

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054988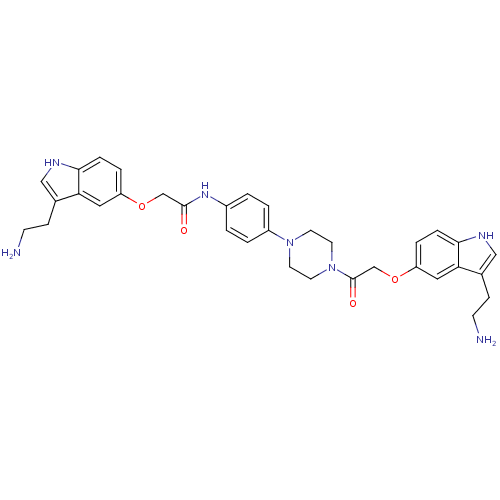 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-[4-(4-{2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054974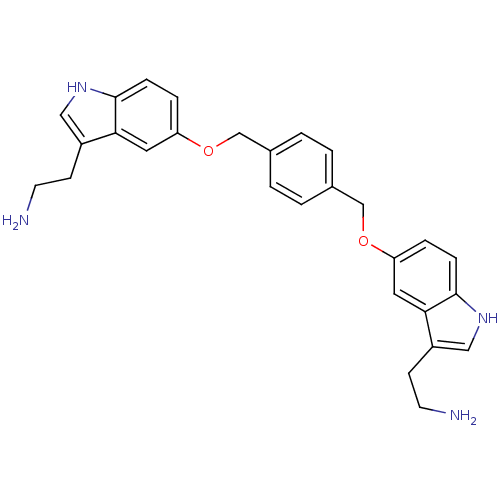 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054974 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054987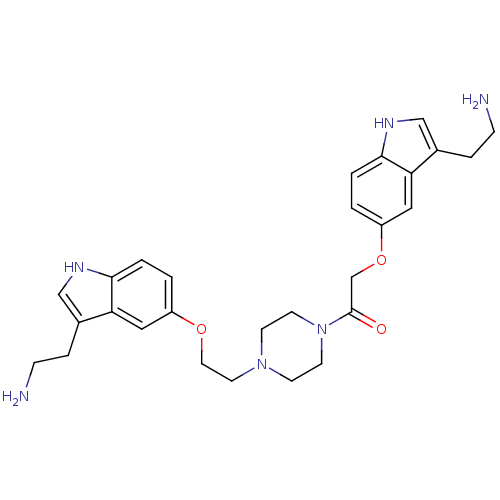 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054981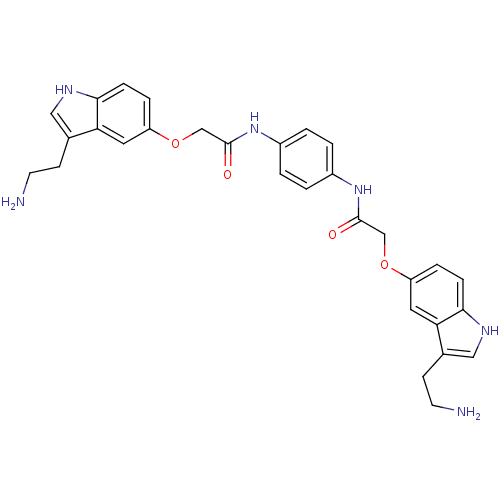 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054988 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-[4-(4-{2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054987 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054976 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-{2-[3-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054976 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-{2-[3-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054983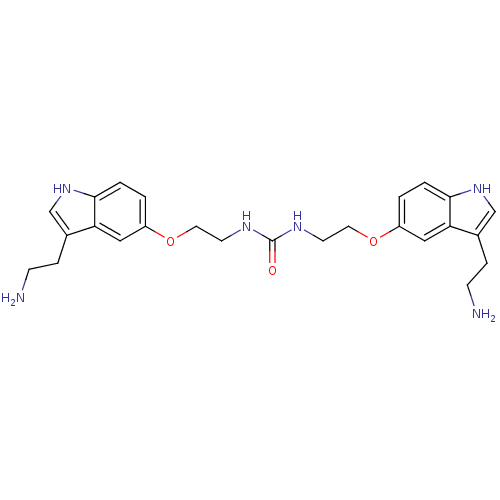 (1,3-Bis-{2-[3-(2-amino-ethyl)-1H-indol-5-yloxy]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054980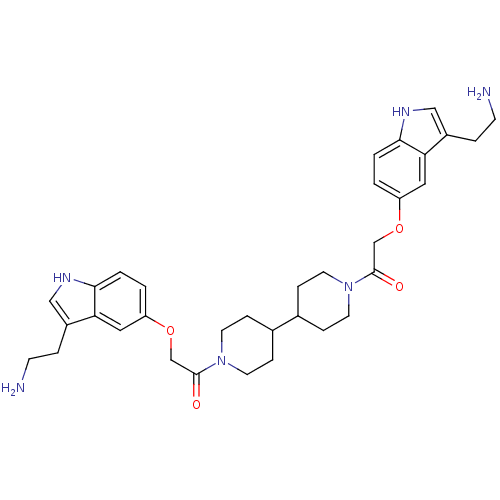 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(1'-{2-[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054979 (2-{5-[2-(4-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054980 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(1'-{2-[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054981 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054973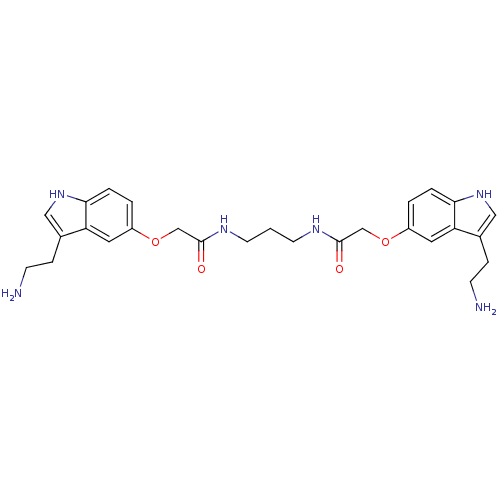 