

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Opioid receptor delta 1 in rat brain homogenates using [3H]DIDI as radioligand | Bioorg Med Chem Lett 7: 151-156 (1997) Article DOI: 10.1016/S0960-894X(96)00599-9 BindingDB Entry DOI: 10.7270/Q2474BBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50494549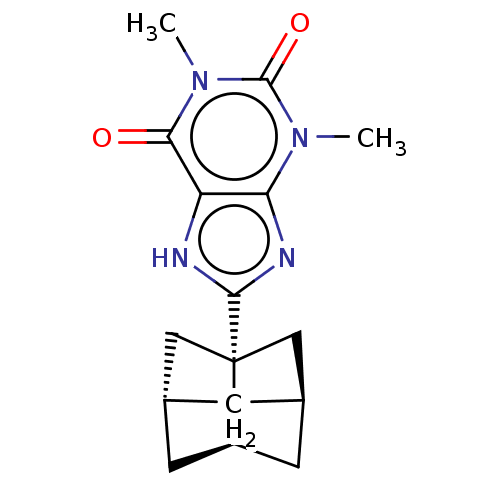 (CHEMBL3093327) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Binding affinity to rat adenosine A1 receptor | Bioorg Med Chem 21: 7435-52 (2013) Article DOI: 10.1016/j.bmc.2013.09.044 BindingDB Entry DOI: 10.7270/Q2TT4TWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087064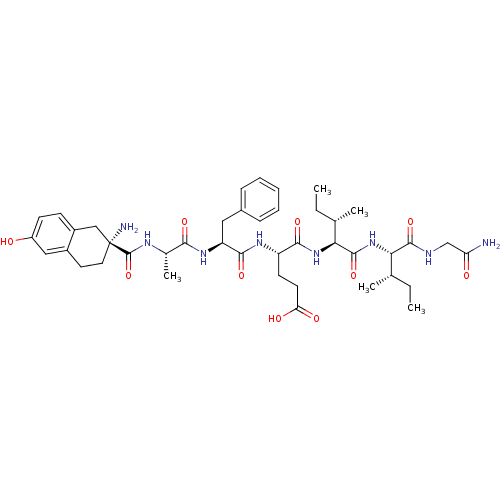 (4-(2-{2-[(2-Amino-6-hydroxy-1,2,3,4-tetrahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of rat brain using [3H]-pClPhe4-DPDPE as radioligand. | J Med Chem 43: 1359-66 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 17: 3037-42 (2009) Article DOI: 10.1016/j.bmc.2009.03.014 BindingDB Entry DOI: 10.7270/Q2SF2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50535225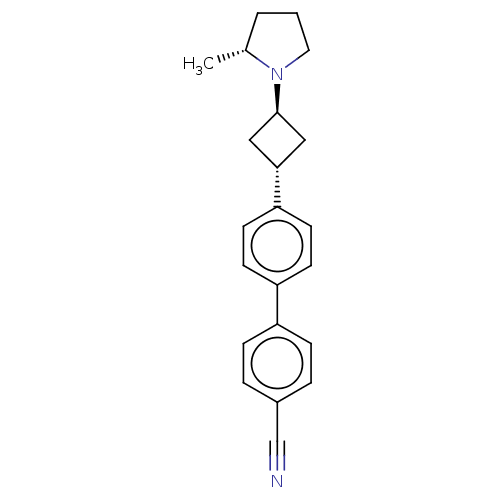 (CHEMBL4516622) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Binding affinity to human H3R | Bioorg Med Chem 25: 5341-5354 (2017) Article DOI: 10.1016/j.bmc.2017.07.058 BindingDB Entry DOI: 10.7270/Q29W0K0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM50455533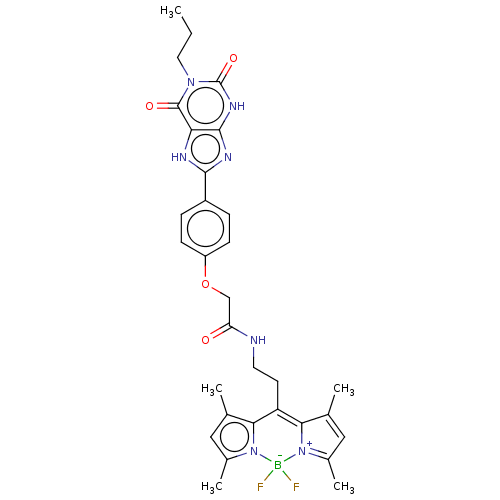 (CHEMBL4205635) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-603 from recombinant rat adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation countin... | J Med Chem 61: 4301-4316 (2018) Article DOI: 10.1021/acs.jmedchem.7b01627 BindingDB Entry DOI: 10.7270/Q23R0WG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting | Bioorg Med Chem 19: 1349-60 (2011) Article DOI: 10.1016/j.bmc.2010.11.051 BindingDB Entry DOI: 10.7270/Q2TT4TS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human H3R | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113041 BindingDB Entry DOI: 10.7270/Q2MP570W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of human H3 