 Found 90 hits with Last Name = 'park' and Initial = 'bk'
Found 90 hits with Last Name = 'park' and Initial = 'bk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM79214
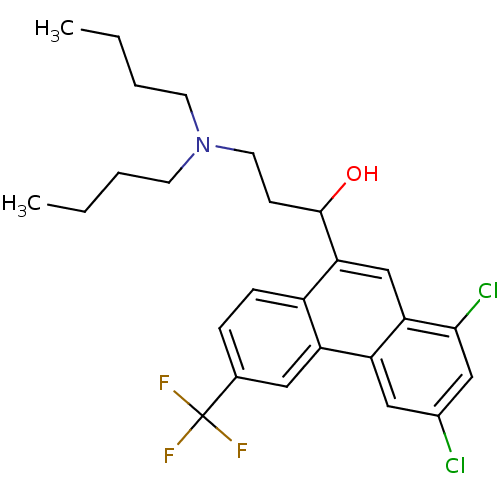
(1-[1,3-bis(chloranyl)-6-(trifluoromethyl)phenanthr...)Show SMILES CCCCN(CCCC)CCC(O)c1cc2c(Cl)cc(Cl)cc2c2cc(ccc12)C(F)(F)F Show InChI InChI=1S/C26H30Cl2F3NO/c1-3-5-10-32(11-6-4-2)12-9-25(33)23-16-22-21(14-18(27)15-24(22)28)20-13-17(26(29,30)31)7-8-19(20)23/h7-8,13-16,25,33H,3-6,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50247629
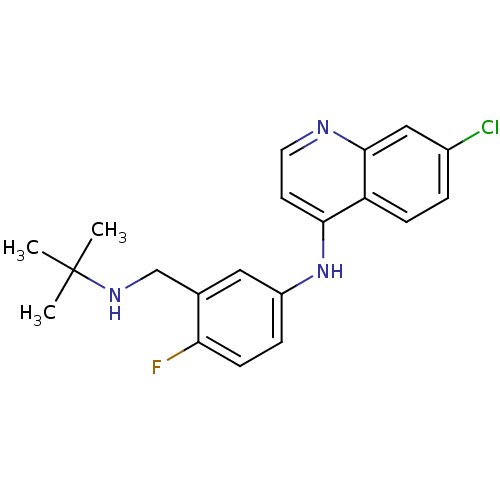
(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254327

(2-(2,4-dimethoxyphenyl)-6-(4-oxo-2-phenyl-4H-chrom...)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C32H22O7/c1-35-20-8-12-24(30(15-20)36-2)32-18-27(34)25-14-21(10-13-28(25)38-32)37-22-9-11-23-26(33)17-29(39-31(23)16-22)19-6-4-3-5-7-19/h3-18H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254381

(2-(4-methoxyphenyl)-6-(4-oxo-2-phenyl-4H-chromen-7...)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C31H20O6/c1-34-21-9-7-20(8-10-21)30-18-27(33)25-15-22(12-14-28(25)36-30)35-23-11-13-24-26(32)17-29(37-31(24)16-23)19-5-3-2-4-6-19/h2-18H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22985
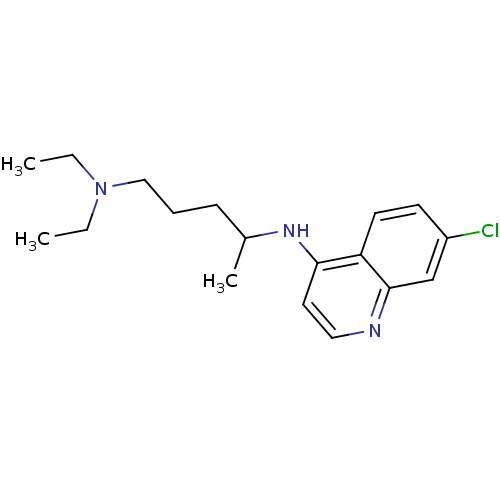
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134936
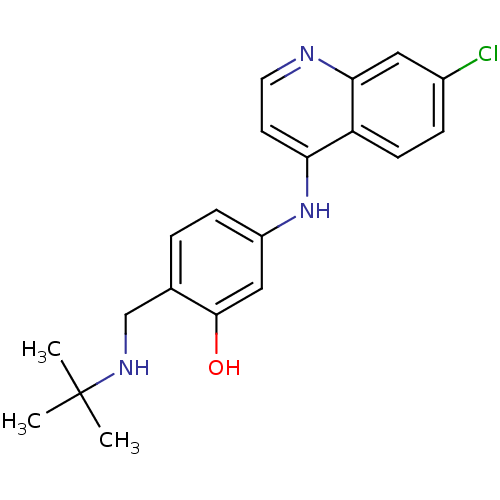
(2-(tert-Butylamino-methyl)-5-(7-chloro-quinolin-4-...)Show InChI InChI=1S/C20H22ClN3O/c1-20(2,3)23-12-13-4-6-15(11-19(13)25)24-17-8-9-22-18-10-14(21)5-7-16(17)18/h4-11,23,25H,12H2,1-3H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134934
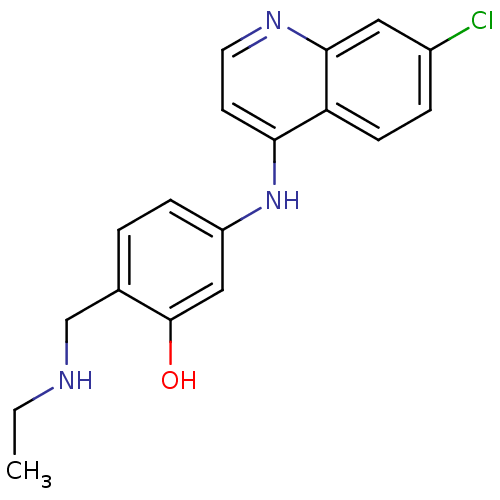
(5-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-3-5-14(10-18(12)23)22-16-7-8-21-17-9-13(19)4-6-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50134931
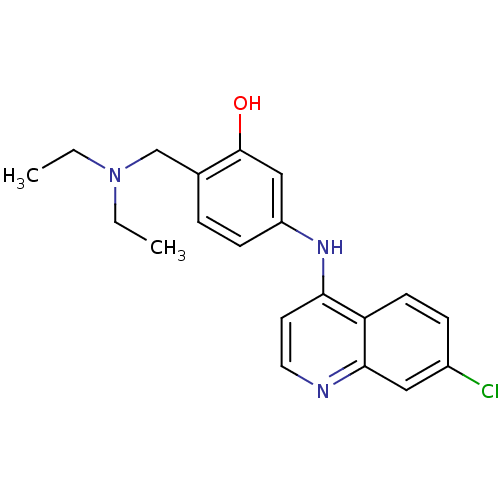
(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50056190
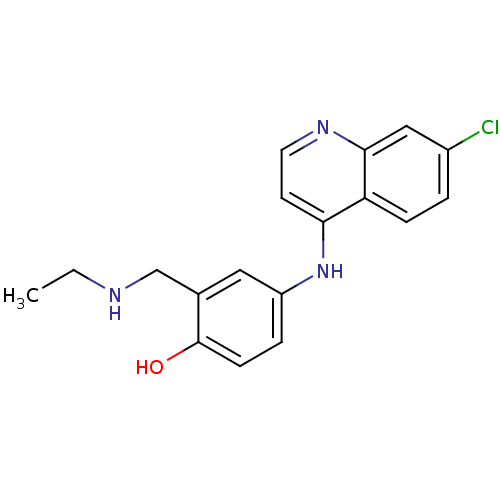
(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50134936

