 Found 220 hits with Last Name = 'parrish' and Initial = 'ca'
Found 220 hits with Last Name = 'parrish' and Initial = 'ca' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769
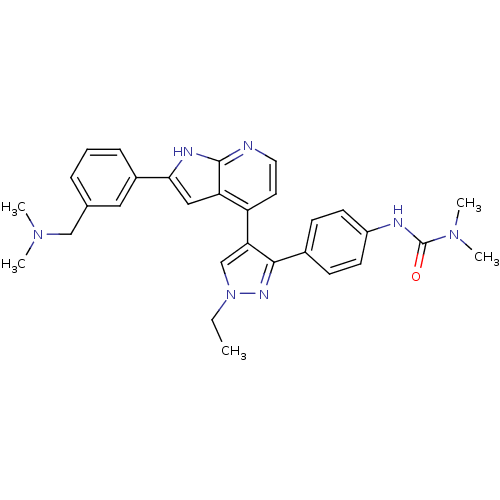
(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM36350
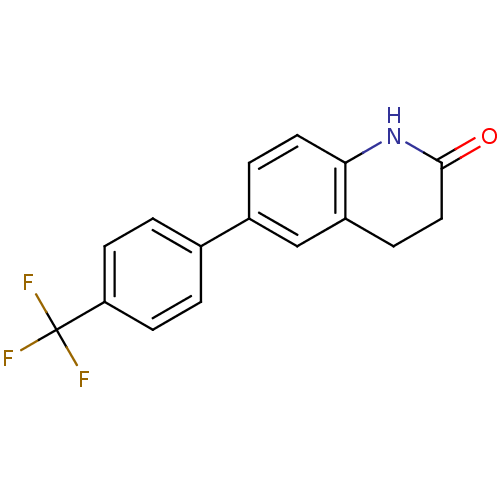
(6-[4-(Trifluoromethyl)phenyl]-3,4-dihydro-2(1H)-qu...)Show InChI InChI=1S/C16H12F3NO/c17-16(18,19)13-5-1-10(2-6-13)11-3-7-14-12(9-11)4-8-15(21)20-14/h1-3,5-7,9H,4,8H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for wild-type cells |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220180
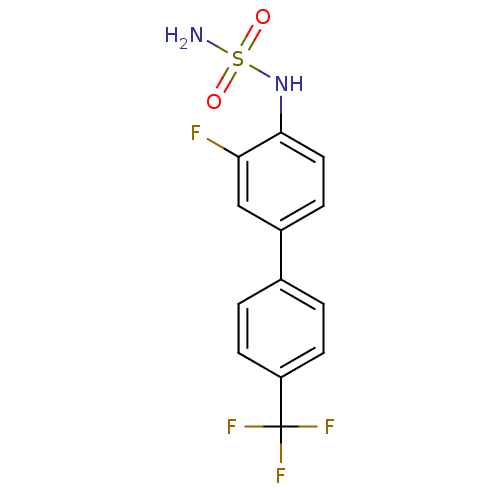
(CHEMBL243669 | N-[3-fluoro-4'-(trifluoromethyl)-4-...)Show InChI InChI=1S/C13H10F4N2O2S/c14-11-7-9(3-6-12(11)19-22(18,20)21)8-1-4-10(5-2-8)13(15,16)17/h1-7,19H,(H2,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220182
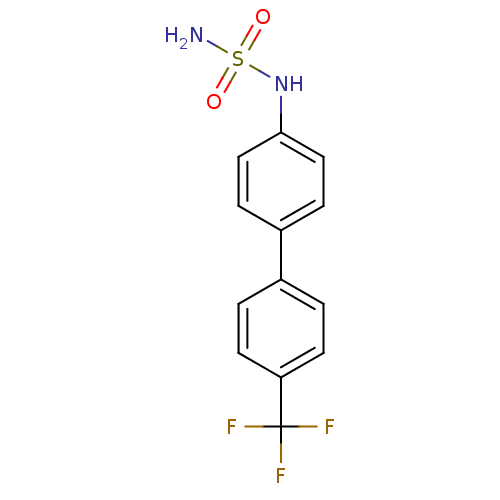
(CHEMBL390629 | N-[4'-(trifluoromethyl)-4-biphenyly...)Show InChI InChI=1S/C13H11F3N2O2S/c14-13(15,16)11-5-1-9(2-6-11)10-3-7-12(8-4-10)18-21(17,19)20/h1-8,18H,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [A356T]
(Homo sapiens (Human)) | BDBM36350

(6-[4-(Trifluoromethyl)phenyl]-3,4-dihydro-2(1H)-qu...)Show InChI InChI=1S/C16H12F3NO/c17-16(18,19)13-5-1-10(2-6-13)11-3-7-14-12(9-11)4-8-15(21)20-14/h1-3,5-7,9H,4,8H2,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
wild-type HCT116 selected at 6WT-6x the IC95 |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kinesin-like protein KIF11 [A356T]
(Homo sapiens (Human)) | BDBM36350