receptor | Eur J Med Chem 152: 223-234 (2018) Article DOI: 10.1016/j.ejmech.2018.04.043 BindingDB Entry DOI: 10.7270/Q2697632 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem 25: 2701-2712 (2017) Article DOI: 10.1016/j.bmc.2017.03.031 BindingDB Entry DOI: 10.7270/Q2833VPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human H3R | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113041 BindingDB Entry DOI: 10.7270/Q2MP570W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50393167 (CHEMBL2153721) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]CPX from adenosine A1 receptor in rat brain cortical membrane | Eur J Med Chem 46: 3590-607 (2011) Article DOI: 10.1016/j.ejmech.2011.05.023 BindingDB Entry DOI: 10.7270/Q2ZC840S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Opioid receptor delta 1 in rat brain homogenates using [3H]naltrindole as radioligand | Bioorg Med Chem Lett 7: 151-156 (1997) Article DOI: 10.1016/S0960-894X(96)00599-9 BindingDB Entry DOI: 10.7270/Q2474BBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087064 (4-(2-{2-[(2-Amino-6-hydroxy-1,2,3,4-tetrahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by ChEMBL | Assay Description Binding affinities against delta 2 opioid receptor of rat brain using [3H]Ile5,6-deltorphin II as radioligand. | J Med Chem 43: 1359-66 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087065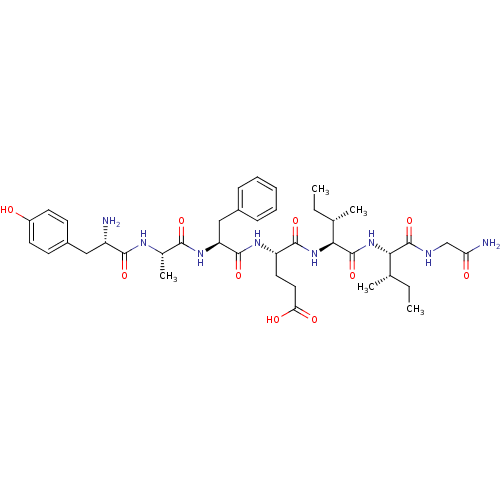 (4-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by ChEMBL | Assay Description Binding affinities against delta 2 opioid receptor of rat brain using [3H]Ile5,6-deltorphin II as radioligand. | J Med Chem 43: 1359-66 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50021325 (4-methyl-(1S,5R,13R,14S)-12-oxa-4-azapentacyclo[9....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Eur J Pharmacol 383: 209-14 (1999) Article DOI: 10.1016/s0014-2999(99)00610-x BindingDB Entry DOI: 10.7270/Q2TT4PJ9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158595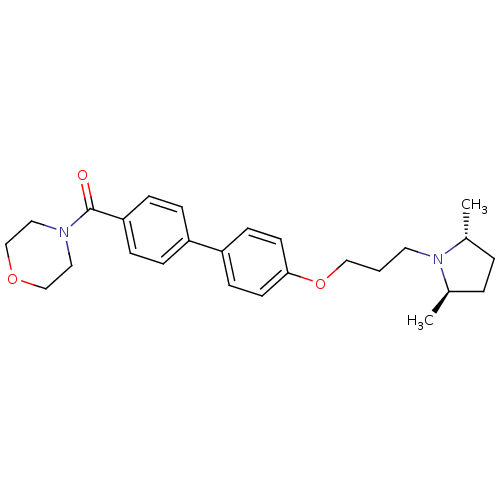 ((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Binding affinity