 Found 1445 hits with Last Name = 'ott' and Initial = 'i'
Found 1445 hits with Last Name = 'ott' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054539
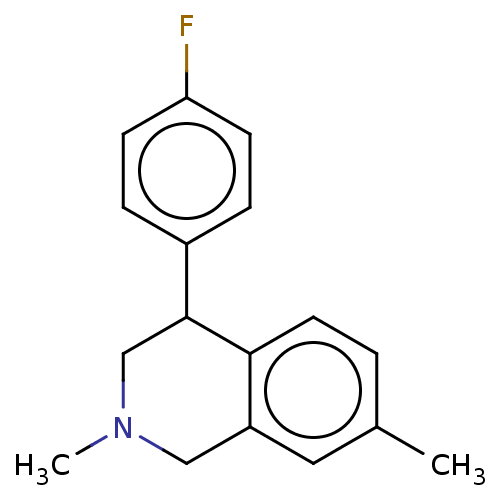
(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308418

(CHEMBL605824 | N-[2-(N',N'-diisopropylamin...)Show InChI InChI=1S/C21H27N3OS/c1-15(2)23(16(3)4)14-13-22-21(25)24-17-9-5-7-11-19(17)26-20-12-8-6-10-18(20)24/h5-12,15-16H,13-14H2,1-4H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50358949
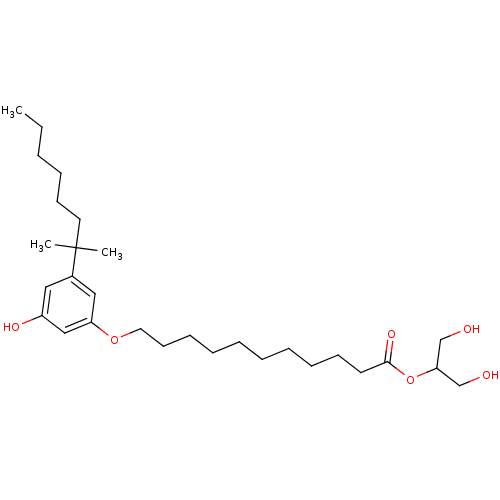
(CHEMBL1923763)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCCCCC(=O)OC(CO)CO)c1 Show InChI InChI=1S/C29H50O6/c1-4-5-6-14-17-29(2,3)24-19-25(32)21-26(20-24)34-18-15-12-10-8-7-9-11-13-16-28(33)35-27(22-30)23-31/h19-21,27,30-32H,4-18,22-23H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308420
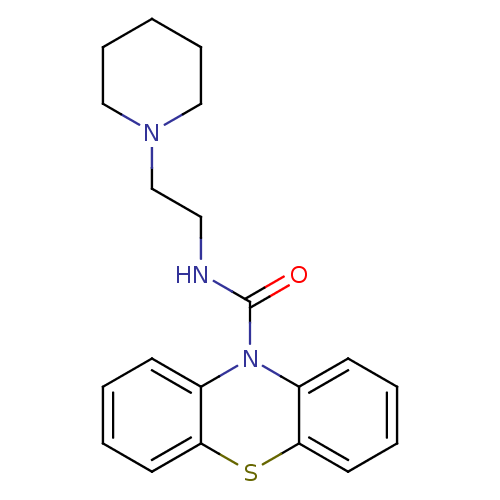
(CHEMBL589563 | N-[2-(Piperidinyl)ethyl]-10H-phenot...)Show InChI InChI=1S/C20H23N3OS/c24-20(21-12-15-22-13-6-1-7-14-22)23-16-8-2-4-10-18(16)25-19-11-5-3-9-17(19)23/h2-5,8-11H,1,6-7,12-15H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50171296
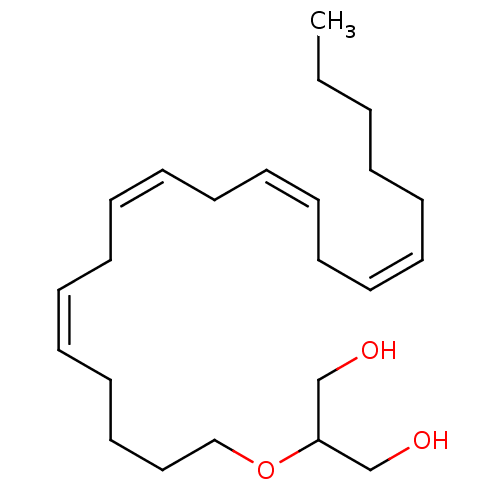
(2-[((11Z,14Z)-Icosa-5,8,11,14-tetraenyl)oxy]-propa...)Show InChI InChI=1S/C23H40O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-26-23(21-24)22-25/h6-7,9-10,12-13,15-16,23-25H,2-5,8,11,14,17-22H2,1H3/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM13211
((3S,6S,9aS)-6-[(2S)-2-aminobutanamido]-N-(diphenyl...)Show SMILES CC[C@H](N)C(=O)N[C@H]1CCC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C27H34N4O3/c1-2-21(28)25(32)29-22-15-9-14-20-16-17-23(31(20)27(22)34)26(33)30-24(18-10-5-3-6-11-18)19-12-7-4-8-13-19/h3-8,10-13,20-24H,2,9,14-17,28H2,1H3,(H,29,32)(H,30,33)/t20-,21-,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | -43.1 | 460 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano
