

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin E (Homo sapiens (Human)) | BDBM50305527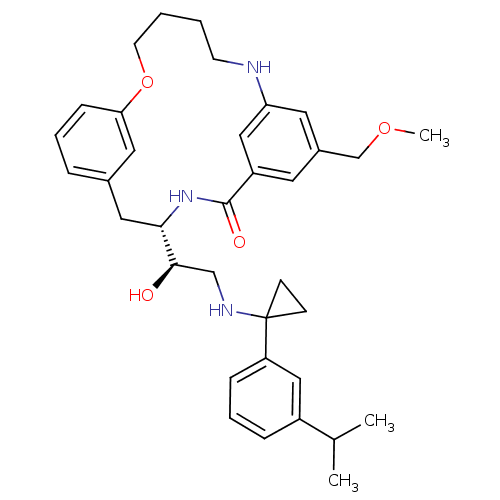 ((4S)-4-[(1R)-1-hydroxy-2-({1-[3-(1-methylethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of cathepsin E | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16047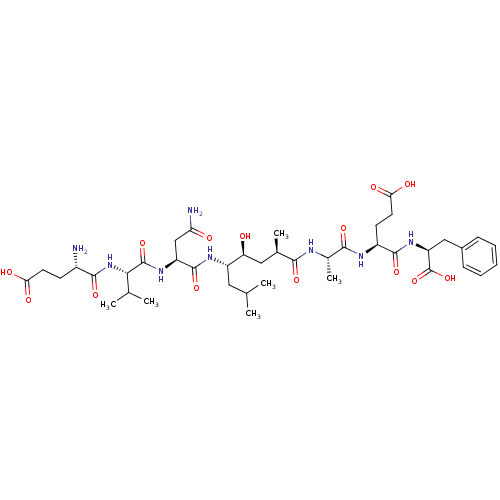 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305542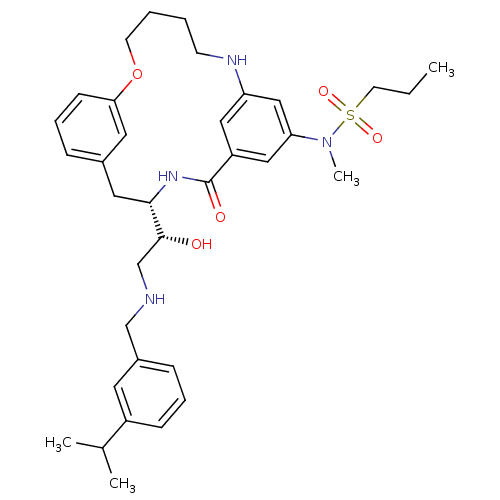 (CHEMBL595016 | Propane-1-sulfonic acid{(S)-4-[(R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50305527 ((4S)-4-[(1R)-1-hydroxy-2-({1-[3-(1-methylethyl)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50294218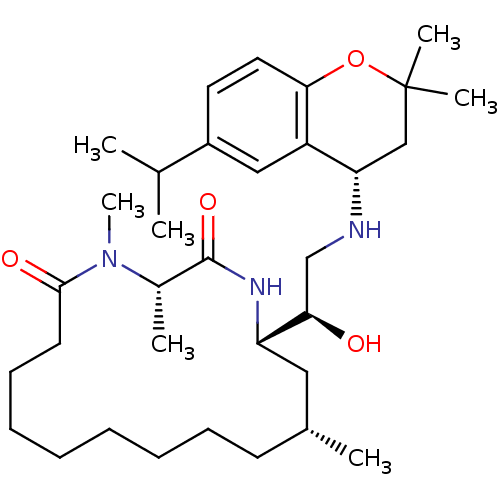 ((3S,14R,16S)-16-((R)-1-hydroxy-2-((S)-6-isopropyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | Bioorg Med Chem Lett 19: 1366-70 (2009) Article DOI: 10.1016/j.bmcl.2009.01.055 BindingDB Entry DOI: 10.7270/Q2SB45S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305544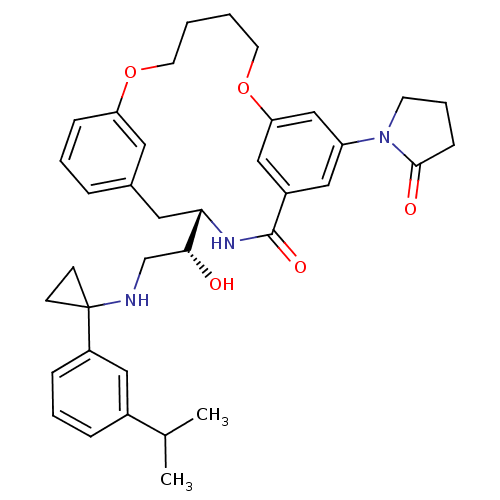 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50294218 ((3S,14R,16S)-16-((R)-1-hydroxy-2-((S)-6-isopropyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 19: 1366-70 (2009) Article DOI: 10.1016/j.bmcl.2009.01.055 BindingDB Entry DOI: 10.7270/Q2SB45S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305531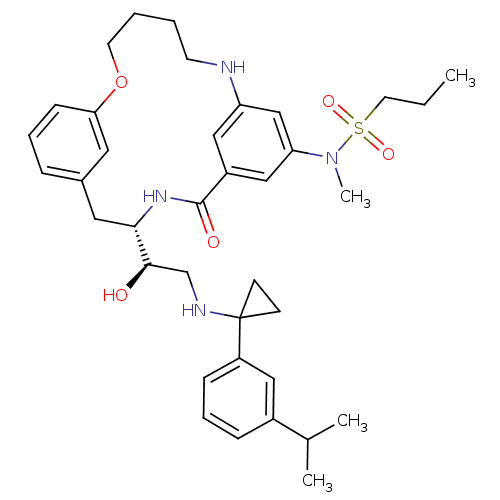 (CHEMBL595136 | Propane-1-sulfonic