to human H3R expressed in rat C6 cells | Bioorg Med Chem 25: 5341-5354 (2017) Article DOI: 10.1016/j.bmc.2017.07.058 BindingDB Entry DOI: 10.7270/Q29W0K0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Effect at histamine H3 receptors (in vitro) on synaptosomes of rat cerebral cortex assayed by functional H3-receptor assay. | J Med Chem 42: 593-600 (1999) Article DOI: 10.1021/jm9804376 BindingDB Entry DOI: 10.7270/Q2GH9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM28583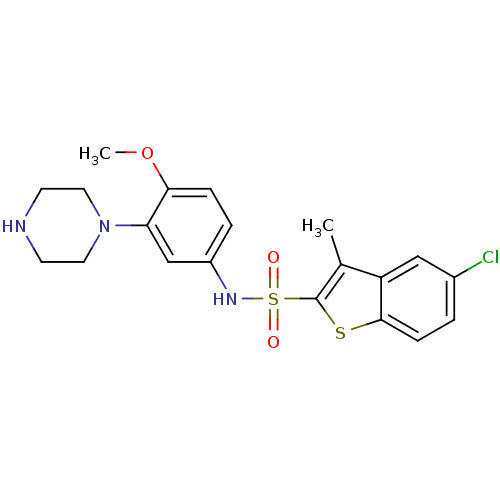 (5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl. Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells after 1 hr by liquid scintillation counting method | Eur J Med Chem 135: 117-124 (2017) Article DOI: 10.1016/j.ejmech.2017.04.033 BindingDB Entry DOI: 10.7270/Q2QN696C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50494549 (CHEMBL3093327) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Binding affinity to human adenosine A1 receptor | Bioorg Med Chem 21: 7435-52 (2013) Article DOI: 10.1016/j.bmc.2013.09.044 BindingDB Entry DOI: 10.7270/Q2TT4TWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human full-length histamine H3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50419052 (SB-399885) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl. Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells | Eur J Med Chem 135: 117-124 (2017) Article DOI: 10.1016/j.ejmech.2017.04.033 BindingDB Entry DOI: 10.7270/Q2QN696C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50290387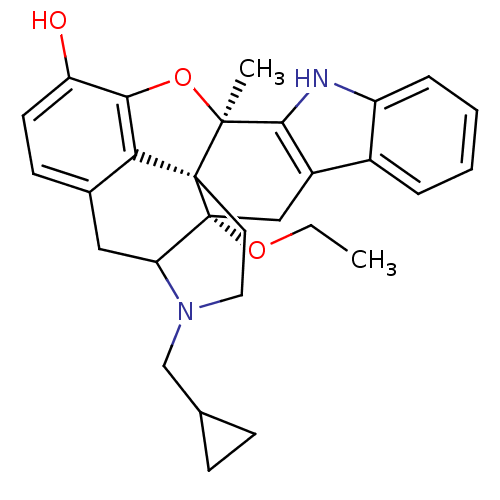 (22-cyclopropylmethyl-2-ethoxy-13-methyl-14-oxa-11,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Opioid receptor delta 1 in rat brain homogenates using [3H]naltrindole as radioligand | Bioorg Med Chem Lett 7: 151-156 (1997) Article DOI: 10.1016/S0960-894X(96)00599-9 BindingDB Entry DOI: 10.7270/Q2474BBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004518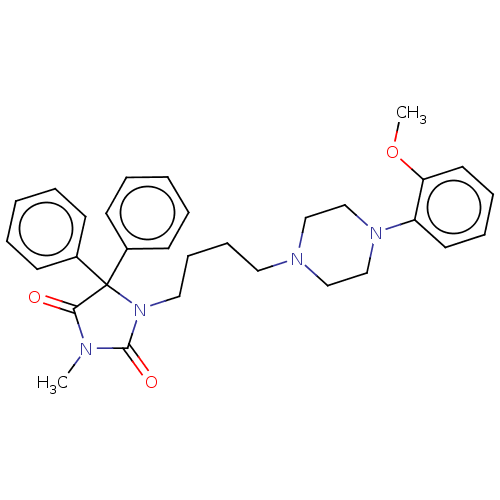 (CHEMBL2079256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in HEK293 cell membrane after 1 hr by Microbeta scintillation countin... | Eur J Med Chem 78: 324-39 (2014) Article DOI: 10.1016/j.ejmech.2014.01.065 BindingDB Entry DOI: 10.7270/Q2J104Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H] raclopride from human recombinant D2L receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting... | Eur J Med Chem 170: 261-275 (2019) Article DOI: 10.1016/j.ejmech.2019.03.017 BindingDB Entry DOI: 10.7270/Q2222Z6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Eur J Pharmacol 383: 209-14 (1999) Article DOI: 10.1016/s0014-2999(99)00610-x BindingDB Entry DOI: 10.7270/Q2TT4PJ9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087063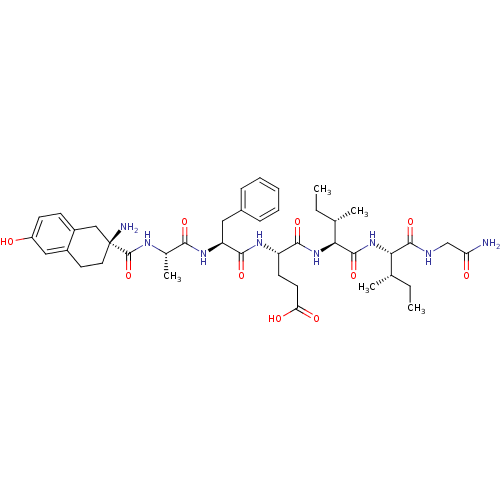 (4-(2-{2-[(2-Amino-6-hydroxy-1,2,3,4-tetrahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of rat brain using [3H]-pClPhe4-DPDPE as radioligand. | J Med Chem 43: 1359-66 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087063 (4-(2-{2-[(2-Amino-6-hydroxy-1,2,3,4-tetrahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by ChEMBL | Assay Description Binding affinities against delta 2 opioid receptor of rat brain using [3H]Ile5,6-deltorphin II as radioligand. | J Med Chem 43: 1359-66 (2001) BindingDB Entry DOI: 10.7270/Q2MS3S0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50514105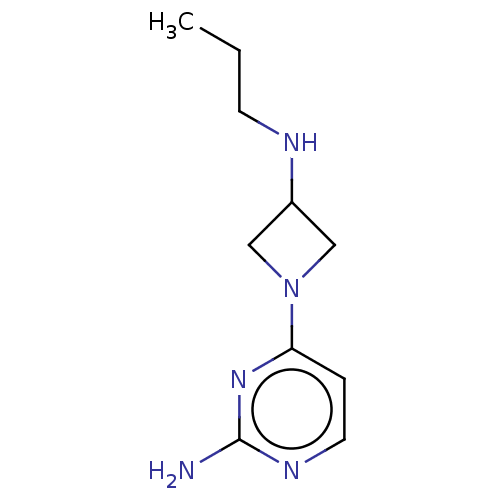 (CHEMBL4441731) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from mouse H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Eur J Pharmacol 383: 209-14 (1999) Article DOI: 10.1016/s0014-2999(99)00610-x BindingDB Entry DOI: 10.7270/Q2TT4PJ9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146835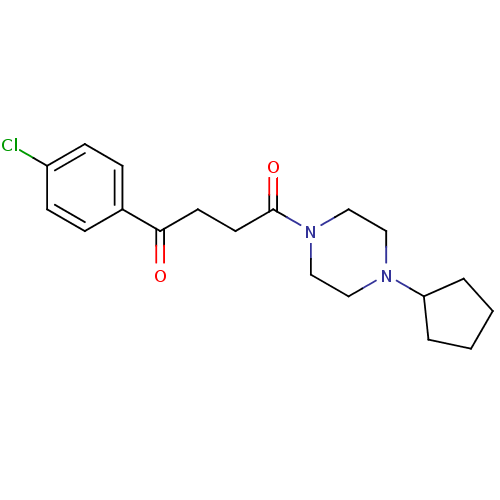 (1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human H3R | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113041 BindingDB Entry DOI: 10.7270/Q2MP570W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Mus musculus) | BDBM50455533 (CHEMBL4205635) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-603 from recombinant mouse adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... | J Med Chem 