| Assay Description
Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... |
Bioorg Med Chem 17: 5834-56 (2009)
Article DOI: 10.1016/j.bmc.2009.07.009
BindingDB Entry DOI: 10.7270/Q28W3BNV |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50062912
(CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...)Show InChI InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50054539

(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50358947
(CHEMBL1923761)Show InChI InChI=1S/C26H44O6/c1-2-3-4-11-14-22-16-17-23(29)19-25(22)31-18-13-10-8-6-5-7-9-12-15-26(30)32-24(20-27)21-28/h16-17,19,24,27-29H,2-15,18,20-21H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM13212
((3S,6S,9aS)-N-(diphenylmethyl)-6-[(2S)-2-(methylam...)Show SMILES CC[C@H](NC)C(=O)N[C@H]1CCC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-3-22(29-2)26(33)30-23-16-10-15-21-17-18-24(32(21)28(23)35)27(34)31-25(19-11-6-4-7-12-19)20-13-8-5-9-14-20/h4-9,11-14,21-25,29H,3,10,15-18H2,1-2H3,(H,30,33)(H,31,34)/t21-,22-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 61 | -40.9 | 530 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano
| Assay Description
Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... |
Bioorg Med Chem 17: 5834-56 (2009)
Article DOI: 10.1016/j.bmc.2009.07.009
BindingDB Entry DOI: 10.7270/Q28W3BNV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM22988

((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...)Show InChI InChI=1S/C22H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(25)23-20-21-24/h6-7,9-10,12-13,15-16,24H,2-5,8,11,14,17-21H2,1H3,(H,23,25)/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells in presence of 100 nM PMSF |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50250422
