 Found 2511 hits with Last Name = 'oku' and Initial = 't'
Found 2511 hits with Last Name = 'oku' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131
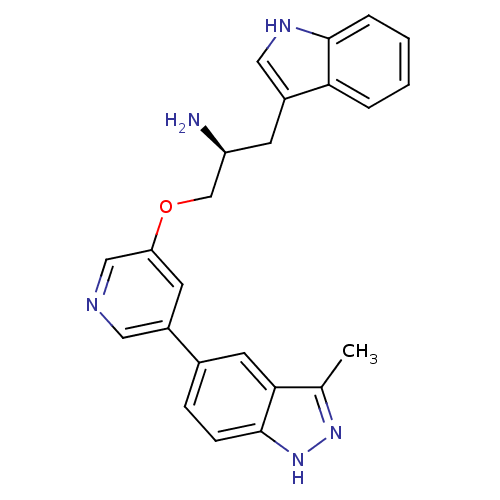
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Nat Chem Biol 5: 484-93 (2009)
Article DOI: 10.1038/nchembio.183
BindingDB Entry DOI: 10.7270/Q2D21XTB |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50523555
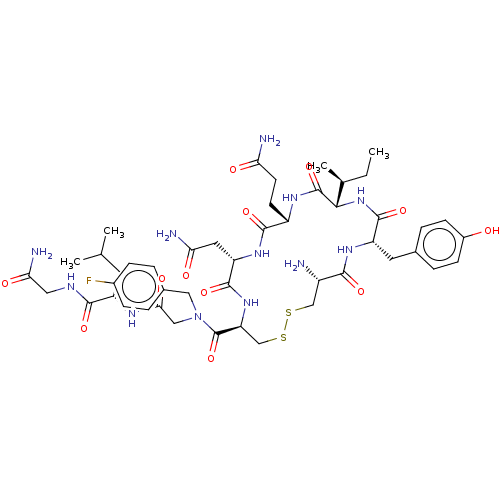
(CHEMBL4474284)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1ccc(F)cc1 |r| Show InChI InChI=1S/C47H67FN12O12S2/c1-5-25(4)40-46(71)55-31(14-15-36(50)62)43(68)57-34(18-37(51)63)44(69)58-35(23-74-73-22-30(49)41(66)56-33(45(70)59-40)17-26-8-12-29(61)13-9-26)47(72)60(20-27-6-10-28(48)11-7-27)21-39(65)54-32(16-24(2)3)42(67)53-19-38(52)64/h6-13,24-25,30-35,40,61H,5,14-23,49H2,1-4H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,67)(H,54,65)(H,55,71)(H,56,66)(H,57,68)(H,58,69)(H,59,70)/t25-,30-,31-,32-,33-,34-,35-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50523556
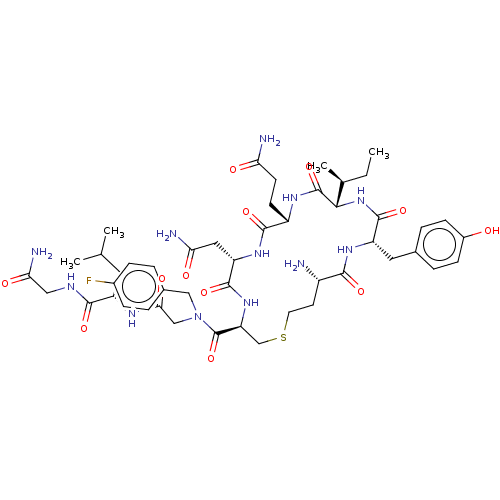
(CHEMBL4458988)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1ccc(F)cc1 |r| Show InChI InChI=1S/C48H69FN12O12S/c1-5-26(4)41-47(72)56-32(14-15-37(51)63)44(69)58-35(20-38(52)64)45(70)59-36(24-74-17-16-31(50)42(67)57-34(46(71)60-41)19-27-8-12-30(62)13-9-27)48(73)61(22-28-6-10-29(49)11-7-28)23-40(66)55-33(18-25(2)3)43(68)54-21-39(53)65/h6-13,25-26,31-36,41,62H,5,14-24,50H2,1-4H3,(H2,51,63)(H2,52,64)(H2,53,65)(H,54,68)(H,55,66)(H,56,72)(H,57,67)(H,58,69)(H,59,70)(H,60,71)/t26-,31-,32-,33-,34-,35-,36-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Cytosolic endo-beta-N-acetylglucosaminidase
(Homo sapiens (Human)) | BDBM50304533
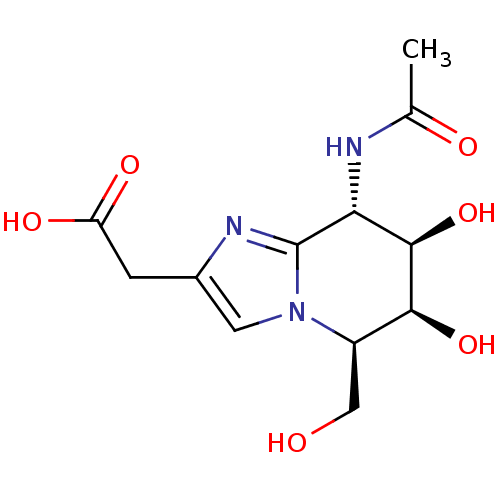
