27,-2.8,;18.28,-4.34,;19.61,-2.02,;15.61,-4.35,;14.28,-5.12,;7.65,-9.01,;9.19,-9.01,;8.42,-10.35,;6.32,-9.79,;4.98,-9.03,;3.65,-9.8,)| Show InChI InChI=1S/C23H28N4O3/c1-13-25-21(24)19-22(26-13)30-23(2,3)20(27-19)17-10-8-16(9-11-17)15-6-4-14(5-7-15)12-18(28)29/h8-11,14-15H,4-7,12H2,1-3H3,(H,28,29)(H2,24,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by cell-based assay |
J Med Chem 57: 3464-83 (2014)
Article DOI: 10.1021/jm500135c
BindingDB Entry DOI: 10.7270/Q23X886W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data