 Found 117 hits with Last Name = 'park' and Initial = 'sb'
Found 117 hits with Last Name = 'park' and Initial = 'sb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM167444

(US9073906, 156)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Cl)cc1Cl |TLB:7:8:10:15.16.17,7:8:15.14.17:10.11.12,18:15:10:8.13.12,THB:16:15:8:10.11.12,16:11:8:15.14.17,14:15:10:8.13.12,14:13:10:15.16.17,18:15:8:10.11.12,(-2.33,2.4,;-2.33,.86,;-.99,.09,;-.99,-1.45,;.34,-2.22,;1.68,-1.45,;1.68,.09,;3.01,-2.22,;4.34,-1.45,;4.11,.62,;3.34,1.95,;4.11,3.28,;4.04,1.9,;5.23,-.19,;6.61,-.17,;6.42,1.95,;5.65,3.28,;5.65,.62,;7.51,3.04,;8.99,2.64,;7.11,4.53,;-2.33,-2.22,;-3.1,-3.55,;-1.56,-3.55,;-3.66,-1.45,;-3.66,.09,;-4.99,-2.22,;-6.33,-1.45,;-6.33,.09,;-7.66,-2.22,;-7.66,-3.76,;-8.99,-4.53,;-6.33,-4.53,;-4.99,-3.76,;-3.66,-4.53,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537919

(CHEMBL4633146)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Br)cc1Cl |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(11.03,-25.79,;11.01,-27.33,;12.33,-28.11,;12.31,-29.65,;13.64,-30.43,;14.98,-29.67,;14.99,-28.13,;16.3,-30.45,;17.64,-29.69,;19.4,-29.74,;19.92,-31.07,;21.11,-31.89,;19.47,-31.98,;18.96,-30.55,;20.14,-29.48,;21.77,-29.44,;22.26,-30.88,;20.51,-28.74,;23.09,-28.66,;24.43,-29.42,;23.08,-27.12,;10.98,-30.41,;11.74,-31.74,;10.2,-31.73,;9.65,-29.62,;9.67,-28.08,;8.31,-30.38,;8.3,-31.91,;9.63,-32.69,;6.96,-32.67,;5.63,-31.88,;4.29,-32.64,;5.65,-30.34,;6.99,-29.59,;7.01,-28.05,)| Show InChI InChI=1S/C23H29BrCl2N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537918

(CHEMBL3951168)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)C(F)(F)F |TLB:18:15:10:8.12.13,7:8:10:15.16.17,THB:16:11:8:14.15.17,16:15:8:10.11.12,18:15:8:10.11.12,14:13:10:15.16.17,14:15:10:8.12.13,(-1.37,-7.72,;-2.51,-7.26,;-2.73,-5.74,;-4.16,-5.17,;-4.38,-3.64,;-3.17,-2.69,;-2.03,-3.15,;-3.39,-1.16,;-2.19,-.22,;-1.2,1.02,;-1.2,2.69,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;1.56,1.02,;1.56,2.59,;.34,.44,;3.07,1.16,;3.58,2.29,;3.79,.16,;-5.37,-6.12,;-5.52,-4.9,;-6.32,-6.91,;-5.15,-7.65,;-3.72,-8.22,;-6.36,-8.6,;-7.79,-8.03,;-7.97,-6.81,;-9,-8.99,;-8.78,-10.51,;-7.34,-11.08,;-6.14,-10.12,;-4.99,-10.58,;-9.98,-11.47,;-11.13,-11.01,;-9.8,-12.69,;-10.95,-12.23,)| Show InChI InChI=1S/C24H29Cl2F3N4O4S/c1-12-9-32(38(36,37)33(10-12)21-17(25)4-16(5-18(21)26)24(27,28)29)11-19(34)31-20-14-2-13-3-15(20)8-23(6-13,7-14)22(30)35/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,30,35)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537922

(CHEMBL4645319)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CCCN(c4c(Cl)cc(cc4Cl)C(F)(F)F)S1(=O)=O)C(C3)C2 |TLB:35:34:8:6.5.4,10:9:6.35.5:36.3.8,10:9:8:6.5.4,THB:35:5:9.34.36:8,4:5:9:36.3.8,4:3:9:6.35.5,(25.97,-44.51,;24.63,-43.75,;24.62,-42.21,;23.31,-44.52,;23.8,-45.96,;22.65,-46.97,;21.46,-46.15,;20.94,-44.82,;22.05,-43.83,;19.18,-44.78,;17.84,-45.53,;16.52,-44.75,;16.53,-43.21,;15.18,-45.51,;13.85,-44.73,;13.87,-43.19,;12.55,-42.41,;11.21,-43.16,;11.19,-44.7,;9.85,-45.46,;9.84,-46.99,;11.17,-47.77,;8.5,-47.75,;7.17,-46.96,;7.19,-45.42,;8.53,-44.67,;8.55,-43.13,;5.83,-47.72,;4.5,-46.94,;5.81,-49.26,;4.49,-48.48,;12.52,-45.49,;13.28,-46.82,;11.74,-46.81,;20.5,-45.63,;21.01,-47.06,;21.68,-44.56,)| Show InChI InChI=1S/C23H27Cl2F3N4O4S/c24-16-6-15(23(26,27)28)7-17(25)20(16)32-3-1-2-31(37(32,35)36)11-18(33)30-19-13-4-12-5-14(19)10-22(8-12,9-13)21(29)34/h6-7,12-14,19H,1-5,8-11H2,(H2,29,34)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537919

(CHEMBL4633146)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Br)cc1Cl |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(11.03,-25.79,;11.01,-27.33,;12.33,-28.11,;12.31,-29.65,;13.64,-30.43,;14.98,-29.67,;14.99,-28.13,;16.3,-30.45,;17.64,-29.69,;19.4,-29.74,;19.92,-31.07,;21.11,-31.89,;19.47,-31.98,;18.96,-30.55,;20.14,-29.48,;21.77,-29.44,;22.26,-30.88,;20.51,-28.74,;23.09,-28.66,;24.43,-29.42,;23.08,-27.12,;10.98,-30.41,;11.74,-31.74,;10.2,-31.73,;9.65,-29.62,;9.67,-28.08,;8.31,-30.38,;8.3,-31.91,;9.63,-32.69,;6.96,-32.67,;5.63,-31.88,;4.29,-32.64,;5.65,-30.34,;6.99,-29.59,;7.01,-28.05,)| Show InChI InChI=1S/C23H29BrCl2N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537918

(CHEMBL3951168)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)C(F)(F)F |TLB:18:15:10:8.12.13,7:8:10:15.16.17,THB:16:11:8:14.15.17,16:15:8:10.11.12,18:15:8:10.11.12,14:13:10:15.16.17,14:15:10:8.12.13,(-1.37,-7.72,;-2.51,-7.26,;-2.73,-5.74,;-4.16,-5.17,;-4.38,-3.64,;-3.17,-2.69,;-2.03,-3.15,;-3.39,-1.16,;-2.19,-.22,;-1.2,1.02,;-1.2,2.69,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;1.56,1.02,;1.56,2.59,;.34,.44,;3.07,1.16,;3.58,2.29,;3.79,.16,;-5.37,-6.12,;-5.52,-4.9,;-6.32,-6.91,;-5.15,-7.65,;-3.72,-8.22,;-6.36,-8.6,;-7.79,-8.03,;-7.97,-6.81,;-9,-8.99,;-8.78,-10.51,;-7.34,-11.08,;-6.14,-10.12,;-4.99,-10.58,;-9.98,-11.47,;-11.13,-11.01,;-9.8,-12.69,;-10.95,-12.23,)| Show InChI InChI=1S/C24H29Cl2F3N4O4S/c1-12-9-32(38(36,37)33(10-12)21-17(25)4-16(5-18(21)26)24(27,28)29)11-19(34)31-20-14-2-13-3-15(20)8-23(6-13,7-14)22(30)35/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,30,35)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537922

(CHEMBL4645319)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)CN1CCCN(c4c(Cl)cc(cc4Cl)C(F)(F)F)S1(=O)=O)C(C3)C2 |TLB:35:34:8:6.5.4,10:9:6.35.5:36.3.8,10:9:8:6.5.4,THB:35:5:9.34.36:8,4:5:9:36.3.8,4:3:9:6.35.5,(25.97,-44.51,;24.63,-43.75,;24.62,-42.21,;23.31,-44.52,;23.8,-45.96,;22.65,-46.97,;21.46,-46.15,;20.94,-44.82,;22.05,-43.83,;19.18,-44.78,;17.84,-45.53,;16.52,-44.75,;16.53,-43.21,;15.18,-45.51,;13.85,-44.73,;13.87,-43.19,;12.55,-42.41,;11.21,-43.16,;11.19,-44.7,;9.85,-45.46,;9.84,-46.99,;11.17,-47.77,;8.5,-47.75,;7.17,-46.96,;7.19,-45.42,;8.53,-44.67,;8.55,-43.13,;5.83,-47.72,;4.5,-46.94,;5.81,-49.26,;4.49,-48.48,;12.52,-45.49,;13.28,-46.82,;11.74,-46.81,;20.5,-45.63,;21.01,-47.06,;21.68,-44.56,)| Show InChI InChI=1S/C23H27Cl2F3N4O4S/c24-16-6-15(23(26,27)28)7-17(25)20(16)32-3-1-2-31(37(32,35)36)11-18(33)30-19-13-4-12-5-14(19)10-22(8-12,9-13)21(29)34/h6-7,12-14,19H,1-5,8-11H2,(H2,29,34)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537921
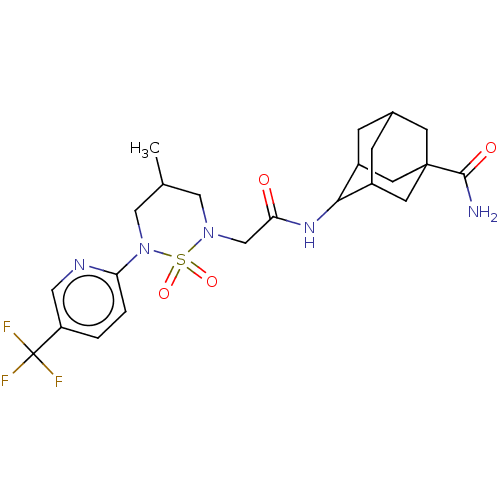
(CHEMBL4649416)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1ccc(cn1)C(F)(F)F |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(62.19,-24.8,;62.17,-26.34,;63.49,-27.12,;63.47,-28.66,;64.79,-29.44,;66.13,-28.68,;66.15,-27.14,;67.46,-29.46,;68.8,-28.71,;70.56,-28.75,;71.07,-30.08,;72.26,-30.9,;70.63,-30.99,;70.12,-29.56,;71.3,-28.49,;72.93,-28.45,;73.42,-29.89,;71.67,-27.76,;74.25,-27.68,;75.59,-28.44,;74.24,-26.14,;62.13,-29.42,;62.9,-30.75,;61.36,-30.74,;60.81,-28.63,;60.82,-27.09,;59.47,-29.39,;59.46,-30.92,;58.12,-31.68,;56.79,-30.89,;56.8,-29.35,;58.15,-28.6,;55.44,-31.65,;54.12,-30.87,;55.43,-33.19,;54.11,-32.41,)| Show InChI InChI=1S/C23H30F3N5O4S/c1-13-10-30(36(34,35)31(11-13)18-3-2-17(9-28-18)23(24,25)26)12-19(32)29-20-15-4-14-5-16(20)8-22(6-14,7-15)21(27)33/h2-3,9,13-16,20H,4-8,10-12H2,1H3,(H2,27,33)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM167444

(US9073906, 156)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(Cl)cc1Cl |TLB:7:8:10:15.16.17,7:8:15.14.17:10.11.12,18:15:10:8.13.12,THB:16:15:8:10.11.12,16:11:8:15.14.17,14:15:10:8.13.12,14:13:10:15.16.17,18:15:8:10.11.12,(-2.33,2.4,;-2.33,.86,;-.99,.09,;-.99,-1.45,;.34,-2.22,;1.68,-1.45,;1.68,.09,;3.01,-2.22,;4.34,-1.45,;4.11,.62,;3.34,1.95,;4.11,3.28,;4.04,1.9,;5.23,-.19,;6.61,-.17,;6.42,1.95,;5.65,3.28,;5.65,.62,;7.51,3.04,;8.99,2.64,;7.11,4.53,;-2.33,-2.22,;-3.1,-3.55,;-1.56,-3.55,;-3.66,-1.45,;-3.66,.09,;-4.99,-2.22,;-6.33,-1.45,;-6.33,.09,;-7.66,-2.22,;-7.66,-3.76,;-8.99,-4.53,;-6.33,-4.53,;-4.99,-3.76,;-3.66,-4.53,)| Show InChI InChI=1S/C23H29Cl3N4O4S/c1-12-9-29(35(33,34)30(10-12)21-17(25)4-16(24)5-18(21)26)11-19(31)28-20-14-2-13-3-15(20)8-23(6-13,7-14)22(27)32/h4-5,12-15,20H,2-3,6-11H2,1H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50384888
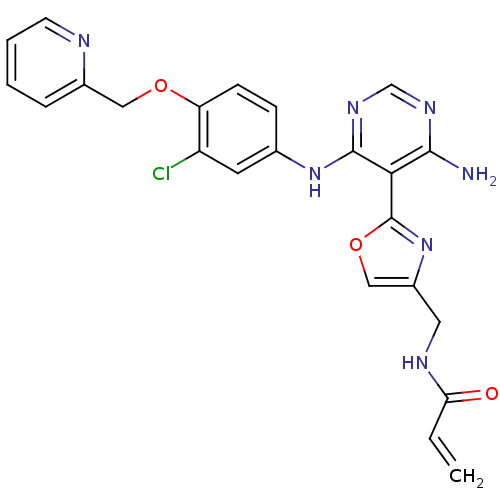
(CHEMBL2035810)Show SMILES Nc1ncnc(Nc2ccc(OCc3ccccn3)c(Cl)c2)c1-c1nc(CNC(=O)C=C)co1 Show InChI InChI=1S/C23H20ClN7O3/c1-2-19(32)27-10-16-12-34-23(31-16)20-21(25)28-13-29-22(20)30-14-6-7-18(17(24)9-14)33-11-15-5-3-4-8-26-15/h2-9,12-13H,1,10-11H2,(H,27,32)(H3,25,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR preincubated for 10 mins followed by incubation for 30 mins by fluorescence polarization assay |
J Med Chem 55: 2846-57 (2012)
Article DOI: 10.1021/jm201758g
BindingDB Entry DOI: 10.7270/Q23T9J8F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537917

(CHEMBL4648389)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(C)cc1Cl |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(36.44,-11.66,;36.42,-13.2,;37.73,-13.98,;37.71,-15.52,;39.04,-16.3,;40.38,-15.54,;40.39,-14,;41.71,-16.33,;43.05,-15.57,;44.8,-15.61,;45.32,-16.94,;46.51,-17.76,;44.88,-17.85,;44.36,-16.42,;45.55,-15.36,;47.17,-15.32,;47.67,-16.75,;45.91,-14.62,;48.5,-14.54,;49.84,-15.3,;48.49,-13,;36.38,-16.28,;37.14,-17.61,;35.61,-17.61,;35.05,-15.49,;35.07,-13.95,;33.72,-16.25,;33.7,-17.78,;35.03,-18.57,;32.37,-18.54,;31.03,-17.76,;29.69,-18.51,;31.05,-16.22,;32.39,-15.46,;32.41,-13.92,)| Show InChI InChI=1S/C24H32Cl2N4O4S/c1-13-3-18(25)22(19(26)4-13)30-11-14(2)10-29(35(30,33)34)12-20(31)28-21-16-5-15-6-17(21)9-24(7-15,8-16)23(27)32/h3-4,14-17,21H,5-12H2,1-2H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50384888

(CHEMBL2035810)Show SMILES Nc1ncnc(Nc2ccc(OCc3ccccn3)c(Cl)c2)c1-c1nc(CNC(=O)C=C)co1 Show InChI InChI=1S/C23H20ClN7O3/c1-2-19(32)27-10-16-12-34-23(31-16)20-21(25)28-13-29-22(20)30-14-6-7-18(17(24)9-14)33-11-15-5-3-4-8-26-15/h2-9,12-13H,1,10-11H2,(H,27,32)(H3,25,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of HER4 preincubated for 10 mins followed by incubation for 30 mins by fluorescence polarization assay |
J Med Chem 55: 2846-57 (2012)
Article DOI: 10.1021/jm201758g
BindingDB Entry DOI: 10.7270/Q23T9J8F |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50300027
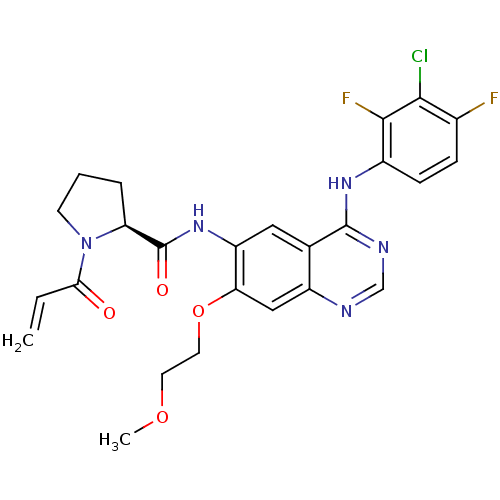
((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her1 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50300026
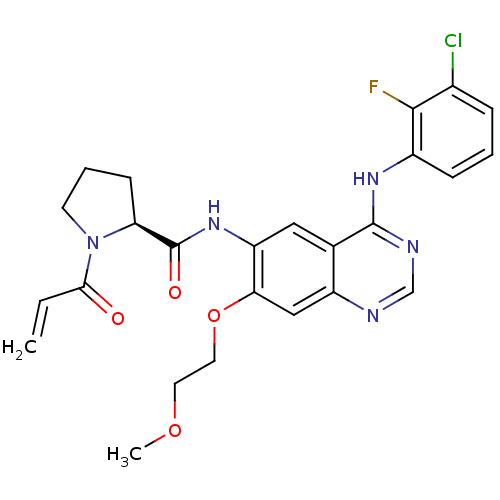
((S)-1-Acryloyl-N-[4-(3-chloro-2-fluorophenylamino)...)Show SMILES COCCOc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H25ClFN5O4/c1-3-22(33)32-9-5-8-20(32)25(34)31-19-12-15-18(13-21(19)36-11-10-35-2)28-14-29-24(15)30-17-7-4-6-16(26)23(17)27/h3-4,6-7,12-14,20H,1,5,8-11H2,2H3,(H,31,34)(H,28,29,30)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her1 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537917

(CHEMBL4648389)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(C)cc1Cl |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(36.44,-11.66,;36.42,-13.2,;37.73,-13.98,;37.71,-15.52,;39.04,-16.3,;40.38,-15.54,;40.39,-14,;41.71,-16.33,;43.05,-15.57,;44.8,-15.61,;45.32,-16.94,;46.51,-17.76,;44.88,-17.85,;44.36,-16.42,;45.55,-15.36,;47.17,-15.32,;47.67,-16.75,;45.91,-14.62,;48.5,-14.54,;49.84,-15.3,;48.49,-13,;36.38,-16.28,;37.14,-17.61,;35.61,-17.61,;35.05,-15.49,;35.07,-13.95,;33.72,-16.25,;33.7,-17.78,;35.03,-18.57,;32.37,-18.54,;31.03,-17.76,;29.69,-18.51,;31.05,-16.22,;32.39,-15.46,;32.41,-13.92,)| Show InChI InChI=1S/C24H32Cl2N4O4S/c1-13-3-18(25)22(19(26)4-13)30-11-14(2)10-29(35(30,33)34)12-20(31)28-21-16-5-15-6-17(21)9-24(7-15,8-16)23(27)32/h3-4,14-17,21H,5-12H2,1-2H3,(H2,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537920

(CHEMBL4632806)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1ccccn1 |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(34.51,-25.75,;34.49,-27.29,;35.81,-28.07,;35.79,-29.61,;37.12,-30.39,;38.46,-29.63,;38.48,-28.09,;39.79,-30.42,;41.13,-29.66,;42.89,-29.7,;43.41,-31.03,;44.6,-31.85,;42.96,-31.95,;42.45,-30.51,;43.63,-29.45,;45.26,-29.41,;45.76,-30.84,;44,-28.71,;46.59,-28.63,;47.93,-29.39,;46.58,-27.09,;34.46,-30.37,;35.22,-31.7,;33.69,-31.7,;33.13,-29.58,;33.15,-28.04,;31.79,-30.34,;31.78,-31.88,;30.44,-32.64,;29.11,-31.85,;29.13,-30.31,;30.47,-29.55,)| Show InChI InChI=1S/C22H31N5O4S/c1-14-11-26(32(30,31)27(12-14)18-4-2-3-5-24-18)13-19(28)25-20-16-6-15-7-17(20)10-22(8-15,9-16)21(23)29/h2-5,14-17,20H,6-13H2,1H3,(H2,23,29)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50300027

((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her2 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50384888

(CHEMBL2035810)Show SMILES Nc1ncnc(Nc2ccc(OCc3ccccn3)c(Cl)c2)c1-c1nc(CNC(=O)C=C)co1 Show InChI InChI=1S/C23H20ClN7O3/c1-2-19(32)27-10-16-12-34-23(31-16)20-21(25)28-13-29-22(20)30-14-6-7-18(17(24)9-14)33-11-15-5-3-4-8-26-15/h2-9,12-13H,1,10-11H2,(H,27,32)(H3,25,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of HER2 preincubated for 10 mins followed by incubation for 30 mins by fluorescence polarization assay |
J Med Chem 55: 2846-57 (2012)
Article DOI: 10.1021/jm201758g
BindingDB Entry DOI: 10.7270/Q23T9J8F |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her2 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of HER2 preincubated for 10 mins followed by incubation for 30 mins by fluorescence polarization assay |
J Med Chem 55: 2846-57 (2012)
Article DOI: 10.1021/jm201758g
BindingDB Entry DOI: 10.7270/Q23T9J8F |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50300026

((S)-1-Acryloyl-N-[4-(3-chloro-2-fluorophenylamino)...)Show SMILES COCCOc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H25ClFN5O4/c1-3-22(33)32-9-5-8-20(32)25(34)31-19-12-15-18(13-21(19)36-11-10-35-2)28-14-29-24(15)30-17-7-4-6-16(26)23(17)27/h3-4,6-7,12-14,20H,1,5,8-11H2,2H3,(H,31,34)(H,28,29,30)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her2 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537916

(CHEMBL4646414)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)-c1ccco1 |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(12.15,-11.25,;12.13,-12.79,;13.45,-13.57,;13.43,-15.11,;14.76,-15.89,;16.1,-15.13,;16.11,-13.59,;17.42,-15.91,;18.76,-15.16,;20.52,-15.2,;21.04,-16.53,;22.23,-17.35,;20.59,-17.44,;20.08,-16.01,;21.26,-14.94,;22.89,-14.9,;23.38,-16.34,;21.63,-14.21,;24.21,-14.12,;25.55,-14.89,;24.2,-12.58,;12.09,-15.87,;12.86,-17.2,;11.32,-17.19,;10.77,-15.08,;10.79,-13.54,;9.43,-15.84,;9.42,-17.37,;10.75,-18.15,;8.08,-18.13,;6.75,-17.34,;6.77,-15.8,;8.11,-15.05,;8.13,-13.51,;5.4,-18.1,;5.22,-19.63,;3.71,-19.93,;2.96,-18.59,;4,-17.46,)| Show InChI InChI=1S/C27H32Cl2N4O5S/c1-15-12-32(14-23(34)31-24-18-5-16-6-19(24)11-27(9-16,10-18)26(30)35)39(36,37)33(13-15)25-20(28)7-17(8-21(25)29)22-3-2-4-38-22/h2-4,7-8,15-16,18-19,24H,5-6,9-14H2,1H3,(H2,30,35)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her1 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 52.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR preincubated for 10 mins followed by incubation for 30 mins by fluorescence polarization assay |
J Med Chem 55: 2846-57 (2012)
Article DOI: 10.1021/jm201758g
BindingDB Entry DOI: 10.7270/Q23T9J8F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537916

(CHEMBL4646414)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)-c1ccco1 |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(12.15,-11.25,;12.13,-12.79,;13.45,-13.57,;13.43,-15.11,;14.76,-15.89,;16.1,-15.13,;16.11,-13.59,;17.42,-15.91,;18.76,-15.16,;20.52,-15.2,;21.04,-16.53,;22.23,-17.35,;20.59,-17.44,;20.08,-16.01,;21.26,-14.94,;22.89,-14.9,;23.38,-16.34,;21.63,-14.21,;24.21,-14.12,;25.55,-14.89,;24.2,-12.58,;12.09,-15.87,;12.86,-17.2,;11.32,-17.19,;10.77,-15.08,;10.79,-13.54,;9.43,-15.84,;9.42,-17.37,;10.75,-18.15,;8.08,-18.13,;6.75,-17.34,;6.77,-15.8,;8.11,-15.05,;8.13,-13.51,;5.4,-18.1,;5.22,-19.63,;3.71,-19.93,;2.96,-18.59,;4,-17.46,)| Show InChI InChI=1S/C27H32Cl2N4O5S/c1-15-12-32(14-23(34)31-24-18-5-16-6-19(24)11-27(9-16,10-18)26(30)35)39(36,37)33(13-15)25-20(28)7-17(8-21(25)29)22-3-2-4-38-22/h2-4,7-8,15-16,18-19,24H,5-6,9-14H2,1H3,(H2,30,35)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM33413
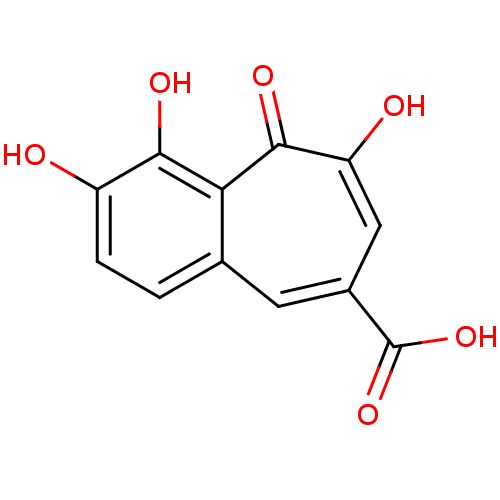
(benzo[7]annulene-8-carboxylic acid, 5)Show InChI InChI=1S/C12H8O6/c13-7-2-1-5-3-6(12(17)18)4-8(14)11(16)9(5)10(7)15/h1-4,13,15H,(H,14,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SHP2 expressed in Escherichia coli using 2P-IRS1 peptide as substrate after 1 hr by microplate reader analysis |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126756
BindingDB Entry DOI: 10.7270/Q26113NZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50300027

((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Ret |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50300027

((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Src |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50300027

((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50300026

((S)-1-Acryloyl-N-[4-(3-chloro-2-fluorophenylamino)...)Show SMILES COCCOc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H25ClFN5O4/c1-3-22(33)32-9-5-8-20(32)25(34)31-19-12-15-18(13-21(19)36-11-10-35-2)28-14-29-24(15)30-17-7-4-6-16(26)23(17)27/h3-4,6-7,12-14,20H,1,5,8-11H2,2H3,(H,31,34)(H,28,29,30)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her4 |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50300027

((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50300027

((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her4 |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50511072
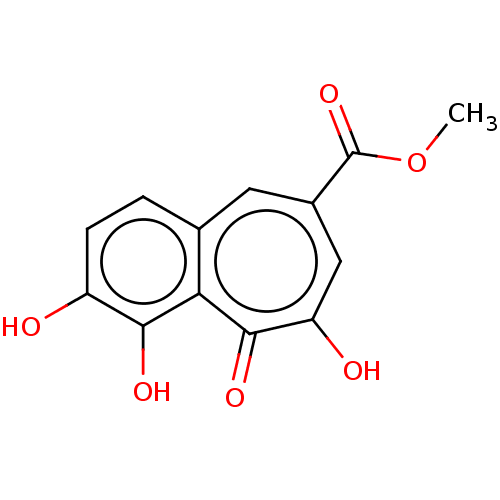
(METHYL 7-DESHYDROXYPYROGALLIN-4-CARBOXYLATE)Show InChI InChI=1S/C13H10O6/c1-19-13(18)7-4-6-2-3-8(14)11(16)10(6)12(17)9(15)5-7/h2-5,14,16H,1H3,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SHP2 expressed in Escherichia coli using 2P-IRS1 peptide as substrate after 1 hr by microplate reader analysis |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126756
BindingDB Entry DOI: 10.7270/Q26113NZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537915
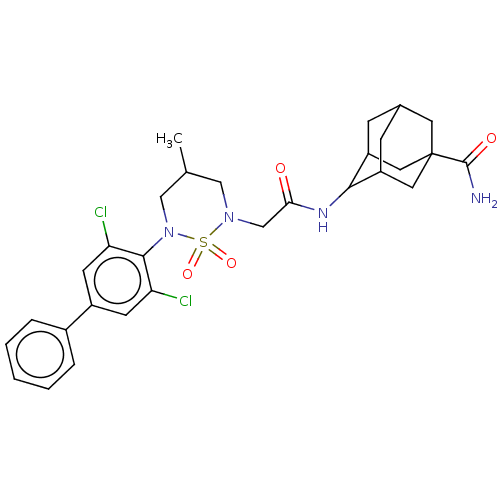
(CHEMBL4638272)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)-c1ccccc1 |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(45.75,.59,;45.73,-.95,;47.05,-1.73,;47.03,-3.27,;48.36,-4.06,;49.7,-3.3,;49.71,-1.76,;51.03,-4.08,;52.37,-3.32,;54.12,-3.37,;54.64,-4.69,;55.83,-5.52,;54.2,-5.61,;53.68,-4.17,;54.87,-3.11,;56.49,-3.07,;56.99,-4.5,;55.24,-2.37,;57.82,-2.29,;59.16,-3.05,;57.81,-.75,;45.69,-4.03,;46.45,-5.36,;44.92,-5.36,;44.36,-3.25,;44.38,-1.7,;43.03,-4,;43.01,-5.54,;44.34,-6.32,;41.68,-6.3,;40.34,-5.51,;40.36,-3.97,;41.7,-3.21,;41.72,-1.67,;39,-6.27,;37.67,-5.48,;36.33,-6.23,;36.31,-7.78,;37.65,-8.56,;38.99,-7.8,)| Show InChI InChI=1S/C29H34Cl2N4O4S/c1-17-14-34(16-25(36)33-26-21-7-18-8-22(26)13-29(11-18,12-21)28(32)37)40(38,39)35(15-17)27-23(30)9-20(10-24(27)31)19-5-3-2-4-6-19/h2-6,9-10,17-18,21-22,26H,7-8,11-16H2,1H3,(H2,32,37)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537915

(CHEMBL4638272)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)-c1ccccc1 |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(45.75,.59,;45.73,-.95,;47.05,-1.73,;47.03,-3.27,;48.36,-4.06,;49.7,-3.3,;49.71,-1.76,;51.03,-4.08,;52.37,-3.32,;54.12,-3.37,;54.64,-4.69,;55.83,-5.52,;54.2,-5.61,;53.68,-4.17,;54.87,-3.11,;56.49,-3.07,;56.99,-4.5,;55.24,-2.37,;57.82,-2.29,;59.16,-3.05,;57.81,-.75,;45.69,-4.03,;46.45,-5.36,;44.92,-5.36,;44.36,-3.25,;44.38,-1.7,;43.03,-4,;43.01,-5.54,;44.34,-6.32,;41.68,-6.3,;40.34,-5.51,;40.36,-3.97,;41.7,-3.21,;41.72,-1.67,;39,-6.27,;37.67,-5.48,;36.33,-6.23,;36.31,-7.78,;37.65,-8.56,;38.99,-7.8,)| Show InChI InChI=1S/C29H34Cl2N4O4S/c1-17-14-34(16-25(36)33-26-21-7-18-8-22(26)13-29(11-18,12-21)28(32)37)40(38,39)35(15-17)27-23(30)9-20(10-24(27)31)19-5-3-2-4-6-19/h2-6,9-10,17-18,21-22,26H,7-8,11-16H2,1H3,(H2,32,37)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50247012
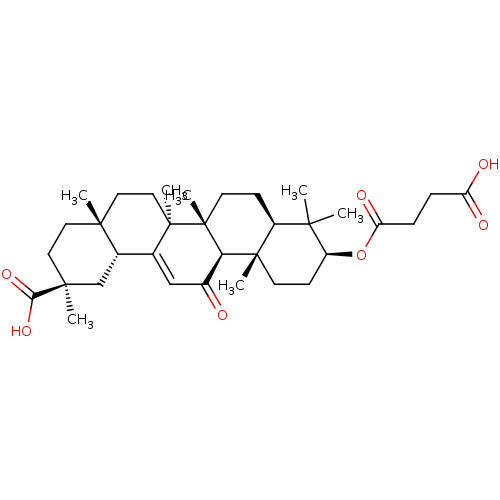
(3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)OC(=O)CCC(O)=O |r,t:18| Show InChI InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50511066
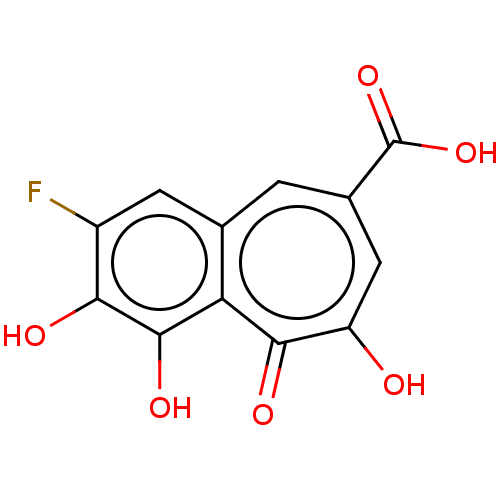
(CHEMBL4467577)Show InChI InChI=1S/C12H7FO6/c13-6-2-4-1-5(12(18)19)3-7(14)10(16)8(4)11(17)9(6)15/h1-3,15,17H,(H,14,16)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SHP2 expressed in Escherichia coli using 2P-IRS1 peptide as substrate after 1 hr by microplate reader analysis |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126756
BindingDB Entry DOI: 10.7270/Q26113NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50300026

((S)-1-Acryloyl-N-[4-(3-chloro-2-fluorophenylamino)...)Show SMILES COCCOc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H25ClFN5O4/c1-3-22(33)32-9-5-8-20(32)25(34)31-19-12-15-18(13-21(19)36-11-10-35-2)28-14-29-24(15)30-17-7-4-6-16(26)23(17)27/h3-4,6-7,12-14,20H,1,5,8-11H2,2H3,(H,31,34)(H,28,29,30)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50300026

((S)-1-Acryloyl-N-[4-(3-chloro-2-fluorophenylamino)...)Show SMILES COCCOc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H25ClFN5O4/c1-3-22(33)32-9-5-8-20(32)25(34)31-19-12-15-18(13-21(19)36-11-10-35-2)28-14-29-24(15)30-17-7-4-6-16(26)23(17)27/h3-4,6-7,12-14,20H,1,5,8-11H2,2H3,(H,31,34)(H,28,29,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537923
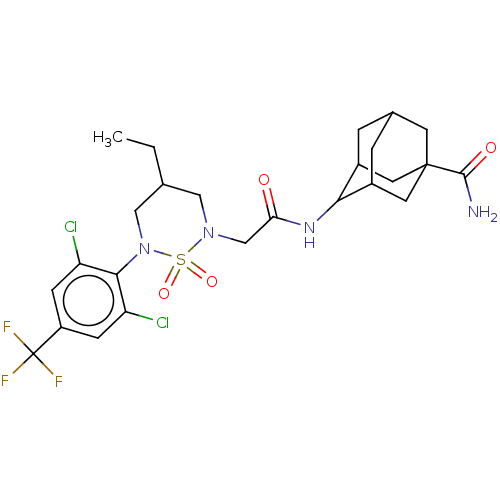
(CHEMBL4638268)Show SMILES CCC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)C(F)(F)F |TLB:13:14:18:11.12.17,13:12:9.14.15:18,8:9:11.13.12:15.16.18,8:9:18:11.12.17,THB:17:12:9:15.16.18,17:16:9:11.13.12,(39.03,-38.53,;37.69,-39.28,;37.67,-40.82,;38.99,-41.6,;38.96,-43.14,;40.29,-43.92,;41.63,-43.16,;41.65,-41.62,;42.96,-43.95,;44.3,-43.19,;46.05,-43.23,;46.57,-44.56,;47.76,-45.38,;46.13,-45.47,;45.61,-44.04,;46.8,-42.98,;48.42,-42.94,;48.92,-44.37,;47.17,-42.24,;49.75,-42.16,;51.09,-42.92,;49.74,-40.62,;37.63,-43.9,;38.39,-45.23,;36.86,-45.22,;36.3,-43.11,;36.32,-41.57,;34.97,-43.87,;34.95,-45.4,;36.28,-46.19,;33.62,-46.16,;32.28,-45.38,;32.3,-43.83,;33.64,-43.08,;33.66,-41.54,;30.94,-46.13,;29.62,-45.35,;30.93,-47.67,;29.6,-46.89,)| Show InChI InChI=1S/C25H31Cl2F3N4O4S/c1-2-13-10-33(39(37,38)34(11-13)22-18(26)5-17(6-19(22)27)25(28,29)30)12-20(35)32-21-15-3-14-4-16(21)9-24(7-14,8-15)23(31)36/h5-6,13-16,21H,2-4,7-12H2,1H3,(H2,31,36)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 529 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50300027

((S)-1-Acryloyl-N-[4-(3-chloro-2,4-difluorophenylam...)Show SMILES COCCOc1cc2ncnc(Nc3ccc(F)c(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H24ClF2N5O4/c1-3-21(34)33-8-4-5-19(33)25(35)32-18-11-14-17(12-20(18)37-10-9-36-2)29-13-30-24(14)31-16-7-6-15(27)22(26)23(16)28/h3,6-7,11-13,19H,1,4-5,8-10H2,2H3,(H,32,35)(H,29,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537923

(CHEMBL4638268)Show SMILES CCC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)C(F)(F)F |TLB:13:14:18:11.12.17,13:12:9.14.15:18,8:9:11.13.12:15.16.18,8:9:18:11.12.17,THB:17:12:9:15.16.18,17:16:9:11.13.12,(39.03,-38.53,;37.69,-39.28,;37.67,-40.82,;38.99,-41.6,;38.96,-43.14,;40.29,-43.92,;41.63,-43.16,;41.65,-41.62,;42.96,-43.95,;44.3,-43.19,;46.05,-43.23,;46.57,-44.56,;47.76,-45.38,;46.13,-45.47,;45.61,-44.04,;46.8,-42.98,;48.42,-42.94,;48.92,-44.37,;47.17,-42.24,;49.75,-42.16,;51.09,-42.92,;49.74,-40.62,;37.63,-43.9,;38.39,-45.23,;36.86,-45.22,;36.3,-43.11,;36.32,-41.57,;34.97,-43.87,;34.95,-45.4,;36.28,-46.19,;33.62,-46.16,;32.28,-45.38,;32.3,-43.83,;33.64,-43.08,;33.66,-41.54,;30.94,-46.13,;29.62,-45.35,;30.93,-47.67,;29.6,-46.89,)| Show InChI InChI=1S/C25H31Cl2F3N4O4S/c1-2-13-10-33(39(37,38)34(11-13)22-18(26)5-17(6-19(22)27)25(28,29)30)12-20(35)32-21-15-3-14-4-16(21)9-24(7-14,8-15)23(31)36/h5-6,13-16,21H,2-4,7-12H2,1H3,(H2,31,36)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 695 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50300026

((S)-1-Acryloyl-N-[4-(3-chloro-2-fluorophenylamino)...)Show SMILES COCCOc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H25ClFN5O4/c1-3-22(33)32-9-5-8-20(32)25(34)31-19-12-15-18(13-21(19)36-11-10-35-2)28-14-29-24(15)30-17-7-4-6-16(26)23(17)27/h3-4,6-7,12-14,20H,1,5,8-11H2,2H3,(H,31,34)(H,28,29,30)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Ret |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50300026

((S)-1-Acryloyl-N-[4-(3-chloro-2-fluorophenylamino)...)Show SMILES COCCOc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1NC(=O)[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C25H25ClFN5O4/c1-3-22(33)32-9-5-8-20(32)25(34)31-19-12-15-18(13-21(19)36-11-10-35-2)28-14-29-24(15)30-17-7-4-6-16(26)23(17)27/h3-4,6-7,12-14,20H,1,5,8-11H2,2H3,(H,31,34)(H,28,29,30)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Src |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50537921

(CHEMBL4649416)Show SMILES CC1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1ccc(cn1)C(F)(F)F |TLB:12:13:17:10.11.16,12:11:8.13.14:17,7:8:10.12.11:14.15.17,7:8:17:10.11.16,THB:16:11:8:14.15.17,16:15:8:10.12.11,(62.19,-24.8,;62.17,-26.34,;63.49,-27.12,;63.47,-28.66,;64.79,-29.44,;66.13,-28.68,;66.15,-27.14,;67.46,-29.46,;68.8,-28.71,;70.56,-28.75,;71.07,-30.08,;72.26,-30.9,;70.63,-30.99,;70.12,-29.56,;71.3,-28.49,;72.93,-28.45,;73.42,-29.89,;71.67,-27.76,;74.25,-27.68,;75.59,-28.44,;74.24,-26.14,;62.13,-29.42,;62.9,-30.75,;61.36,-30.74,;60.81,-28.63,;60.82,-27.09,;59.47,-29.39,;59.46,-30.92,;58.12,-31.68,;56.79,-30.89,;56.8,-29.35,;58.15,-28.6,;55.44,-31.65,;54.12,-30.87,;55.43,-33.19,;54.11,-32.41,)| Show InChI InChI=1S/C23H30F3N5O4S/c1-13-10-30(36(34,35)31(11-13)18-3-2-17(9-28-18)23(24,25)26)12-19(32)29-20-15-4-14-5-16(20)8-22(6-14,7-15)21(27)33/h2-3,9,13-16,20H,4-8,10-12H2,1H3,(H2,27,33)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50511068
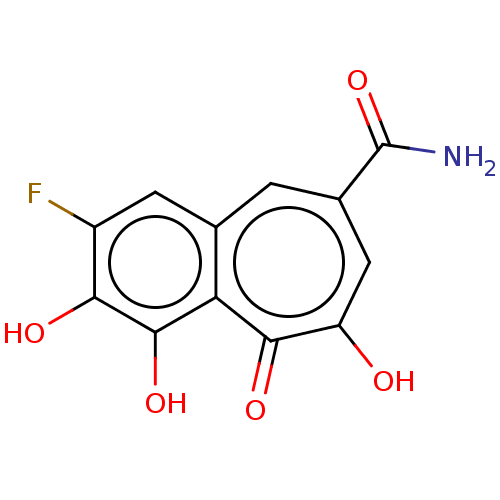
(CHEMBL4459515)Show InChI InChI=1S/C12H8FNO5/c13-6-2-4-1-5(12(14)19)3-7(15)10(17)8(4)11(18)9(6)16/h1-3,16,18H,(H2,14,19)(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 949 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SHP2 expressed in Escherichia coli using 2P-IRS1 peptide as substrate after 1 hr by microplate reader analysis |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126756
BindingDB Entry DOI: 10.7270/Q26113NZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50537924
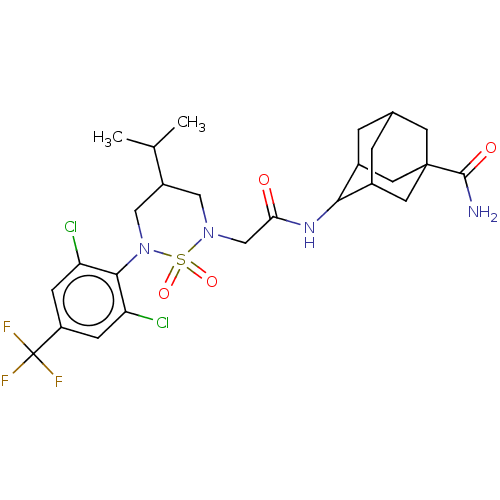
(CHEMBL4647977)Show SMILES CC(C)C1CN(CC(=O)NC2C3CC4CC2CC(C4)(C3)C(N)=O)S(=O)(=O)N(C1)c1c(Cl)cc(cc1Cl)C(F)(F)F |TLB:14:15:19:12.13.18,14:13:10.15.16:19,9:10:12.14.13:16.17.19,9:10:19:12.13.18,THB:18:13:10:16.17.19,18:17:10:12.14.13,(64.15,-35.48,;62.8,-36.23,;61.48,-35.44,;62.78,-37.77,;64.1,-38.55,;64.08,-40.09,;65.41,-40.87,;66.75,-40.11,;66.76,-38.58,;68.07,-40.9,;69.41,-40.14,;71.17,-40.18,;71.69,-41.51,;72.88,-42.33,;71.24,-42.42,;70.73,-40.99,;71.91,-39.93,;73.54,-39.89,;74.03,-41.32,;72.28,-39.19,;74.86,-39.11,;76.2,-39.87,;74.85,-37.57,;62.75,-40.85,;63.51,-42.18,;61.97,-42.18,;61.42,-40.06,;61.44,-38.52,;60.08,-40.82,;60.07,-42.35,;61.4,-43.14,;58.73,-43.11,;57.4,-42.33,;57.42,-40.79,;58.76,-40.03,;58.78,-38.49,;56.06,-43.08,;54.73,-42.3,;56.04,-44.62,;54.72,-43.84,)| Show InChI InChI=1S/C26H33Cl2F3N4O4S/c1-13(2)17-10-34(40(38,39)35(11-17)23-19(27)5-18(6-20(23)28)26(29,30)31)12-21(36)33-22-15-3-14-4-16(22)9-25(7-14,8-15)24(32)37/h5-6,13-17,22H,3-4,7-12H2,1-2H3,(H2,32,37)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 981 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126787
BindingDB Entry DOI: 10.7270/Q2TX3JWS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50511075
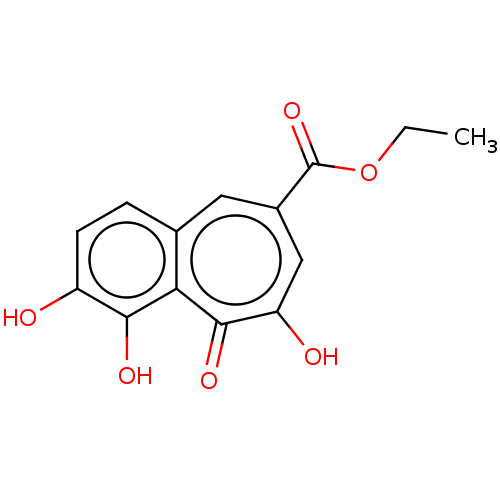
(CHEMBL4447485)Show InChI InChI=1S/C14H12O6/c1-2-20-14(19)8-5-7-3-4-9(15)12(17)11(7)13(18)10(16)6-8/h3-6,15,17H,2H2,1H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 985 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SHP2 expressed in Escherichia coli using 2P-IRS1 peptide as substrate after 1 hr by microplate reader analysis |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126756
BindingDB Entry DOI: 10.7270/Q26113NZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her1 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanmi Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Her2 by fluorescence polarization assay |
J Med Chem 52: 6880-8 (2009)
Article DOI: 10.1021/jm901146p
BindingDB Entry DOI: 10.7270/Q28W3DDJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data