61: 4301-4316 (2018) Article DOI: 10.1021/acs.jmedchem.7b01627 BindingDB Entry DOI: 10.7270/Q23R0WG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (GUINEA PIG) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Eur J Pharmacol 383: 209-14 (1999) Article DOI: 10.1016/s0014-2999(99)00610-x BindingDB Entry DOI: 10.7270/Q2TT4PJ9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM30712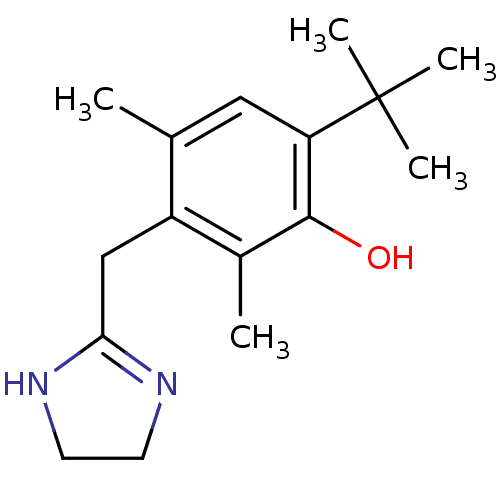 (6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-clonidine from alpha2-adrenergic receptor in rat brain cortex after 30 mins by Microbeta scintillation counting method | Eur J Med Chem 147: 102-114 (2018) Article DOI: 10.1016/j.ejmech.2018.01.093 BindingDB Entry DOI: 10.7270/Q29P346S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human full-length histamine H4 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50455533 (CHEMBL4205635) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... | J Med Chem 61: 4301-4316 (2018) Article DOI: 10.1021/acs.jmedchem.7b01627 BindingDB Entry DOI: 10.7270/Q23R0WG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50533199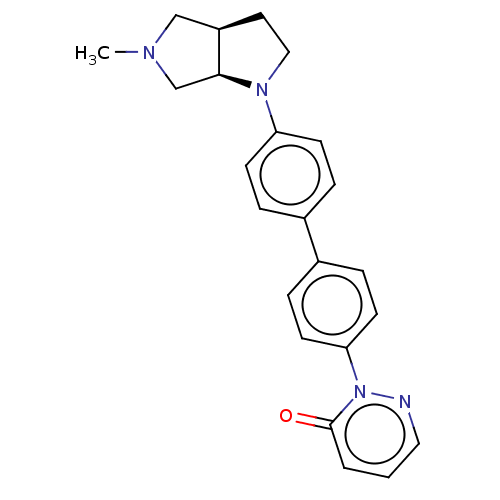 (CHEMBL4527011) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Antagonist activity at human H3R expressed in rat C6 cells incubated for 20 mins by [35S]GTPgammaS binding assay | Bioorg Med Chem 25: 5341-5354 (2017) Article DOI: 10.1016/j.bmc.2017.07.058 BindingDB Entry DOI: 10.7270/Q29W0K0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50533199 (CHEMBL4527011) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from full-length human histamine H3 receptor expressed in HEK cells after 30 mins by liquid scintillation ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 BindingDB Entry DOI: 10.7270/Q2XD155T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50533199 (CHEMBL4527011) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from full-length human histamine H3 receptor expressed in HEK cells after 30 mins by liquid scintillation ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 BindingDB Entry DOI: 