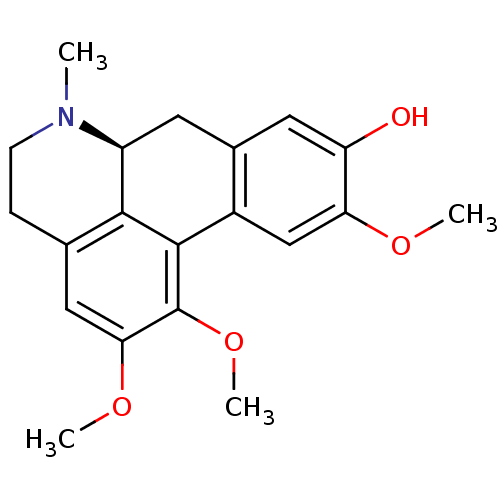
((+)-N-methyl-laurotetanine | CHEMBL464099 | N-meth...)Show SMILES COc1cc-2c(C[C@@H]3N(C)CCc4cc(OC)c(OC)c-2c34)cc1O |r| Show InChI InChI=1S/C20H23NO4/c1-21-6-5-11-9-17(24-3)20(25-4)19-13-10-16(23-2)15(22)8-12(13)7-14(21)18(11)19/h8-10,14,22H,5-7H2,1-4H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in CHO cells |
J Nat Prod 69: 432-5 (2006)
Article DOI: 10.1021/np058114h
BindingDB Entry DOI: 10.7270/Q2V98907 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50054470
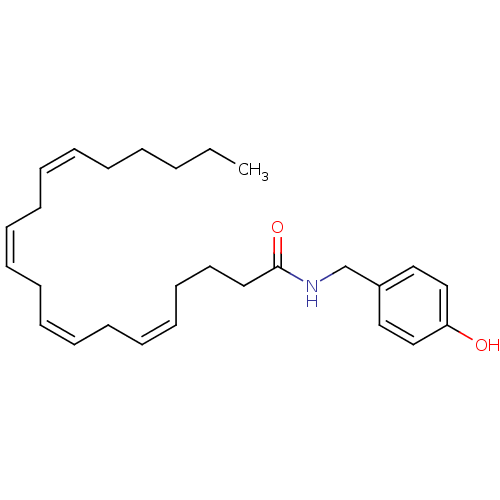
((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid 4-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCc1ccc(O)cc1 Show InChI InChI=1S/C27H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-27(30)28-24-25-20-22-26(29)23-21-25/h6-7,9-10,12-13,15-16,20-23,29H,2-5,8,11,14,17-19,24H2,1H3,(H,28,30)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358958
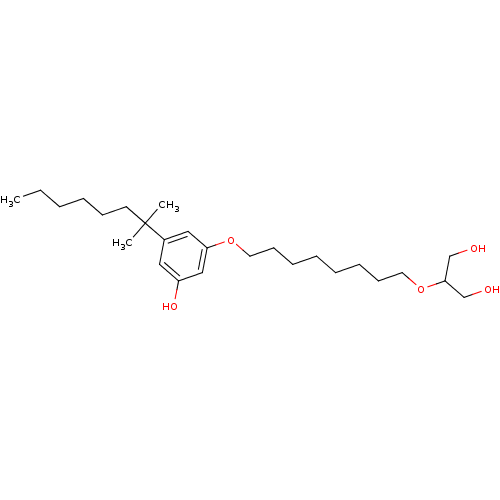
(CHEMBL1923772)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCCOC(CO)CO)c1 Show InChI InChI=1S/C26H46O5/c1-4-5-6-11-14-26(2,3)22-17-23(29)19-24(18-22)30-15-12-9-7-8-10-13-16-31-25(20-27)21-28/h17-19,25,27-29H,4-16,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308409
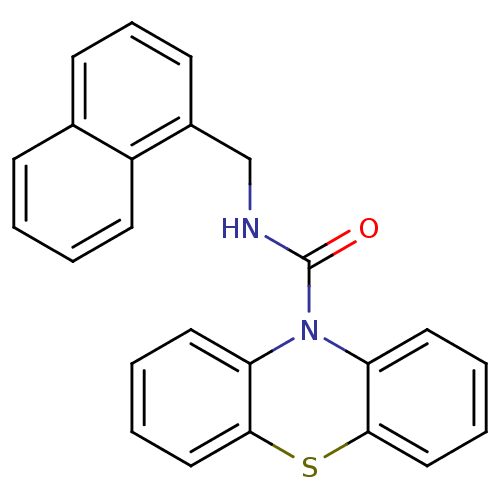
(CHEMBL592673 | N-(Naphthalen-1-ylmethyl)-1'H-pheno...)Show InChI InChI=1S/C24H18N2OS/c27-24(25-16-18-10-7-9-17-8-1-2-11-19(17)18)26-20-12-3-5-14-22(20)28-23-15-6-4-13-21(23)26/h1-15H,16H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50358957
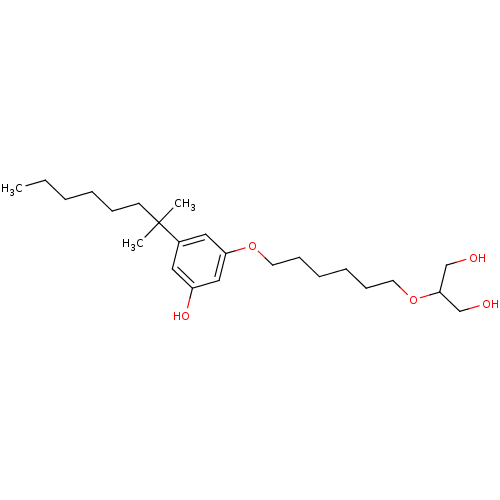
(CHEMBL1923771)Show InChI InChI=1S/C24H42O5/c1-4-5-6-9-12-24(2,3)20-15-21(27)17-22(16-20)28-13-10-7-8-11-14-29-23(18-25)19-26/h15-17,23,25-27H,4-14,18-19H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308413