(2-((5R,6S,7R,8S)-8-acetamido-6,7-dihydroxy-5-methy...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)n2cc(CC(O)=O)nc12 |r| Show InChI InChI=1S/C12H17N3O6/c1-5(17)13-9-11(21)10(20)7(4-16)15-3-6(2-8(18)19)14-12(9)15/h3,7,9-11,16,20-21H,2,4H2,1H3,(H,13,17)(H,18,19)/t7-,9-,10+,11-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.925 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human placenta beta-N-acetylglucosaminidase by pNP-GlcNAc substrate hydrolysis assay |
Bioorg Med Chem 17: 7248-53 (2009)
Article DOI: 10.1016/j.bmc.2009.08.052
BindingDB Entry DOI: 10.7270/Q20865D3 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044686
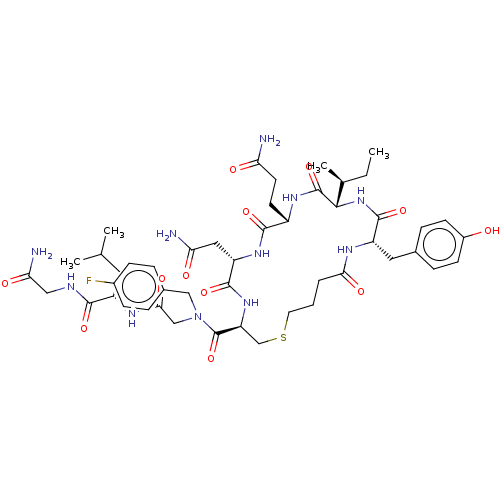
(CHEMBL3354594)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1ccc(F)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C48H68FN11O12S/c1-5-27(4)42-47(71)56-32(16-17-37(50)62)44(68)57-35(21-38(51)63)45(69)58-36(25-73-18-6-7-40(65)54-34(46(70)59-42)20-28-10-14-31(61)15-11-28)48(72)60(23-29-8-12-30(49)13-9-29)24-41(66)55-33(19-26(2)3)43(67)53-22-39(52)64/h8-15,26-27,32-36,42,61H,5-7,16-25H2,1-4H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,67)(H,54,65)(H,55,66)(H,56,71)(H,57,68)(H,58,69)(H,59,70)/t27-,32-,33-,34-,35-,36-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Cytosolic endo-beta-N-acetylglucosaminidase
(Homo sapiens (Human)) | BDBM50304532
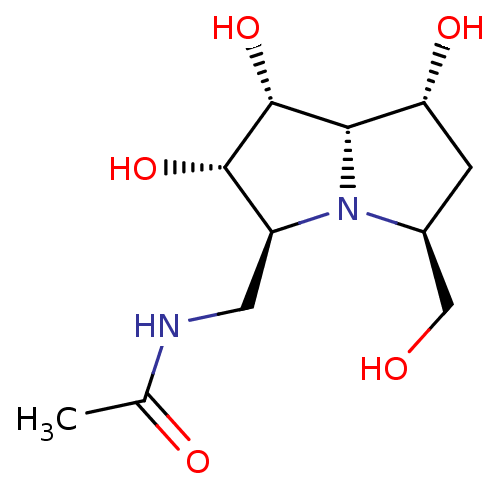
((1R*, 3S*, 5S*, 6S*, 7R*, 7a S*)-5-acetamidomethyl...)Show SMILES CC(=O)NC[C@H]1[C@H](O)[C@H](O)[C@@H]2[C@H](O)C[C@@H](CO)N12 |r| Show InChI InChI=1S/C11H20N2O5/c1-5(15)12-3-7-10(17)11(18)9-8(16)2-6(4-14)13(7)9/h6-11,14,16-18H,2-4H2,1H3,(H,12,15)/t6-,7-,8+,9-,10-,11+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human placenta beta-N-acetylglucosaminidase by pNP-GlcNAc substrate hydrolysis assay |
Bioorg Med Chem 17: 7248-53 (2009)
Article DOI: 10.1016/j.bmc.2009.08.052
BindingDB Entry DOI: 10.7270/Q20865D3 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50523557