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(3-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054973 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(3-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054978 (2-(5-{3-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054979 (2-{5-[2-(4-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054978 (2-(5-{3-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054986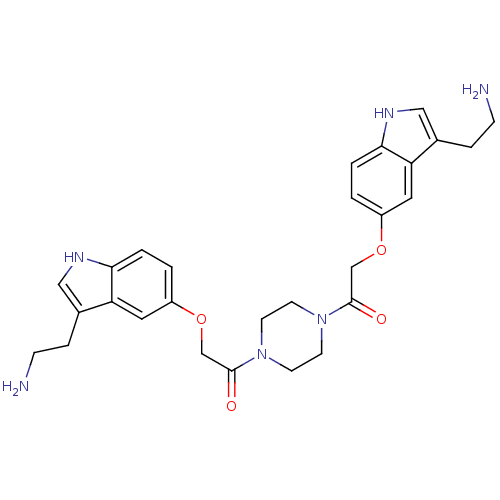 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054985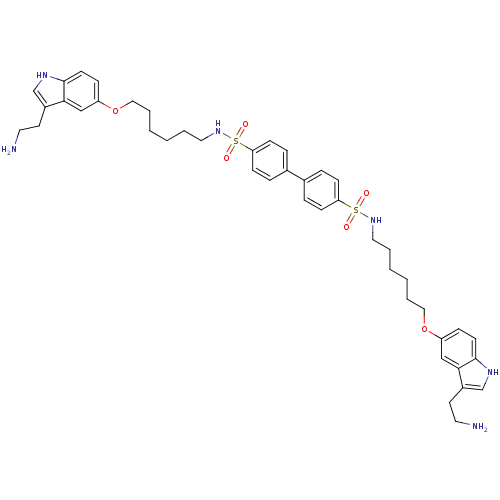 (Biphenyl-4,4'-disulfonic acid bis-({6-[3-(2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054983 (1,3-Bis-{2-[3-(2-amino-ethyl)-1H-indol-5-yloxy]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070409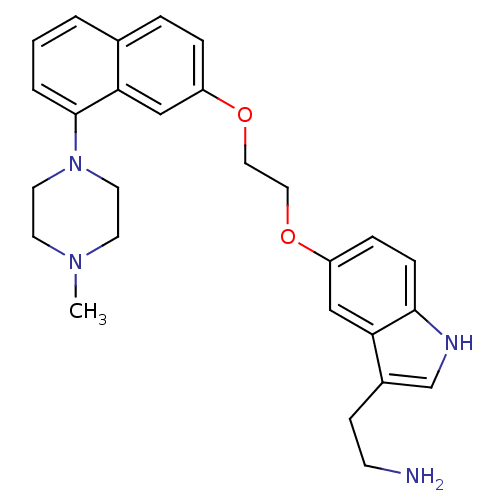 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054986 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054985 (Biphenyl-4,4'-disulfonic acid bis-({6-[3-(2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054977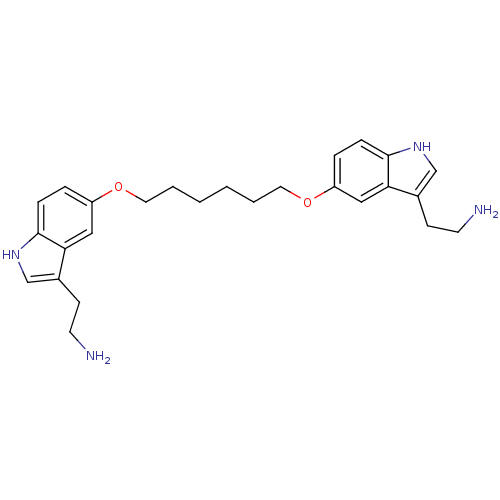 (2-(5-{6-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-hexyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070406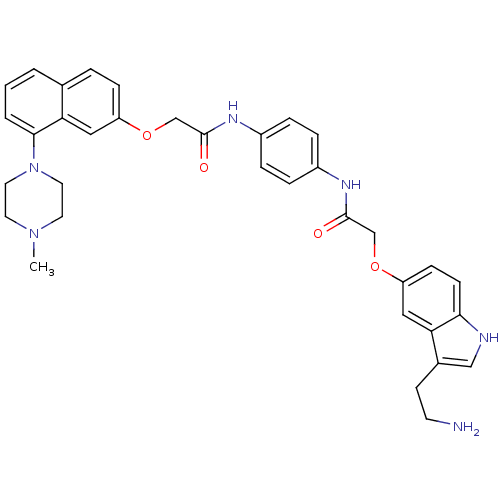 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054977 (2-(5-{6-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-hexyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070406 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054978 (2-(5-{3-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054982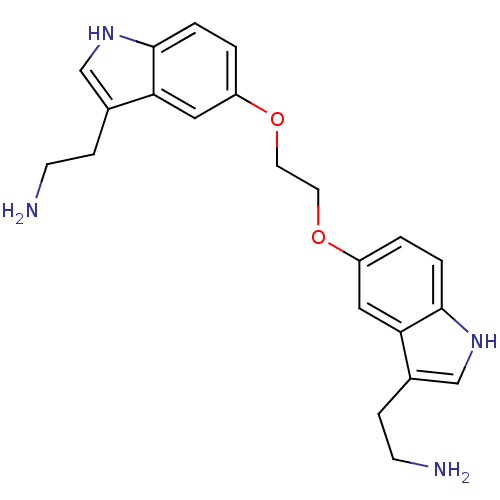 (2-(5-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070408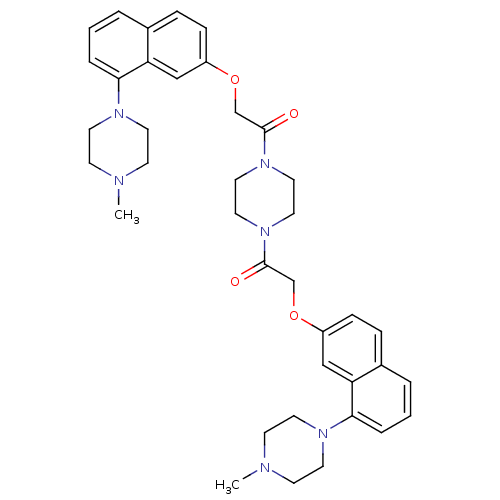 (2-[8-(4-Methyl-piperazin-1-yl)-naphthalen-2-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054984 (2-(5-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054974 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054985 (Biphenyl-4,4'-disulfonic acid bis-({6-[3-(2-amino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50033442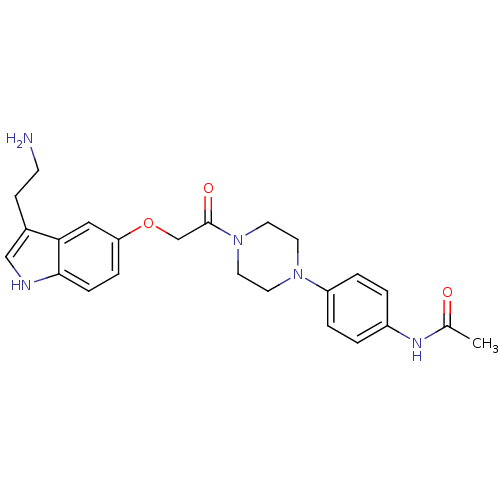 (CHEMBL331240 | N-[4-(4-{2-[3-(2-Amino-ethyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054984 (2-(5-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070405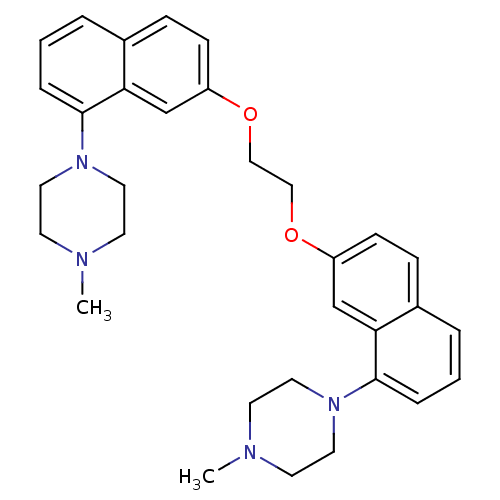 (1,2-di[8-(4-methylhexahydro-1-pyrazinyl)-2-naphthy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054977 (2-(5-{6-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-hexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070405 (1,2-di[8-(4-methylhexahydro-1-pyrazinyl)-2-naphthy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054983 (1,3-Bis-{2-[3-(2-amino-ethyl)-1H-indol-5-yloxy]-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054976 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-{2-[3-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054982 (2-(5-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070408 (2-[8-(4-Methyl-piperazin-1-yl)-naphthalen-2-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50033442 (CHEMBL331240 | N-[4-(4-{2-[3-(2-Amino-ethyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |