

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579331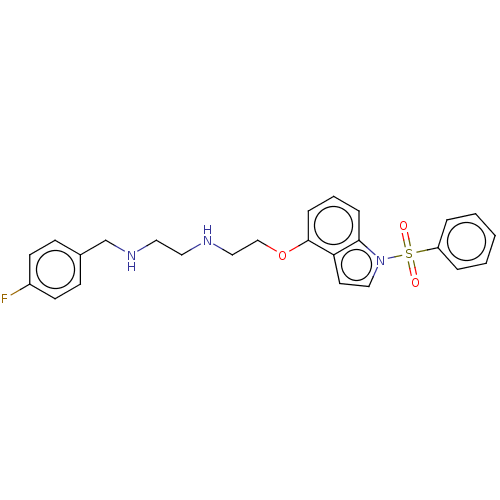 (CHEMBL4852099) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530711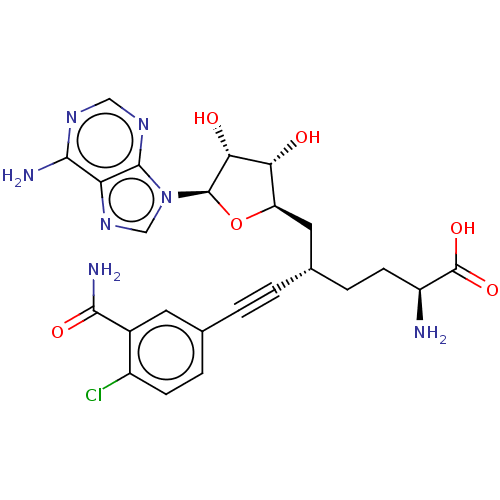 (CHEMBL4553052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530711 (CHEMBL4553052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50056419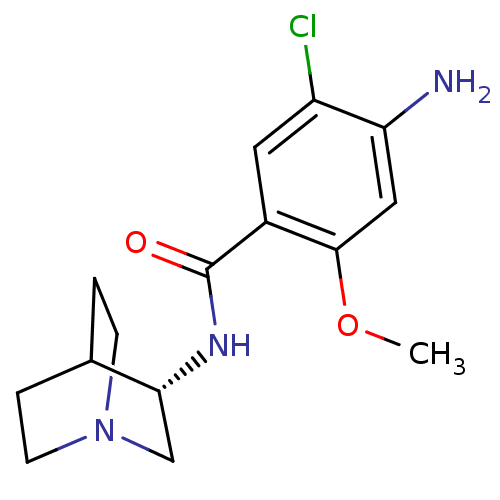 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530731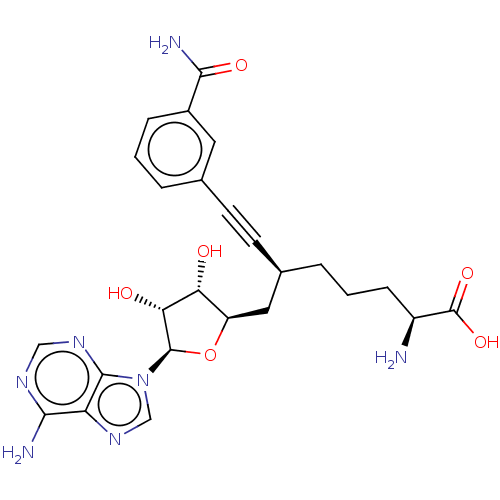 (CHEMBL4580446) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530731 (CHEMBL4580446) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM78940 (METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712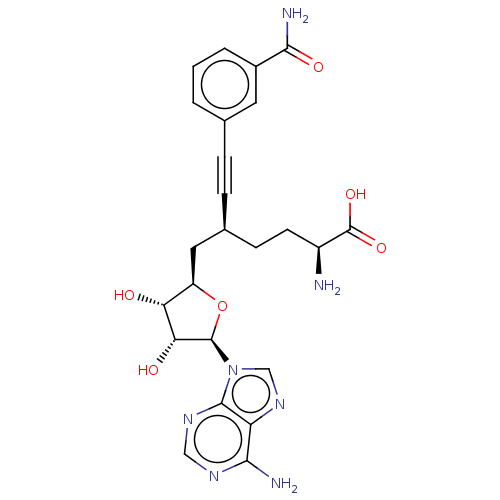 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to adenosine A2a receptor of rat brain tissue | J Med Chem 48: 2009-18 (2005) Article DOI: 10.1021/jm0498396 BindingDB Entry DOI: 10.7270/Q2NZ88FB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579329 (CHEMBL4863218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50181836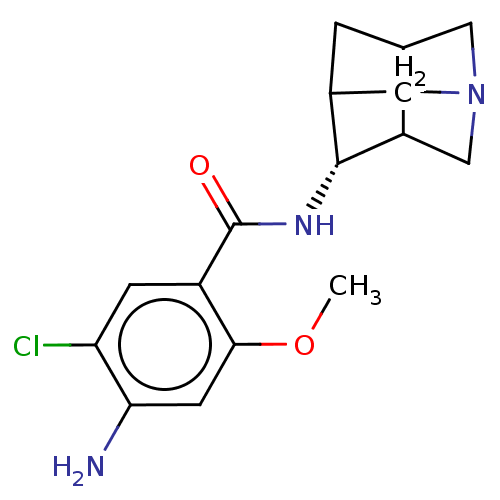 (4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50402702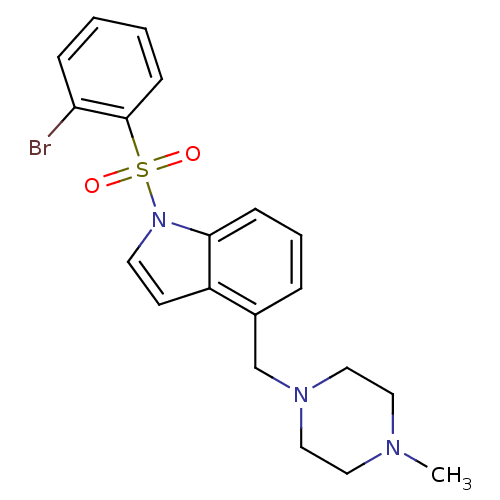 (CHEMBL2207386) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Limited Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 7431-5 (2012) Article DOI: 10.1016/j.bmcl.2012.10.057 BindingDB Entry DOI: 10.7270/Q25140DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579335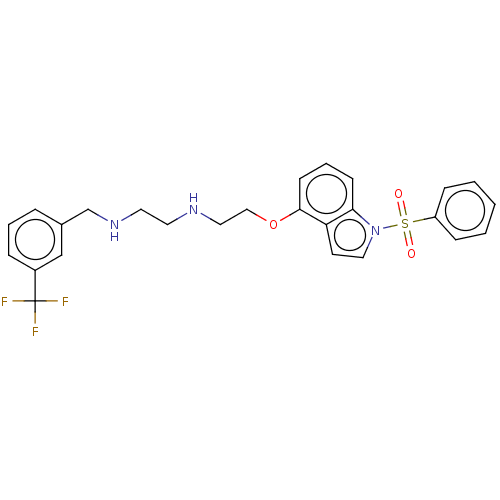 (CHEMBL4854972) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530723 (CHEMBL4455627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530723 (CHEMBL4455627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50533199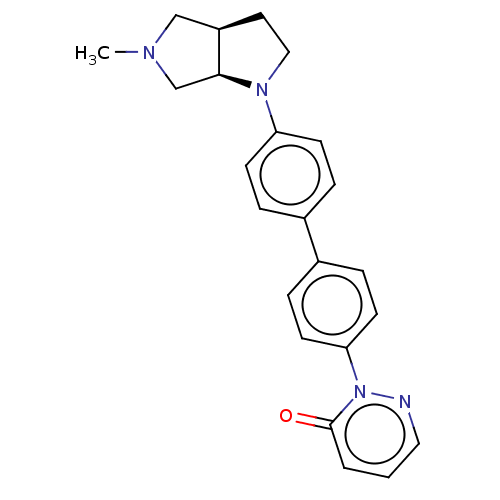 (CHEMBL4527011) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from full-length human histamine H3 receptor expressed in HEK cells after 30 mins by liquid scintillation ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 BindingDB Entry DOI: 10.7270/Q2XD155T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50533199 (CHEMBL4527011) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from full-length human histamine H3 receptor expressed in HEK cells after 30 mins by liquid scintillation ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 BindingDB Entry DOI: 10.7270/Q2XD155T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579334 (CHEMBL4864206) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50163407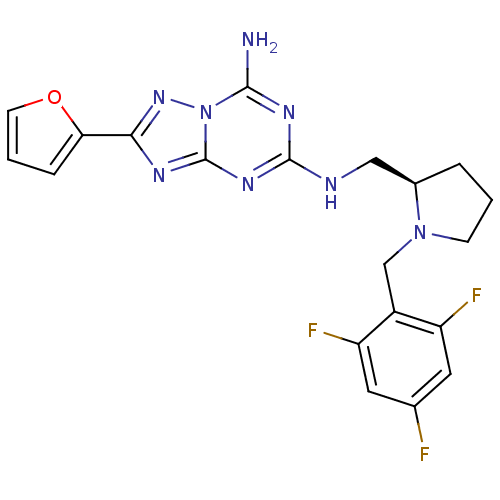 (2-Furan-2-yl-N*5*-[(R)-1-(2,4,6-trifluoro-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to adenosine A2a receptor of rat brain tissue | J Med Chem 48: 2009-18 (2005) Article DOI: 10.1021/jm0498396 BindingDB Entry DOI: 10.7270/Q2NZ88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579332 (CHEMBL4851595) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579327 (CHEMBL4847532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345760 (1-(Benzenesulfonyl)-5-(piperazin-1-yl-methyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345777 (1-(2-Bromo benzenesulfonyl)-5-(4-ethylpiperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345758 (3-chloro-1-(4-fluoro benzenesulfonyl)-5-(piperazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530737 (CHEMBL4449355) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530737 (CHEMBL4449355) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50402705 (CHEMBL2207368) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Limited Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 7431-5 (2012) Article DOI: 10.1016/j.bmcl.2012.10.057 BindingDB Entry DOI: 10.7270/Q25140DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345754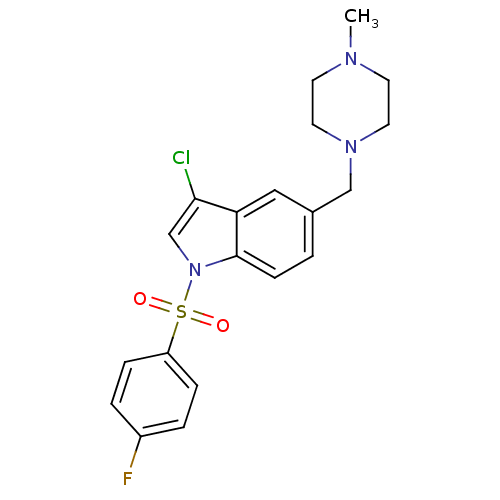 (3-chloro-1-(4-fluoro benzenesulfonyl)-5-(4-methylp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50151182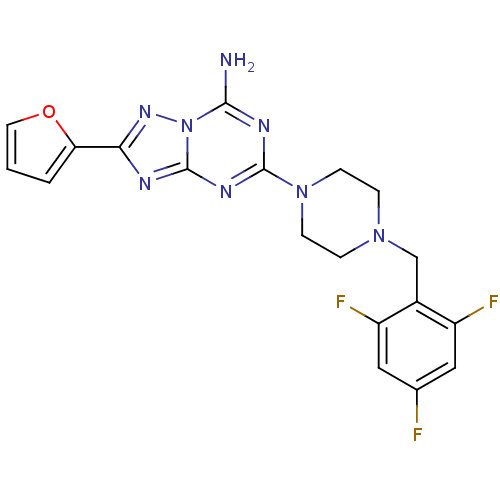 (2-Furan-2-yl-5-[4-(2,4,6-trifluoro-benzyl)-piperaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to adenosine A2a receptor of rat brain tissue | J Med Chem 48: 2009-18 (2005) Article DOI: 10.1021/jm0498396 BindingDB Entry DOI: 10.7270/Q2NZ88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579328 (CHEMBL4878383) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50163432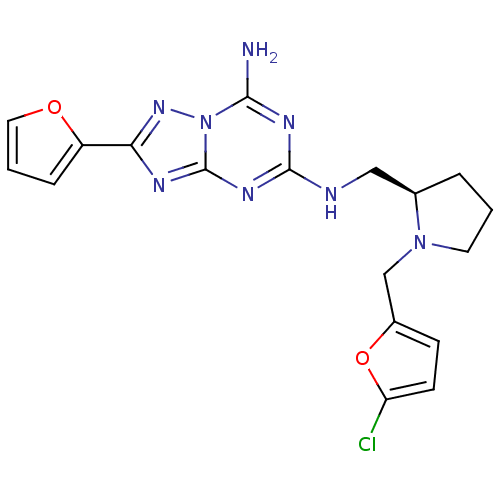 (CHEMBL179478 | N*5*-[(R)-1-(5-Chloro-furan-2-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to adenosine A2a receptor of rat brain tissue | J Med Chem 48: 2009-18 (2005) Article DOI: 10.1021/jm0498396 BindingDB Entry DOI: 10.7270/Q2NZ88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579323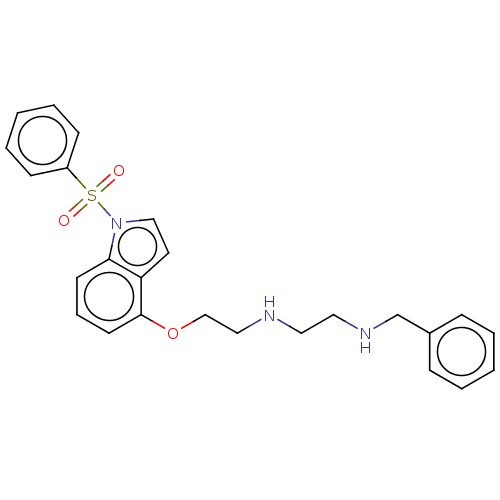 (CHEMBL4878361) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345763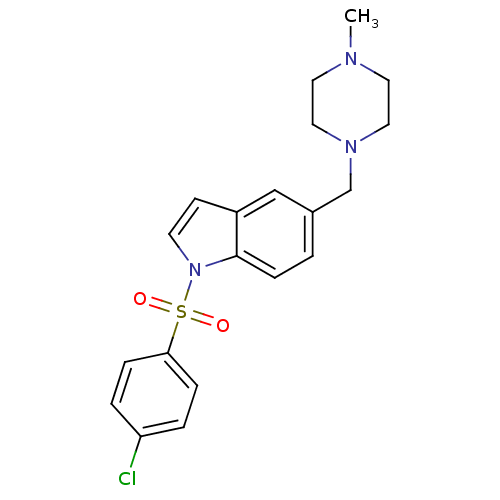 (1-(4-Chloro benzenesulfonyl)-5-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345762 (1-(3-trifluoromethyl benzenesulfonyl)-5-(4-methylp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345772 (1-(4-Fluoro benzenesulfonyl)-5-(4-ethylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50402701 (CHEMBL2207388) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Limited Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 22: 7431-5 (2012) Article DOI: 10.1016/j.bmcl.2012.10.057 BindingDB Entry DOI: 10.7270/Q25140DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345753 (3-Chloro-1-(2,4-difluorobenzenesulfonyl)-5-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530724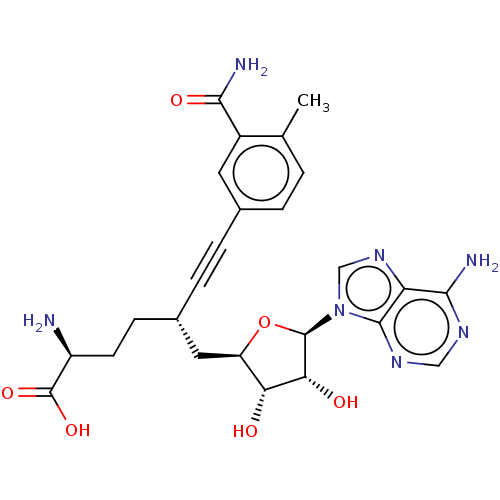 (CHEMBL4519184) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50530724 (CHEMBL4519184) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50334248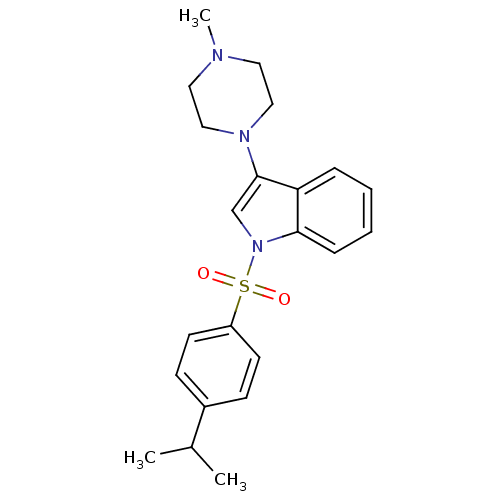 (1-(4-Isopropylbenzenesulfonyl)-3-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells by glass fiber filtration assay | Bioorg Med Chem Lett 21: 346-9 (2010) Article DOI: 10.1016/j.bmcl.2010.11.001 BindingDB Entry DOI: 10.7270/Q2P84C5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579320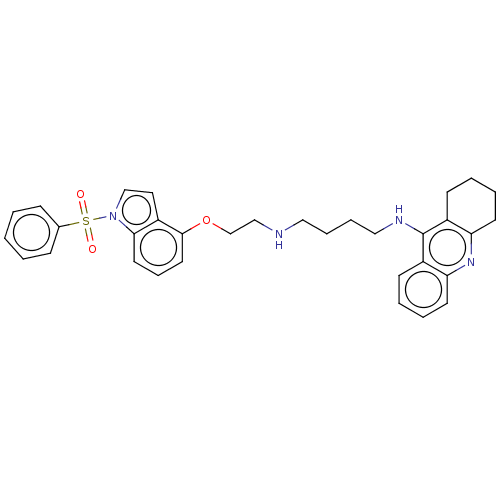 (CHEMBL4874513) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579333 (CHEMBL4868597) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50345769 (1-Benzenesulfonyl-5-(4-methylpiperazin-1-yl-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | ACS Med Chem Lett 1: 340-344 (2010) Article DOI: 10.1021/ml100101u BindingDB Entry DOI: 10.7270/Q2CR5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50449570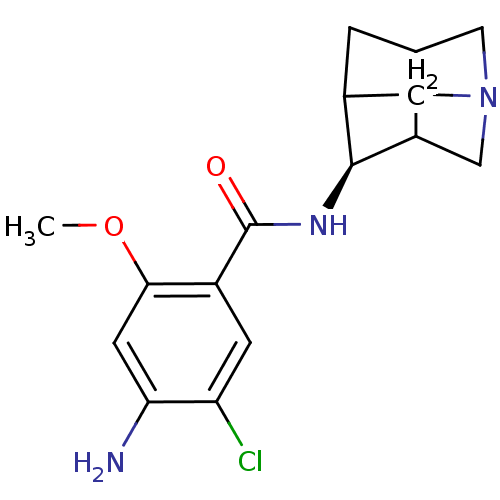 (CHEMBL2448137) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50156604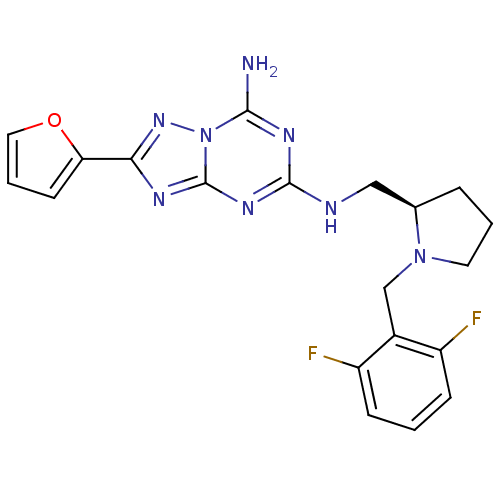 (CHEMBL173672 | N*5*-[(R)-1-(2,6-Difluoro-benzyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to adenosine A2a receptor of rat brain tissue | J Med Chem 48: 2009-18 (2005) Article DOI: 10.1021/jm0498396 BindingDB Entry DOI: 10.7270/Q2NZ88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50163404 (CHEMBL177615 | N*5*-[(R)-1-(2-Chloro-3,6-difluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to adenosine A2a receptor of rat brain tissue | J Med Chem 48: 2009-18 (2005) Article DOI: 10.1021/jm0498396 BindingDB Entry DOI: 10.7270/Q2NZ88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6124 total ) | Next | Last >> |