acid((S)-4-{(R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50305536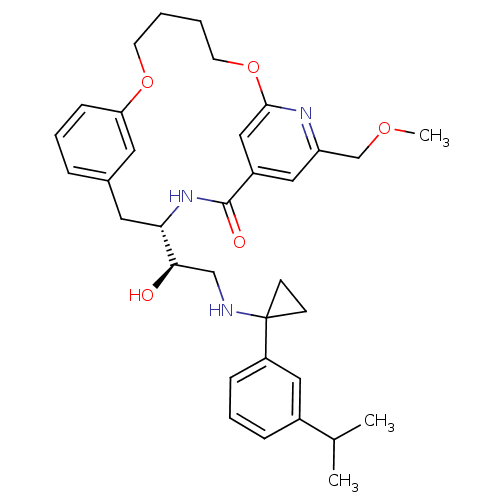 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50305537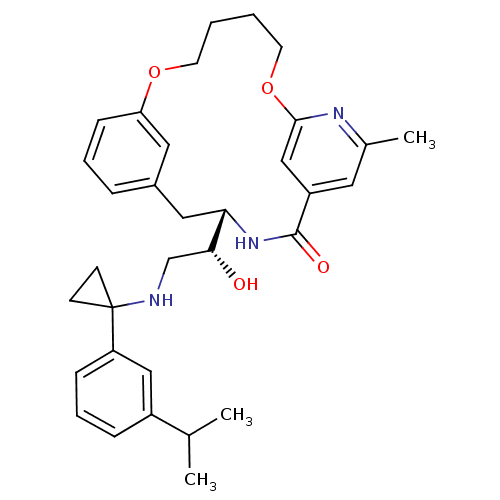 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of cathepsin E | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50305536 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of cathepsin E | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50305533 ((S)-4-[(R)-1-Hydroxy-2-(3-isopropyl-benzylamino)-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of cathepsin E | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305536 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16054 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16057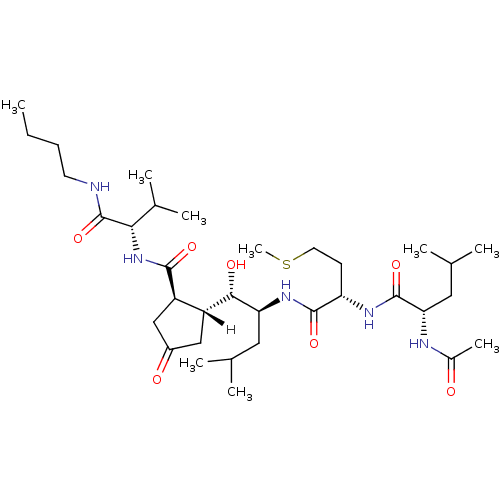 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16060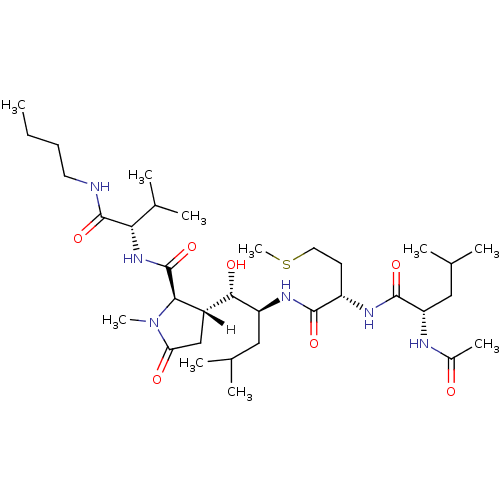 ((2R,3R)-3-[(1S,2S)-2-[[(2S)-2-[[(2S)-2-acetamido-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16055 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16049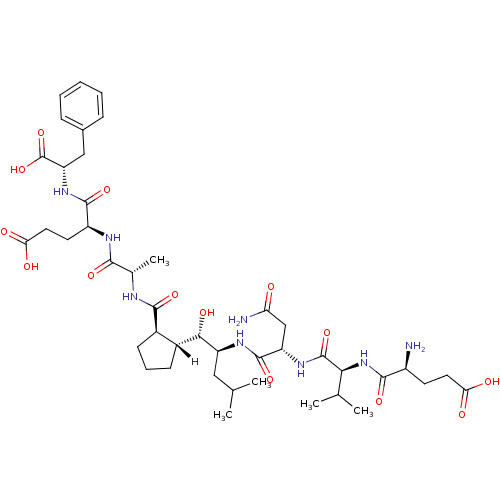 ((4S)-4-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50305537 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16048 ((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16049 ((4S)-4-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305527 ((4S)-4-[(1R)-1-hydroxy-2-({1-[3-(1-methylethyl)phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305537 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305543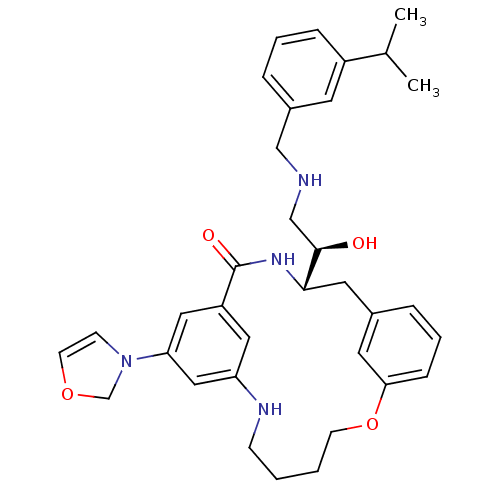 ((S)-4-[(R)-1-Hydroxy-2-(3-isopropyl-benzylamino)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305527 ((4S)-4-[(1R)-1-hydroxy-2-({1-[3-(1-methylethyl)phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of BACE1 expressed in APP-overexpressing CHO cells | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16053 ((2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S)-2-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16055 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50294219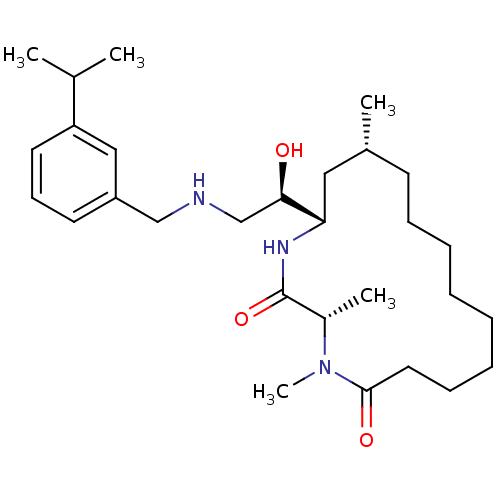 ((3S,14R,16S)-16-((R)-1-hydroxy-2-(3-isopropylbenzy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 19: 1366-70 (2009) Article DOI: 10.1016/j.bmcl.2009.01.055 BindingDB Entry DOI: 10.7270/Q2SB45S3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305534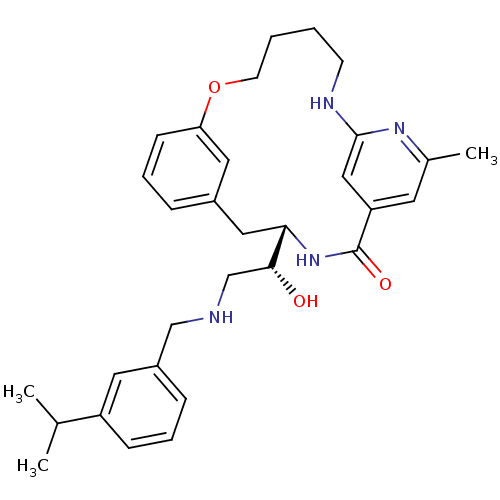 ((S)-4-[(R)-1-Hydroxy-2-(3-isopropyl-benzylamino)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16051 ((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16050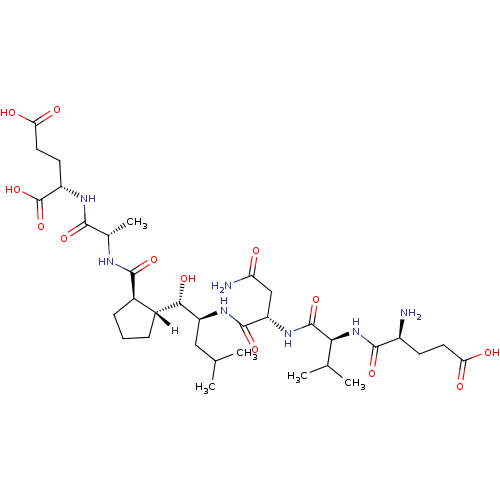 ((2S)-2-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305533 ((S)-4-[(R)-1-Hydroxy-2-(3-isopropyl-benzylamino)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50294219 ((3S,14R,16S)-16-((R)-1-hydroxy-2-(3-isopropylbenzy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 expressed in CHO cells expressing human recombinant APP assessed as amyloid beta40 aggregation | Bioorg Med Chem Lett 19: 1366-70 (2009) Article DOI: 10.1016/j.bmcl.2009.01.055 BindingDB Entry DOI: 10.7270/Q2SB45S3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305536 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of BACE1 expressed in APP-overexpressing CHO cells | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305530 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-1-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305528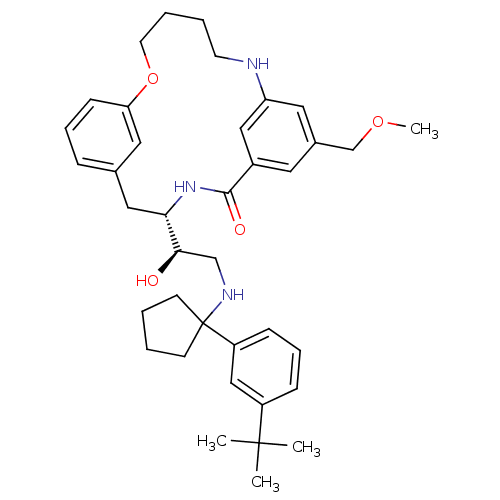 ((S)-4-{(R)-2-[1-(3-tert-Butyl-phenyl)-cyclopentyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305533 ((S)-4-[(R)-1-Hydroxy-2-(3-isopropyl-benzylamino)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of BACE1 expressed in APP-overexpressing CHO cells | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16057 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16056 ((1R,2R)-N-butyl-2-[(1S,2S)-2-[(2S)-2-[(2S)-2-aceta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16050 ((2S)-2-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305535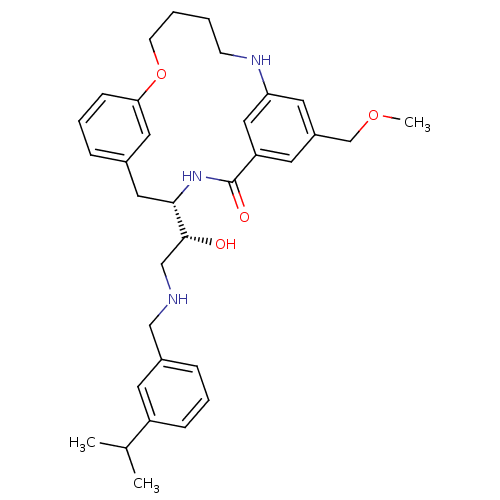 ((S)-4-[(R)-1-Hydroxy-2-(3-isopropyl-benzylamino)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305529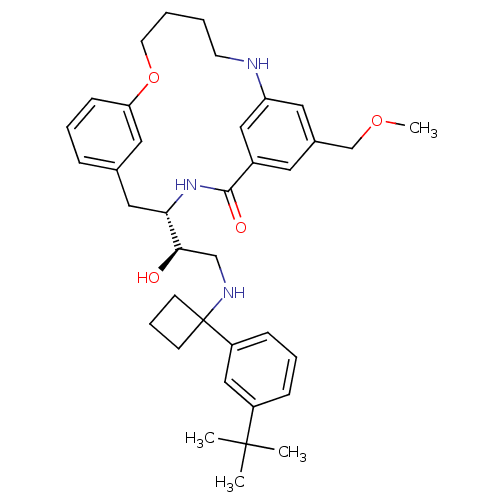 ((S)-4-{(R)-2-[1-(3-tert-Butyl-phenyl)-cyclobutylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16060 ((2R,3R)-3-[(1S,2S)-2-[[(2S)-2-[[(2S)-2-acetamido-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305537 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of BACE1 expressed in APP-overexpressing CHO cells | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50305540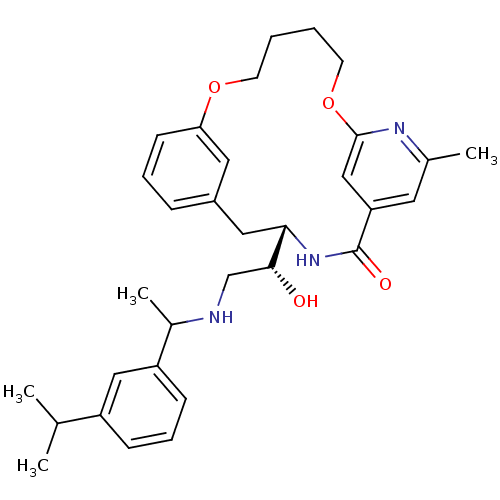 ((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in Escherichia coli | Bioorg Med Chem Lett 20: 603-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.092 BindingDB Entry DOI: 10.7270/Q2H1324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16052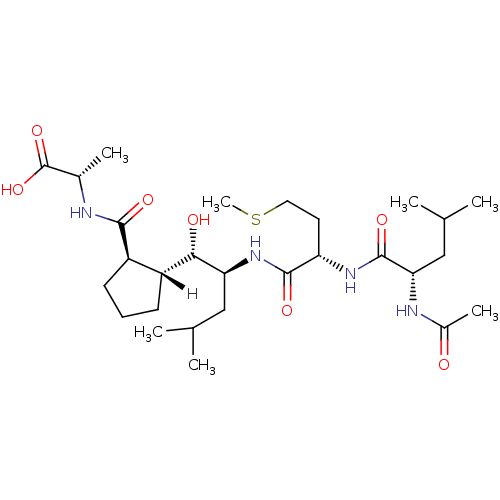 ((2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S)-2-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16048 ((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1R...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16059 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(3S,4R)-4-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29754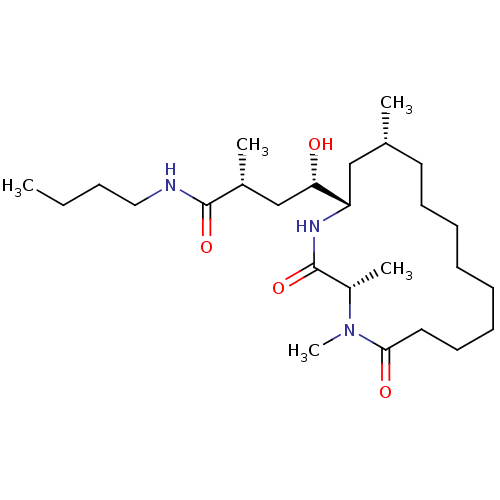 (macrocyclic peptidomimetic, 3h) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 19: 1366-70 (2009) Article DOI: 10.1016/j.bmcl.2009.01.055 BindingDB Entry DOI: 10.7270/Q2SB45S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 112 total ) | Next | Last >> |