(2-(tert-Butylamino-methyl)-5-(7-chloro-quinolin-4-...)Show InChI InChI=1S/C20H22ClN3O/c1-20(2,3)23-12-13-4-6-15(11-19(13)25)24-17-8-9-22-18-10-14(21)5-7-16(17)18/h4-11,23,25H,12H2,1-3H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134934

(5-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-3-5-14(10-18(12)23)22-16-7-8-21-17-9-13(19)4-6-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50247629

(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human cloned ERG |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50247629

(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50253965

(2-(4-hydroxyphenyl)-7-(2-(2-hydroxyphenyl)-4-oxo-4...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2ccc(Oc3ccc4oc(cc(=O)c4c3)-c3ccccc3O)cc2o1 Show InChI InChI=1S/C30H18O7/c31-18-7-5-17(6-8-18)28-15-25(33)22-11-9-20(14-29(22)37-28)35-19-10-12-27-23(13-19)26(34)16-30(36-27)21-3-1-2-4-24(21)32/h1-16,31-32H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254380
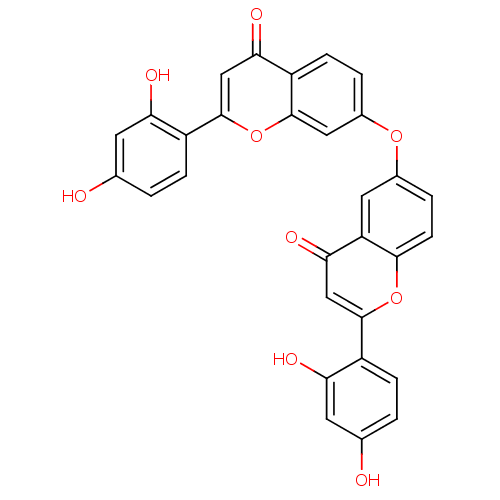
(6,7'-oxybis(2-(2,4-dihydroxyphenyl)-4H-chromen-4-o...)Show SMILES Oc1ccc(c(O)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(O)cc3O)ccc2o1 Show InChI InChI=1S/C30H18O9/c31-15-1-5-19(23(33)9-15)29-14-26(36)22-11-17(4-8-27(22)38-29)37-18-3-7-21-25(35)13-30(39-28(21)12-18)20-6-2-16(32)10-24(20)34/h1-14,31-34H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254380

(6,7'-oxybis(2-(2,4-dihydroxyphenyl)-4H-chromen-4-o...)Show SMILES Oc1ccc(c(O)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(O)cc3O)ccc2o1 Show InChI InChI=1S/C30H18O9/c31-15-1-5-19(23(33)9-15)29-14-26(36)22-11-17(4-8-27(22)38-29)37-18-3-7-21-25(35)13-30(39-28(21)12-18)20-6-2-16(32)10-24(20)34/h1-14,31-34H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50134934

(5-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-3-5-14(10-18(12)23)22-16-7-8-21-17-9-13(19)4-6-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50134936

(2-(tert-Butylamino-methyl)-5-(7-chloro-quinolin-4-...)Show InChI InChI=1S/C20H22ClN3O/c1-20(2,3)23-12-13-4-6-15(11-19(13)25)24-17-8-9-22-18-10-14(21)5-7-16(17)18/h4-11,23,25H,12H2,1-3H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using DEF substrate |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50134936

(2-(tert-Butylamino-methyl)-5-(7-chloro-quinolin-4-...)Show InChI InChI=1S/C20H22ClN3O/c1-20(2,3)23-12-13-4-6-15(11-19(13)25)24-17-8-9-22-18-10-14(21)5-7-16(17)18/h4-11,23,25H,12H2,1-3H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using N-N,diethyl-formamide as substrate |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using N-N,diethyl-formamide as substrate |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using DEF substrate |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50056190

(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134936

(2-(tert-Butylamino-methyl)-5-(7-chloro-quinolin-4-...)Show InChI InChI=1S/C20H22ClN3O/c1-20(2,3)23-12-13-4-6-15(11-19(13)25)24-17-8-9-22-18-10-14(21)5-7-16(17)18/h4-11,23,25H,12H2,1-3H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM22985

(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
J Med Chem 45: 4975-83 (2002)
BindingDB Entry DOI: 10.7270/Q2P55PQ4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50134931

(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
J Med Chem 52: 1408-15 (2010)
Article DOI: 10.1021/jm8012618
BindingDB Entry DOI: 10.7270/Q2348KCX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254285

(6,7'-oxybis(2-phenyl-4H-chromen-4-one) | CHEMBL466...)Show SMILES O=c1cc(oc2cc(Oc3ccc4oc(cc(=O)c4c3)-c3ccccc3)ccc12)-c1ccccc1 Show InChI InChI=1S/C30H18O5/c31-25-17-29(20-9-5-2-6-10-20)35-30-16-22(11-13-23(25)30)33-21-12-14-27-24(15-21)26(32)18-28(34-27)19-7-3-1-4-8-19/h1-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50253965

(2-(4-hydroxyphenyl)-7-(2-(2-hydroxyphenyl)-4-oxo-4...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2ccc(Oc3ccc4oc(cc(=O)c4c3)-c3ccccc3O)cc2o1 Show InChI InChI=1S/C30H18O7/c31-18-7-5-17(6-8-18)28-15-25(33)22-11-9-20(14-29(22)37-28)35-19-10-12-27-23(13-19)26(34)16-30(36-27)21-3-1-2-4-24(21)32/h1-16,31-32H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254424
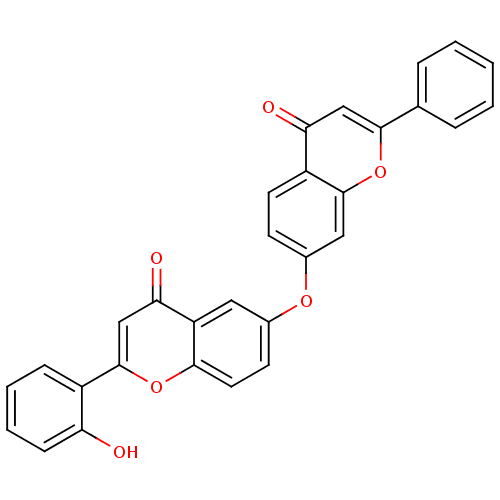
(2-(2-hydroxyphenyl)-6-(4-oxo-2-phenyl-4H-chromen-7...)Show SMILES Oc1ccccc1-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C30H18O6/c31-24-9-5-4-8-21(24)30-17-26(33)23-14-19(11-13-27(23)35-30)34-20-10-12-22-25(32)16-28(36-29(22)15-20)18-6-2-1-3-7-18/h1-17,31H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254423
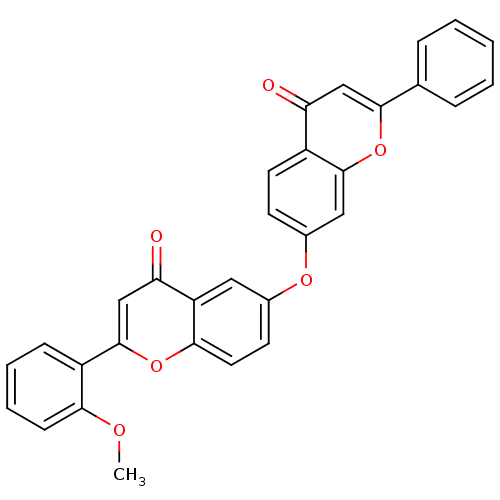
(2-(2-methoxyphenyl)-6-(4-oxo-2-phenyl-4H-chromen-7...)Show SMILES COc1ccccc1-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C31H20O6/c1-34-27-10-6-5-9-23(27)31-18-26(33)24-15-20(12-14-28(24)36-31)35-21-11-13-22-25(32)17-29(37-30(22)16-21)19-7-3-2-4-8-19/h2-18H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data