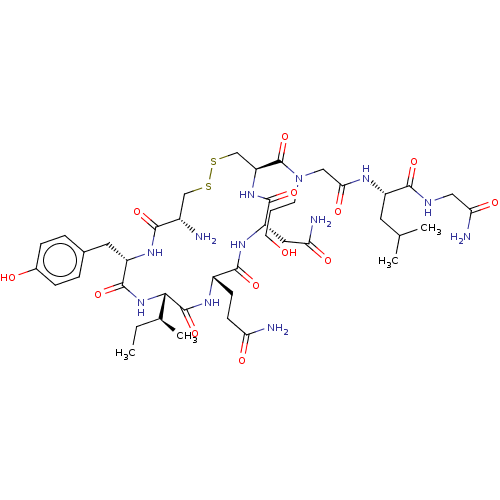
(CHEMBL4583231)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCCO)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H68N12O13S2/c1-5-23(4)36-42(67)50-27(11-12-32(45)58)39(64)52-30(17-33(46)59)40(65)53-31(21-70-69-20-26(44)37(62)51-29(41(66)54-36)16-24-7-9-25(57)10-8-24)43(68)55(13-6-14-56)19-35(61)49-28(15-22(2)3)38(63)48-18-34(47)60/h7-10,22-23,26-31,36,56-57H,5-6,11-21,44H2,1-4H3,(H2,45,58)(H2,46,59)(H2,47,60)(H,48,63)(H,49,61)(H,50,67)(H,51,62)(H,52,64)(H,53,65)(H,54,66)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044742
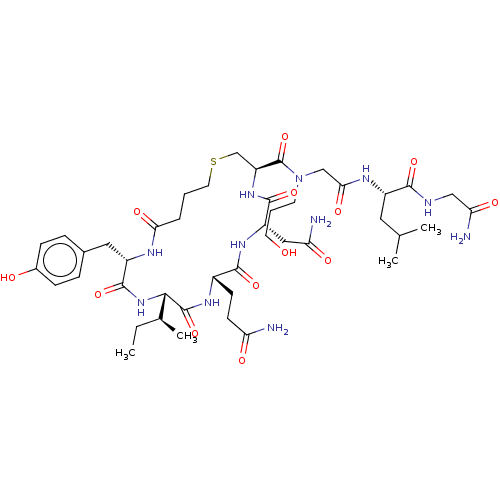
(CHEMBL3354579)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCCO)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C44H69N11O13S/c1-5-25(4)38-43(67)51-28(13-14-33(45)58)40(64)52-31(20-34(46)59)41(65)53-32(23-69-17-6-8-36(61)49-30(42(66)54-38)19-26-9-11-27(57)12-10-26)44(68)55(15-7-16-56)22-37(62)50-29(18-24(2)3)39(63)48-21-35(47)60/h9-12,24-25,28-32,38,56-57H,5-8,13-23H2,1-4H3,(H2,45,58)(H2,46,59)(H2,47,60)(H,48,63)(H,49,61)(H,50,62)(H,51,67)(H,52,64)(H,53,65)(H,54,66)/t25-,28-,29-,30-,31-,32-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044677
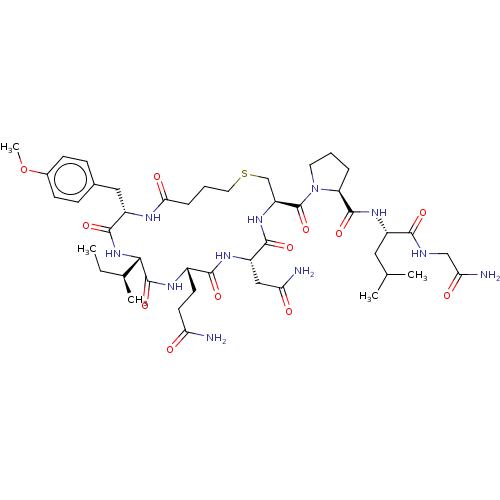
(CHEBI:59204 | Carbetocin)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC Show InChI InChI=1S/C45H69N11O12S/c1-6-25(4)38-44(66)51-28(15-16-34(46)57)40(62)52-31(21-35(47)58)41(63)54-32(23-69-18-8-10-37(60)50-30(42(64)55-38)20-26-11-13-27(68-5)14-12-26)45(67)56-17-7-9-33(56)43(65)53-29(19-24(2)3)39(61)49-22-36(48)59/h11-14,24-25,28-33,38H,6-10,15-23H2,1-5H3,(H2,46,57)(H2,47,58)(H2,48,59)(H,49,61)(H,50,60)(H,51,66)(H,52,62)(H,53,65)(H,54,63)(H,55,64)/t25-,28-,29-,30-,31-,32-,33-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50058163
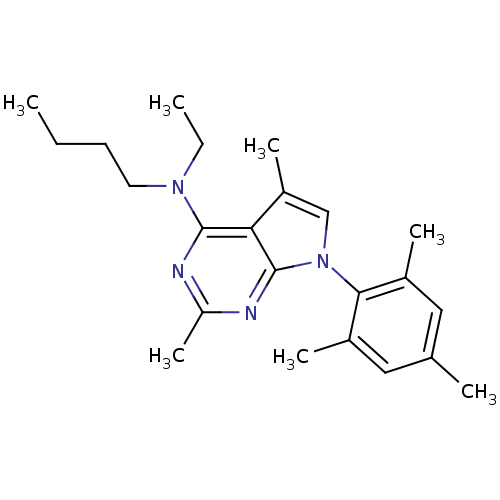
(Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...)Show SMILES CCCCN(CC)c1nc(C)nc2n(cc(C)c12)-c1c(C)cc(C)cc1C |(-10.62,2.69,;-9.29,3.46,;-7.95,2.7,;-6.62,3.48,;-5.28,2.71,;-3.95,3.49,;-3.96,5.03,;-5.27,1.17,;-6.6,.4,;-6.6,-1.14,;-7.94,-1.91,;-5.27,-1.91,;-3.92,-1.14,;-2.44,-1.61,;-1.54,-.35,;-2.46,.9,;-1.99,2.37,;-3.93,.41,;-1.96,-3.07,;-2.99,-4.22,;-4.49,-3.91,;-2.5,-5.68,;-.99,-5.99,;-.51,-7.46,;.03,-4.83,;-.46,-3.38,;.56,-2.22,)| Show InChI InChI=1S/C23H32N4/c1-8-10-11-26(9-2)22-20-18(6)14-27(23(20)25-19(7)24-22)21-16(4)12-15(3)13-17(21)5/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044777
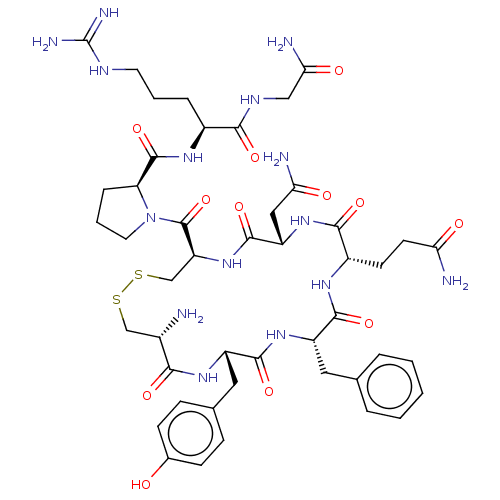
(Arginine Vasopressin | Beta-Hypophamine | CHEBI:34...)Show SMILES N[C@H]1CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50523554
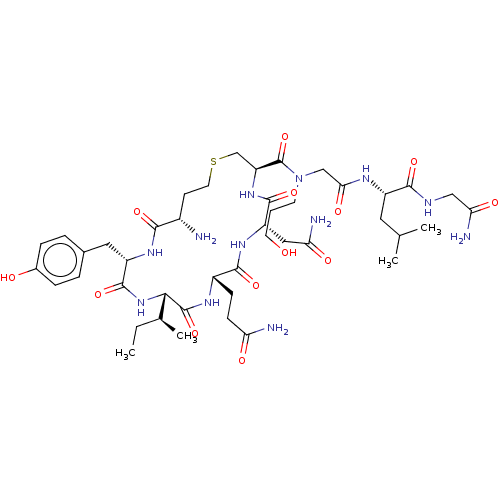
(CHEMBL4467042)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCCO)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C44H70N12O13S/c1-5-24(4)37-43(68)51-28(11-12-33(46)59)40(65)53-31(19-34(47)60)41(66)54-32(22-70-16-13-27(45)38(63)52-30(42(67)55-37)18-25-7-9-26(58)10-8-25)44(69)56(14-6-15-57)21-36(62)50-29(17-23(2)3)39(64)49-20-35(48)61/h7-10,23-24,27-32,37,57-58H,5-6,11-22,45H2,1-4H3,(H2,46,59)(H2,47,60)(H2,48,61)(H,49,64)(H,50,62)(H,51,68)(H,52,63)(H,53,65)(H,54,66)(H,55,67)/t24-,27-,28-,29-,30-,31-,32-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50074456
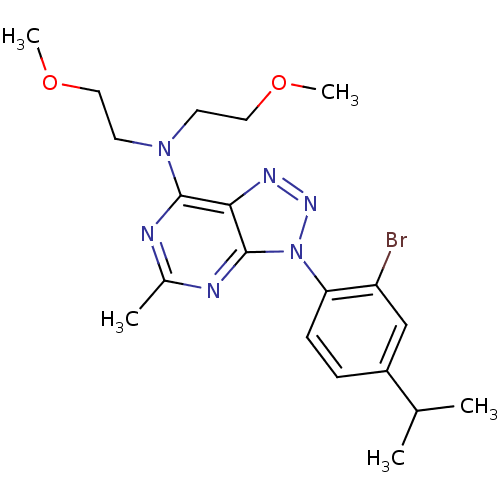
(CHEMBL10504 | SC241 | [3-(2-Bromo-4-isopropyl-phen...)Show SMILES COCCN(CCOC)c1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C20H27BrN6O2/c1-13(2)15-6-7-17(16(21)12-15)27-20-18(24-25-27)19(22-14(3)23-20)26(8-10-28-4)9-11-29-5/h6-7,12-13H,8-11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50197875
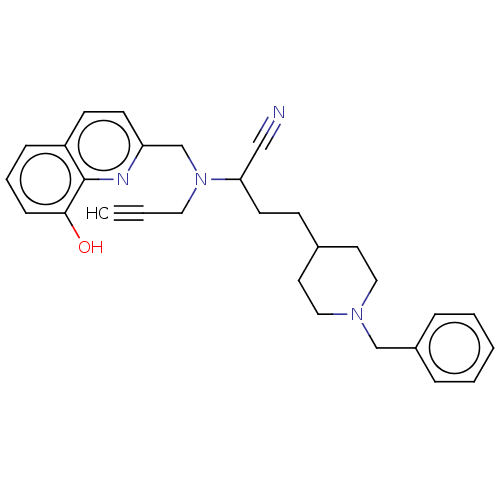