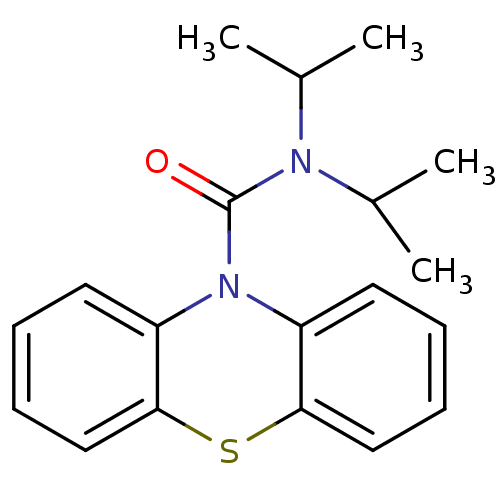
(CHEMBL588172 | N,N-Diisopropyl-1'H-phenothiazine-1...)Show InChI InChI=1S/C19H22N2OS/c1-13(2)20(14(3)4)19(22)21-15-9-5-7-11-17(15)23-18-12-8-6-10-16(18)21/h5-14H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50564589
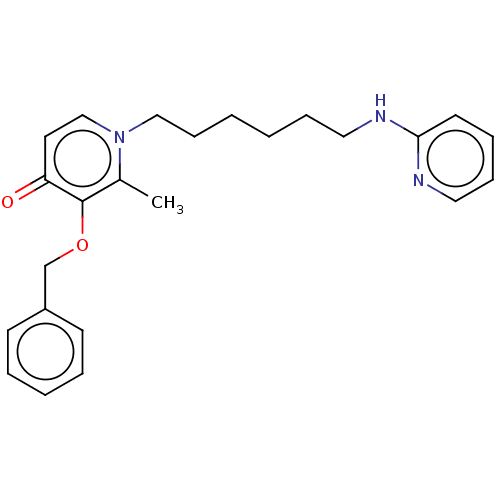
(CHEMBL4783436) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112350
BindingDB Entry DOI: 10.7270/Q25X2DP5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358957

(CHEMBL1923771)Show InChI InChI=1S/C24H42O5/c1-4-5-6-9-12-24(2,3)20-15-21(27)17-22(16-20)28-13-10-7-8-11-14-29-23(18-25)19-26/h15-17,23,25-27H,4-14,18-19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308408
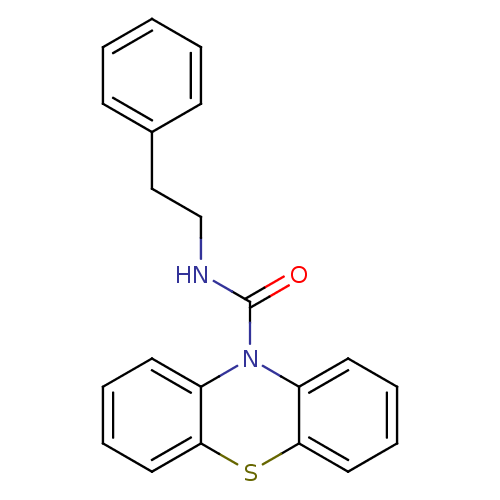
(CHEMBL602314 | N-(2-Phenylethyl)-1'H-phenothiazine...)Show InChI InChI=1S/C21H18N2OS/c24-21(22-15-14-16-8-2-1-3-9-16)23-17-10-4-6-12-19(17)25-20-13-7-5-11-18(20)23/h1-13H,14-15H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50054470

((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid 4-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCc1ccc(O)cc1 Show InChI InChI=1S/C27H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-27(30)28-24-25-20-22-26(29)23-21-25/h6-7,9-10,12-13,15-16,20-23,29H,2-5,8,11,14,17-19,24H2,1H3,(H,28,30)/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308419
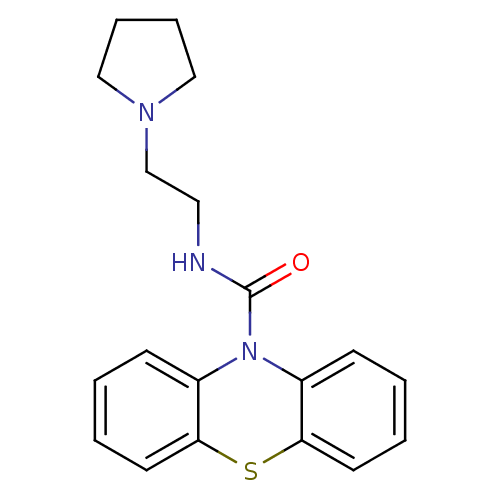
(CHEMBL589536 | N-[2-(Pyrrolidinyl)ethyl]-10H-pheno...)Show InChI InChI=1S/C19H21N3OS/c23-19(20-11-14-21-12-5-6-13-21)22-15-7-1-3-9-17(15)24-18-10-4-2-8-16(18)22/h1-4,7-10H,5-6,11-14H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054539

(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308424
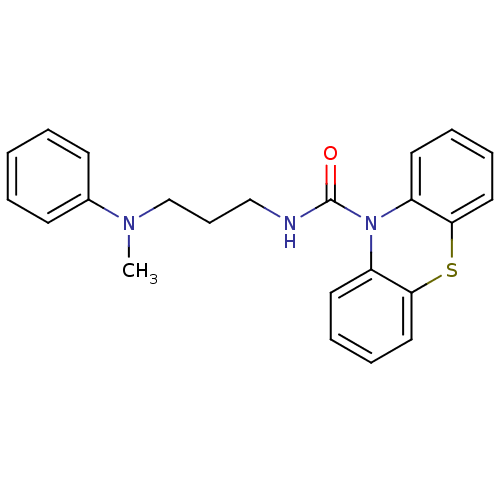
(CHEMBL589805 | N-(3-(Methyl(phenyl)amino)propyl)-1...)Show InChI InChI=1S/C23H23N3OS/c1-25(18-10-3-2-4-11-18)17-9-16-24-23(27)26-19-12-5-7-14-21(19)28-22-15-8-6-13-20(22)26/h2-8,10-15H,9,16-17H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50564591
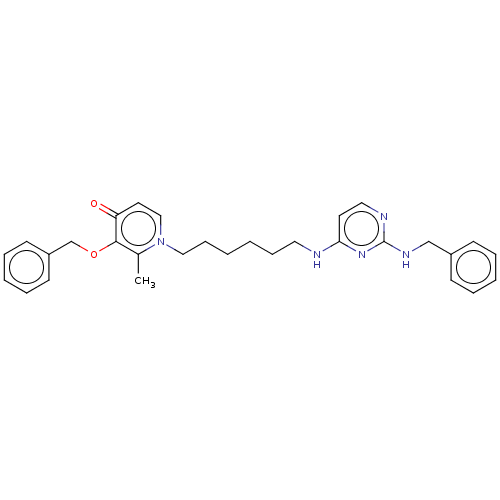
(CHEMBL4799735)Show SMILES Cc1c(OCc2ccccc2)c(=O)ccn1CCCCCCNc1ccnc(NCc2ccccc2)n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112350
BindingDB Entry DOI: 10.7270/Q25X2DP5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50358958

(CHEMBL1923772)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCCOC(CO)CO)c1 Show InChI InChI=1S/C26H46O5/c1-4-5-6-11-14-26(2,3)22-17-23(29)19-24(18-22)30-15-12-9-7-8-10-13-16-31-25(20-27)21-28/h17-19,25,27-29H,4-16,20-21H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50246638

(CHEMBL472897 | N-(1H-indazol-5-yl)icosa-5,8,11,14-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc2[nH]ncc2c1 Show InChI InChI=1S/C27H37N3O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-27(31)29-25-20-21-26-24(22-25)23-28-30-26/h6-7,9-10,12-13,15-16,20-23H,2-5,8,11,14,17-19H2,1H3,(H,28,30)(H,29,31)/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308402
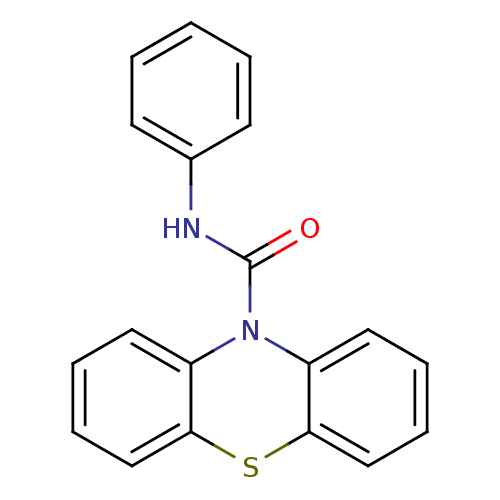
(CHEMBL590537 | N-Phenyl-1'H-phenothiazine-1'-carbo...)Show InChI InChI=1S/C19H14N2OS/c22-19(20-14-8-2-1-3-9-14)21-15-10-4-6-12-17(15)23-18-13-7-5-11-16(18)21/h1-13H,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358964
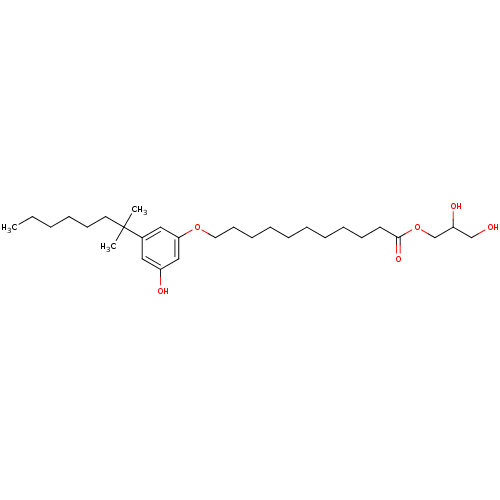
(CHEMBL1923778)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCCCCC(=O)OCC(O)CO)c1 Show InChI InChI=1S/C29H50O6/c1-4-5-6-14-17-29(2,3)24-19-25(31)21-27(20-24)34-18-15-12-10-8-7-9-11-13-16-28(33)35-23-26(32)22-30/h19-21,26,30-32H,4-18,22-23H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50062912

(CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...)Show InChI InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308401
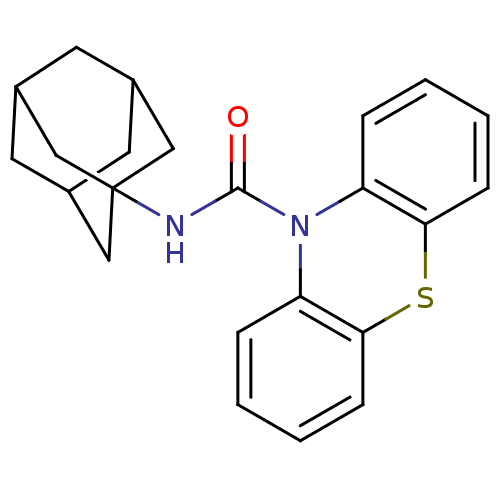
(CHEMBL591468 | N-(Adamant-1-yl)-1'H-phenothiazine-...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)N1c2ccccc2Sc2ccccc12 |TLB:6:7:4.5.10:11,THB:6:5:11:12.7.8,8:7:4:10.9.11,8:9:4:12.6.7| Show InChI InChI=1S/C23H24N2OS/c26-22(24-23-12-15-9-16(13-23)11-17(10-15)14-23)25-18-5-1-3-7-20(18)27-21-8-4-2-6-19(21)25/h1-8,15-17H,9-14H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358959
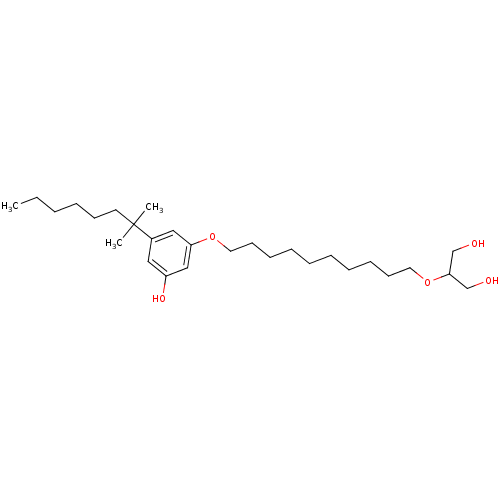
(CHEMBL1923773)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCCCCOC(CO)CO)c1 Show InChI InChI=1S/C28H50O5/c1-4-5-6-13-16-28(2,3)24-19-25(31)21-26(20-24)32-17-14-11-9-7-8-10-12-15-18-33-27(22-29)23-30/h19-21,27,29-31H,4-18,22-23H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308412
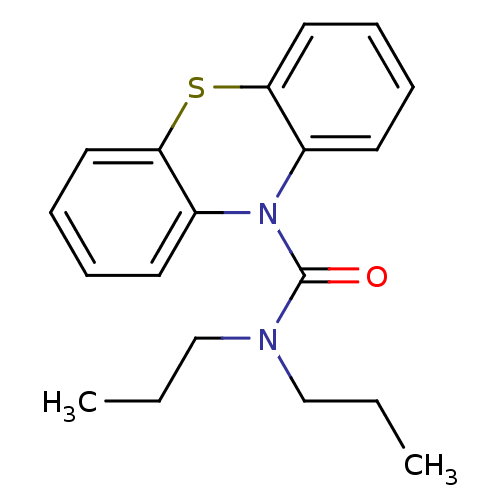
(CHEMBL589063 | N,N-Dipropyl-1'H-phenothiazine-1'-c...)Show InChI InChI=1S/C19H22N2OS/c1-3-13-20(14-4-2)19(22)21-15-9-5-7-11-17(15)23-18-12-8-6-10-16(18)21/h5-12H,3-4,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50358951
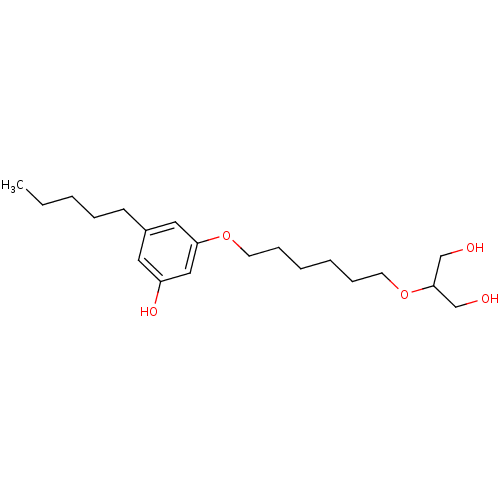
(CHEMBL1923765)Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-17-12-18(23)14-19(13-17)24-10-7-4-5-8-11-25-20(15-21)16-22/h12-14,20-23H,2-11,15-16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50564588
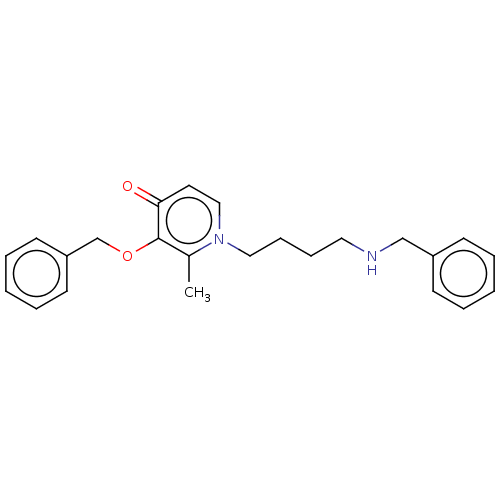
(CHEMBL4785902) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112350
BindingDB Entry DOI: 10.7270/Q25X2DP5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM26144
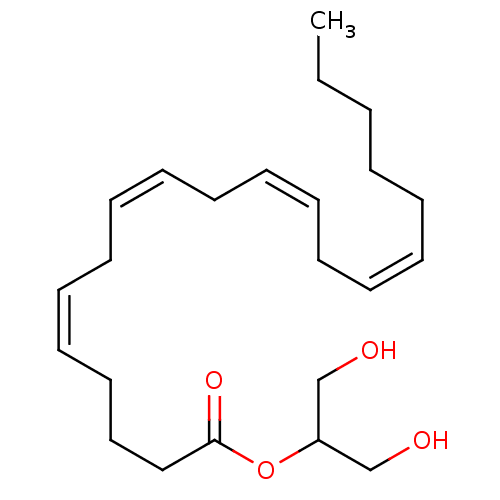