10.7270/Q2XD155T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268815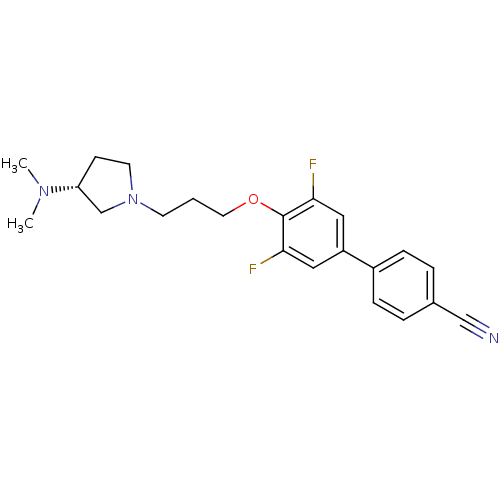 ((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human H3R | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113041 BindingDB Entry DOI: 10.7270/Q2MP570W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50240709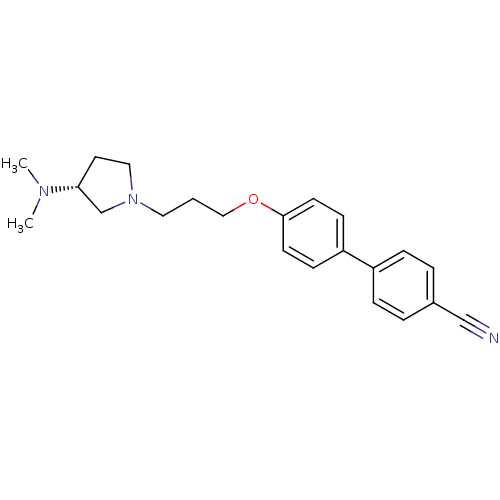 ((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human H3R | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113041 BindingDB Entry DOI: 10.7270/Q2MP570W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-(R)alpha-MeHA from rat brain H3 receptor | Bioorg Med Chem 26: 2573-2585 (2018) Article DOI: 10.1016/j.bmc.2018.04.023 BindingDB Entry DOI: 10.7270/Q2TQ641P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human full-length histamine H3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfran from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem 17: 3037-42 (2009) Article DOI: 10.1016/j.bmc.2009.03.014 BindingDB Entry DOI: 10.7270/Q2SF2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50378000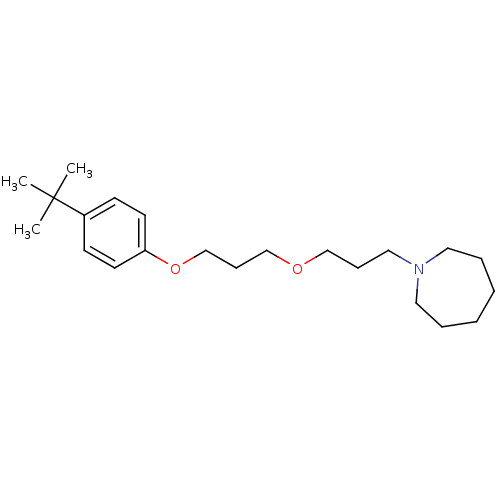 (CHEMBL1202078) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfran from human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 17: 3037-42 (2009) Article DOI: 10.1016/j.bmc.2009.03.014 BindingDB Entry DOI: 10.7270/Q2SF2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Eur J Pharmacol 383: 209-14 (1999) Article DOI: 10.1016/s0014-2999(99)00610-x BindingDB Entry DOI: 10.7270/Q2TT4PJ9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from human striatal full length H3 receptor after 60 mins by gamma counting method | Bioorg Med Chem 26: 2573-2585 (2018) Article DOI: 10.1016/j.bmc.2018.04.023 BindingDB Entry DOI: 10.7270/Q2TQ641P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfran from human histamine H3 receptor expressed in rat C6 cells | Bioorg Med Chem 17: 3037-42 (2009) Article DOI: 10.1016/j.bmc.2009.03.014 BindingDB Entry DOI: 10.7270/Q2SF2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7542 total ) | Next | Last >> |