(CHEMBL3910142)Show SMILES Oc1cccc2ccc(CN(CC#C)C(CCC3CCN(Cc4ccccc4)CC3)C#N)nc12 Show InChI InChI=1S/C29H32N4O/c1-2-17-33(22-26-13-12-25-9-6-10-28(34)29(25)31-26)27(20-30)14-11-23-15-18-32(19-16-23)21-24-7-4-3-5-8-24/h1,3-10,12-13,23,27,34H,11,14-19,21-22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... |
Eur J Med Chem 121: 864-879 (2016)
Article DOI: 10.1016/j.ejmech.2015.10.001
BindingDB Entry DOI: 10.7270/Q2B27X8T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50197875

(CHEMBL3910142)Show SMILES Oc1cccc2ccc(CN(CC#C)C(CCC3CCN(Cc4ccccc4)CC3)C#N)nc12 Show InChI InChI=1S/C29H32N4O/c1-2-17-33(22-26-13-12-25-9-6-10-28(34)29(25)31-26)27(20-30)14-11-23-15-18-32(19-16-23)21-24-7-4-3-5-8-24/h1,3-10,12-13,23,27,34H,11,14-19,21-22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins follow... |
Eur J Med Chem 121: 864-879 (2016)
Article DOI: 10.1016/j.ejmech.2015.10.001
BindingDB Entry DOI: 10.7270/Q2B27X8T |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85397
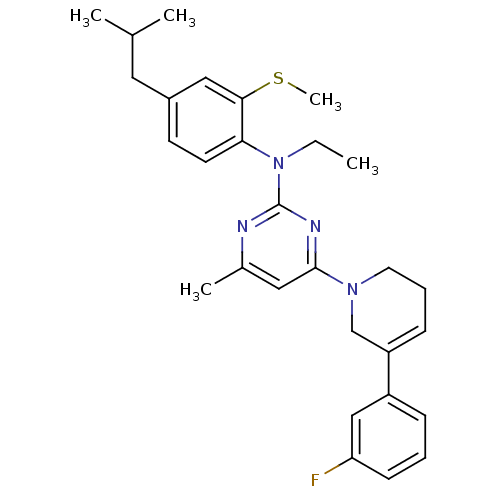
(CRA1000)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1SC |c:14| Show InChI InChI=1S/C29H35FN4S/c1-6-34(26-13-12-22(15-20(2)3)17-27(26)35-5)29-31-21(4)16-28(32-29)33-14-8-10-24(19-33)23-9-7-11-25(30)18-23/h7,9-13,16-18,20H,6,8,14-15,19H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85398
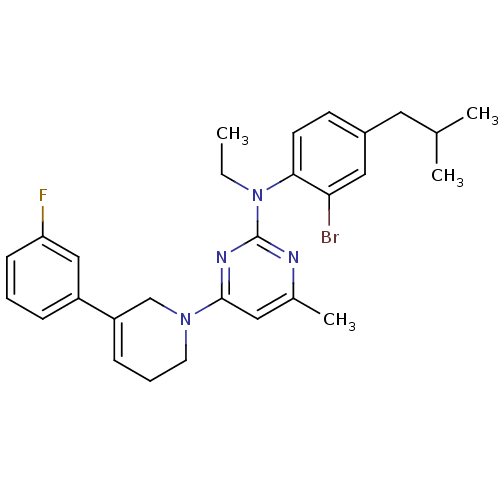
(CRA1001)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1Br |c:14| Show InChI InChI=1S/C28H32BrFN4/c1-5-34(26-12-11-21(14-19(2)3)16-25(26)29)28-31-20(4)15-27(32-28)33-13-7-9-23(18-33)22-8-6-10-24(30)17-22/h6,8-12,15-17,19H,5,7,13-14,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358251
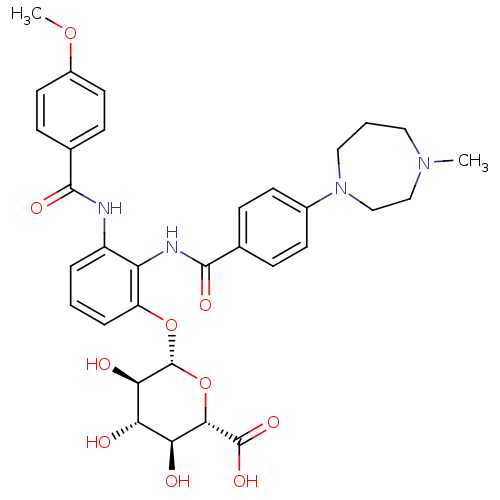
(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85397

(CRA1000)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1SC |c:14| Show InChI InChI=1S/C29H35FN4S/c1-6-34(26-13-12-22(15-20(2)3)17-27(26)35-5)29-31-21(4)16-28(32-29)33-14-8-10-24(19-33)23-9-7-11-25(30)18-23/h7,9-13,16-18,20H,6,8,14-15,19H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 20.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50074456

(CHEMBL10504 | SC241 | [3-(2-Bromo-4-isopropyl-phen...)Show SMILES COCCN(CCOC)c1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C20H27BrN6O2/c1-13(2)15-6-7-17(16(21)12-15)27-20-18(24-25-27)19(22-14(3)23-20)26(8-10-28-4)9-11-29-5/h6-7,12-13H,8-11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85398

(CRA1001)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1Br |c:14| Show InChI InChI=1S/C28H32BrFN4/c1-5-34(26-12-11-21(14-19(2)3)16-25(26)29)28-31-20(4)15-27(32-28)33-13-7-9-23(18-33)22-8-6-10-24(30)17-22/h6,8-12,15-17,19H,5,7,13-14,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 22.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358252
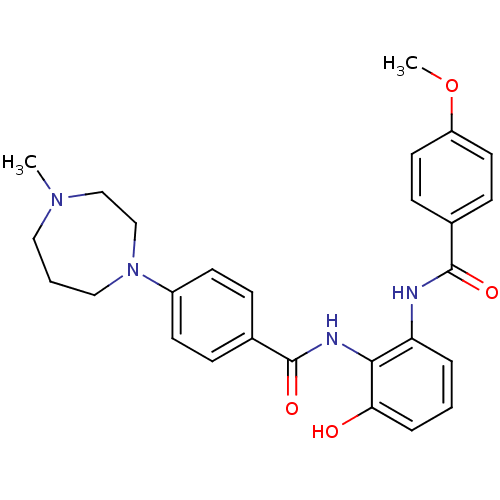
(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50197875

(CHEMBL3910142)Show SMILES Oc1cccc2ccc(CN(CC#C)C(CCC3CCN(Cc4ccccc4)CC3)C#N)nc12 Show InChI InChI=1S/C29H32N4O/c1-2-17-33(22-26-13-12-25-9-6-10-28(34)29(25)31-26)27(20-30)14-11-23-15-18-32(19-16-23)21-24-7-4-3-5-8-24/h1,3-10,12-13,23,27,34H,11,14-19,21-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... |
Eur J Med Chem 121: 864-879 (2016)
Article DOI: 10.1016/j.ejmech.2015.10.001
BindingDB Entry DOI: 10.7270/Q2B27X8T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50197875

(CHEMBL3910142)Show SMILES Oc1cccc2ccc(CN(CC#C)C(CCC3CCN(Cc4ccccc4)CC3)C#N)nc12 Show InChI InChI=1S/C29H32N4O/c1-2-17-33(22-26-13-12-25-9-6-10-28(34)29(25)31-26)27(20-30)14-11-23-15-18-32(19-16-23)21-24-7-4-3-5-8-24/h1,3-10,12-13,23,27,34H,11,14-19,21-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins fo... |
Eur J Med Chem 121: 864-879 (2016)
Article DOI: 10.1016/j.ejmech.2015.10.001
BindingDB Entry DOI: 10.7270/Q2B27X8T |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 2
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(RAT) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Monkey) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50018671
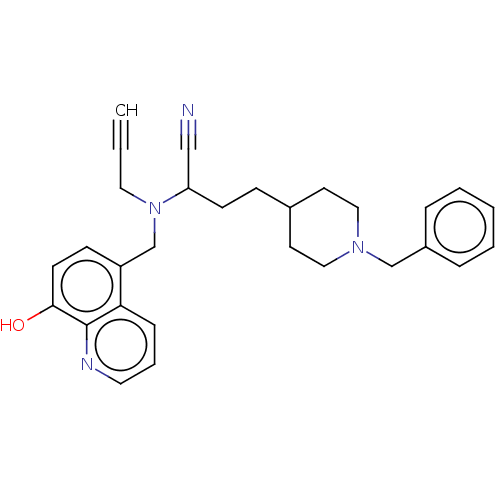
(CHEMBL3291019)Show SMILES Oc1ccc(CN(CC#C)C(CCC2CCN(Cc3ccccc3)CC2)C#N)c2cccnc12 Show InChI InChI=1S/C29H32N4O/c1-2-17-33(22-25-11-13-28(34)29-27(25)9-6-16-31-29)26(20-30)12-10-23-14-18-32(19-15-23)21-24-7-4-3-5-8-24/h1,3-9,11,13,16,23,26,34H,10,12,14-15,17-19,21-22H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Reversible inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 80: 543-61 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.078
BindingDB Entry DOI: 10.7270/Q2BK1DWK |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1A4
(Rattus norvegicus) | BDBM18957
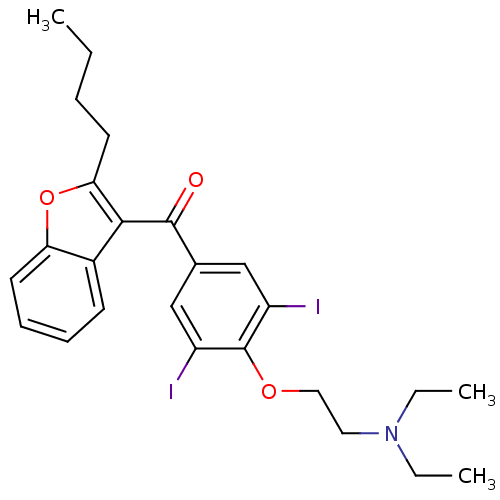
((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...)Show SMILES CCCCc1oc2ccccc2c1C(=O)c1cc(I)c(OCCN(CC)CC)c(I)c1 Show InChI InChI=1S/C25H29I2NO3/c1-4-7-11-22-23(18-10-8-9-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-13-28(5-2)6-3/h8-10,12,15-16H,4-7,11,13-14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of Digoxin uptake in Xenopus laevis oocytes |
Endocrinology 142: 2005-12 (2001)
Article DOI: 10.1210/endo.142.5.8115
BindingDB Entry DOI: 10.7270/Q2GF0XB3 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM85398

(CRA1001)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1Br |c:14| Show InChI InChI=1S/C28H32BrFN4/c1-5-34(26-12-11-21(14-19(2)3)16-25(26)29)28-31-20(4)15-27(32-28)33-13-7-9-23(18-33)22-8-6-10-24(30)17-22/h6,8-12,15-17,19H,5,7,13-14,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM50074456

(CHEMBL10504 | SC241 | [3-(2-Bromo-4-isopropyl-phen...)Show SMILES COCCN(CCOC)c1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C20H27BrN6O2/c1-13(2)15-6-7-17(16(21)12-15)27-20-18(24-25-27)19(22-14(3)23-20)26(8-10-28-4)9-11-29-5/h6-7,12-13H,8-11H2,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM50058163

(Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...)Show SMILES CCCCN(CC)c1nc(C)nc2n(cc(C)c12)-c1c(C)cc(C)cc1C |(-10.62,2.69,;-9.29,3.46,;-7.95,2.7,;-6.62,3.48,;-5.28,2.71,;-3.95,3.49,;-3.96,5.03,;-5.27,1.17,;-6.6,.4,;-6.6,-1.14,;-7.94,-1.91,;-5.27,-1.91,;-3.92,-1.14,;-2.44,-1.61,;-1.54,-.35,;-2.46,.9,;-1.99,2.37,;-3.93,.41,;-1.96,-3.07,;-2.99,-4.22,;-4.49,-3.91,;-2.5,-5.68,;-.99,-5.99,;-.51,-7.46,;.03,-4.83,;-.46,-3.38,;.56,-2.22,)| Show InChI InChI=1S/C23H32N4/c1-8-10-11-26(9-2)22-20-18(6)14-27(23(20)25-19(7)24-22)21-16(4)12-15(3)13-17(21)5/h12-14H,8-11H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM85397

(CRA1000)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1SC |c:14| Show InChI InChI=1S/C29H35FN4S/c1-6-34(26-13-12-22(15-20(2)3)17-27(26)35-5)29-31-21(4)16-28(32-29)33-14-8-10-24(19-33)23-9-7-11-25(30)18-23/h7,9-13,16-18,20H,6,8,14-15,19H2,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5
(Homo sapiens (Human)) | BDBM50366926
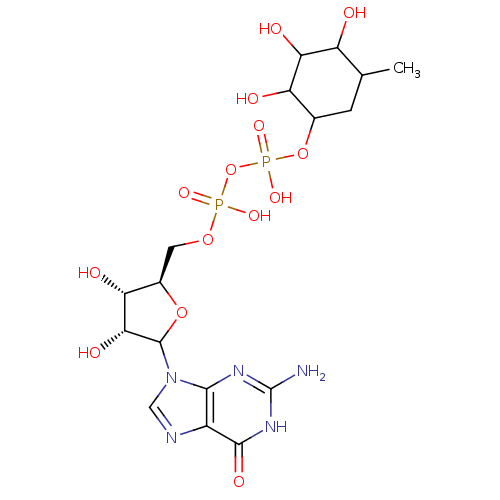
(CHEMBL609638)Show SMILES CC1CC(OP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c2nc(N)[nH]c3=O)C(O)C(O)C1O |r| Show InChI InChI=1S/C17H27N5O14P2/c1-5-2-6(10(24)12(26)9(5)23)35-38(31,32)36-37(29,30)33-3-7-11(25)13(27)16(34-7)22-4-19-8-14(22)20-17(18)21-15(8)28/h4-7,9-13,16,23-27H,2-3H2,1H3,(H,29,30)(H,31,32)(H3,18,20,21,28)/t5?,6?,7-,9?,10?,11-,12?,13-,16?/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California
Curated by ChEMBL
| Assay Description
Inhibition of Fucosyltransferase 5 by the compound was evaluated; Competitive inhibition |
Bioorg Med Chem Lett 14: 571-3 (2004)
BindingDB Entry DOI: 10.7270/Q2MS3TBP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data