(1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)OC(CO)CO Show InChI InChI=1S/C23H38O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23(26)27-22(20-24)21-25/h6-7,9-10,12-13,15-16,22,24-25H,2-5,8,11,14,17-21H2,1H3/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308417
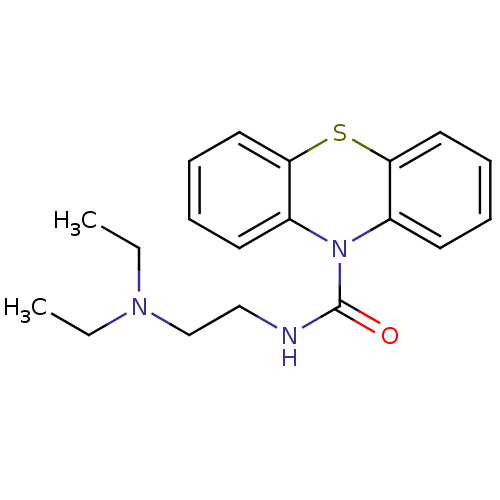
(CHEMBL601282 | N-[2-(N',N'-Diethylamino)ethyl]-1'H...)Show InChI InChI=1S/C19H23N3OS/c1-3-21(4-2)14-13-20-19(23)22-15-9-5-7-11-17(15)24-18-12-8-6-10-16(18)22/h5-12H,3-4,13-14H2,1-2H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308414
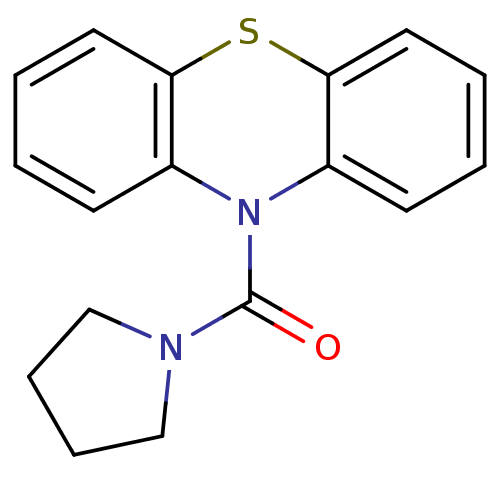
((1'H-Phenothiazin-1'-yl)(pyrrolidin-1-yl)methanone...)Show InChI InChI=1S/C17H16N2OS/c20-17(18-11-5-6-12-18)19-13-7-1-3-9-15(13)21-16-10-4-2-8-14(16)19/h1-4,7-10H,5-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308420

(CHEMBL589563 | N-[2-(Piperidinyl)ethyl]-10H-phenot...)Show InChI InChI=1S/C20H23N3OS/c24-20(21-12-15-22-13-6-1-7-14-22)23-16-8-2-4-10-18(16)25-19-11-5-3-9-17(19)23/h2-5,8-11H,1,6-7,12-15H2,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308406
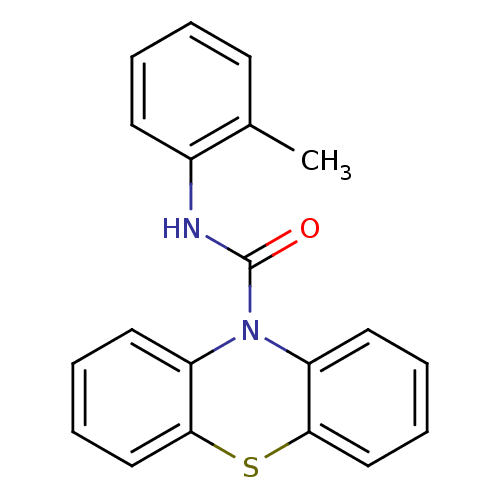
(CHEMBL600460 | N-o-Tolyl-1'H-phenothiazine-1'-carb...)Show InChI InChI=1S/C20H16N2OS/c1-14-8-2-3-9-15(14)21-20(23)22-16-10-4-6-12-18(16)24-19-13-7-5-11-17(19)22/h2-13H,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50564584
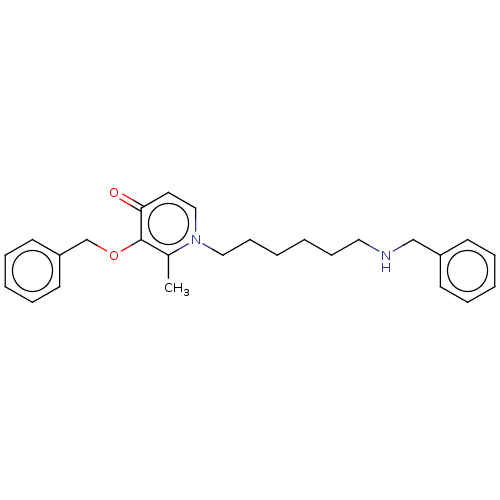
(CHEMBL4791565) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 788 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112350
BindingDB Entry DOI: 10.7270/Q25X2DP5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358948

(CHEMBL1923762)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCC(=O)OC(CO)CO)c1 Show InChI InChI=1S/C26H44O6/c1-4-5-6-11-14-26(2,3)21-16-22(29)18-23(17-21)31-15-12-9-7-8-10-13-25(30)32-24(19-27)20-28/h16-18,24,27-29H,4-15,19-20H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358949

(CHEMBL1923763)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCCCCC(=O)OC(CO)CO)c1 Show InChI InChI=1S/C29H50O6/c1-4-5-6-14-17-29(2,3)24-19-25(32)21-26(20-24)34-18-15-12-10-8-7-9-11-13-16-28(33)35-27(22-30)23-31/h19-21,27,30-32H,4-18,22-23H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50358946
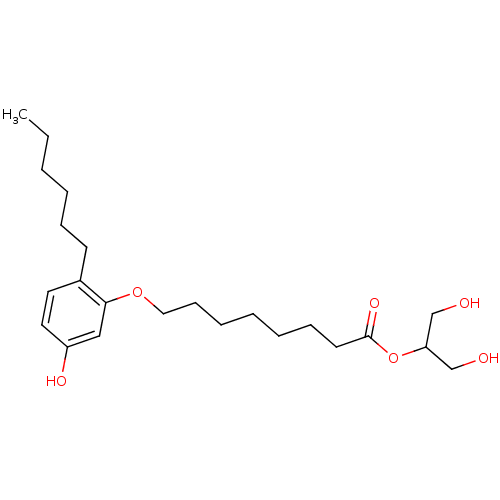
(CHEMBL1923760)Show InChI InChI=1S/C23H38O6/c1-2-3-4-8-11-19-13-14-20(26)16-22(19)28-15-10-7-5-6-9-12-23(27)29-21(17-24)18-25/h13-14,16,21,24-26H,2-12,15,17-18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358950
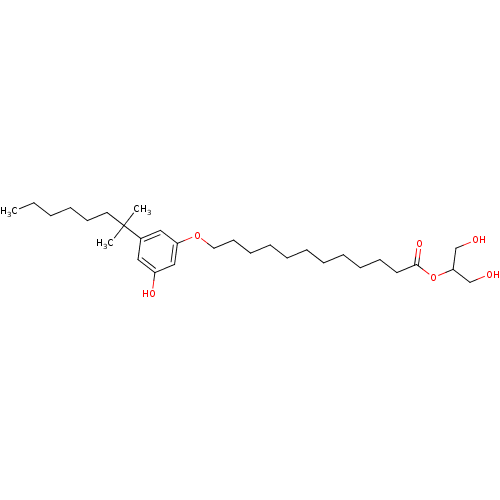
(CHEMBL1923764)Show SMILES CCCCCCC(C)(C)c1cc(O)cc(OCCCCCCCCCCCC(=O)OC(CO)CO)c1 Show InChI InChI=1S/C30H52O6/c1-4-5-6-15-18-30(2,3)25-20-26(33)22-27(21-25)35-19-16-13-11-9-7-8-10-12-14-17-29(34)36-28(23-31)24-32/h20-22,28,31-33H,4-19,23-24H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308418

(CHEMBL605824 | N-[2-(N',N'-diisopropylamin...)Show InChI InChI=1S/C21H27N3OS/c1-15(2)23(16(3)4)14-13-22-21(25)24-17-9-5-7-11-19(17)26-20-12-8-6-10-18(20)24/h5-12,15-16H,13-14H2,1-4H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358943
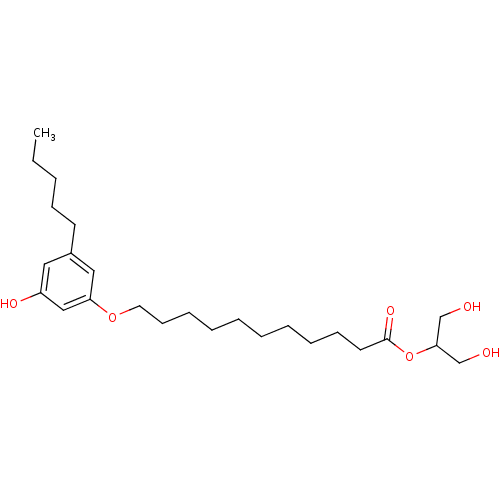
(CHEMBL1923757)Show InChI InChI=1S/C25H42O6/c1-2-3-10-13-21-16-22(28)18-23(17-21)30-15-12-9-7-5-4-6-8-11-14-25(29)31-24(19-26)20-27/h16-18,24,26-28H,2-15,19-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50358951

(CHEMBL1923765)Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-17-12-18(23)14-19(13-17)24-10-7-4-5-8-11-25-20(15-21)16-22/h12-14,20-23H,2-11,15-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells |
J Med Chem 54: 8278-88 (2011)
Article DOI: 10.1021/jm200529h
BindingDB Entry DOI: 10.7270/Q23J3DC3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data