(6-[4-(Trifluoromethyl)phenyl]-3,4-dihydro-2(1H)-qu...)Show InChI InChI=1S/C16H12F3NO/c17-16(18,19)13-5-1-10(2-6-13)11-3-7-14-12(9-11)4-8-15(21)20-14/h1-3,5-7,9H,4,8H2,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
wild-type HCT116 selected at 3 the IC95 |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220169
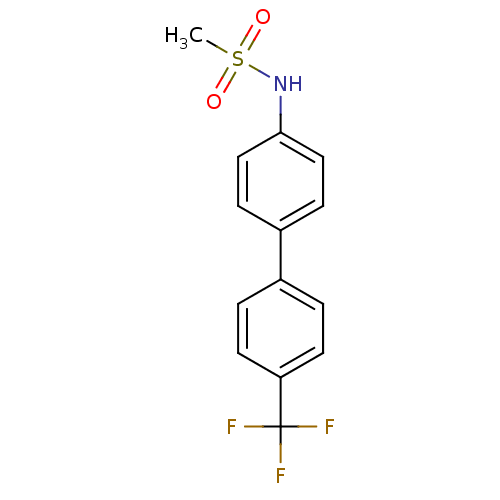
(CHEMBL244933 | N-[4'-(trifluoromethyl)-4-biphenyly...)Show InChI InChI=1S/C14H12F3NO2S/c1-21(19,20)18-13-8-4-11(5-9-13)10-2-6-12(7-3-10)14(15,16)17/h2-9,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora A ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM36351
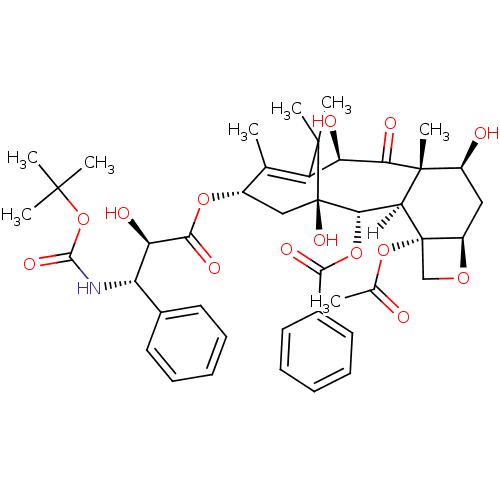
(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for wild-type cells |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [D130V]
(Homo sapiens (Human)) | BDBM36351

(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for ispinesib-resistant DCT116-D130V cell |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [D130V]
(Homo sapiens (Human)) | BDBM36351

(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for ispinesib-resistant DCT116-D130V cell |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM36351

(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for wild-type cells |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM36351

(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for wild-type cells |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [D130V]
(Homo sapiens (Human)) | BDBM36351

(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for ispinesib-resistant DCT116-D130V cell |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [D130V]
(Homo sapiens (Human)) | BDBM36350

(6-[4-(Trifluoromethyl)phenyl]-3,4-dihydro-2(1H)-qu...)Show InChI InChI=1S/C16H12F3NO/c17-16(18,19)13-5-1-10(2-6-13)11-3-7-14-12(9-11)4-8-15(21)20-14/h1-3,5-7,9H,4,8H2,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for ispinesib-resistant DCT116-D130V cell |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220156
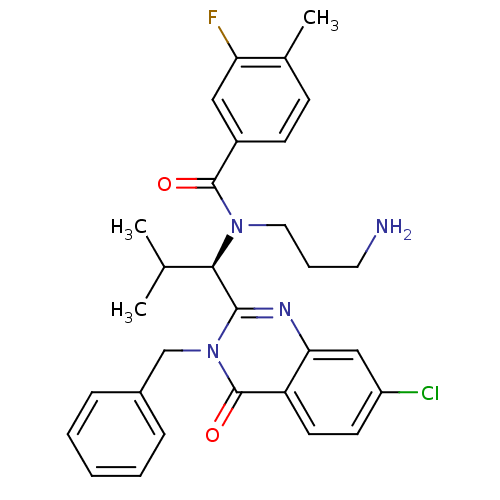
((R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-ox...)Show SMILES CC(C)[C@@H](N(CCCN)C(=O)c1ccc(C)c(F)c1)c1nc2cc(Cl)ccc2c(=O)n1Cc1ccccc1 Show InChI InChI=1S/C30H32ClFN4O2/c1-19(2)27(35(15-7-14-33)29(37)22-11-10-20(3)25(32)16-22)28-34-26-17-23(31)12-13-24(26)30(38)36(28)18-21-8-5-4-6-9-21/h4-6,8-13,16-17,19,27H,7,14-15,18,33H2,1-3H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for wild-type cells |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [D130V]
(Homo sapiens (Human)) | BDBM36351

(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for ispinesib-resistant DCT116-D130V cell |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220156

((R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-ox...)Show SMILES CC(C)[C@@H](N(CCCN)C(=O)c1ccc(C)c(F)c1)c1nc2cc(Cl)ccc2c(=O)n1Cc1ccccc1 Show InChI InChI=1S/C30H32ClFN4O2/c1-19(2)27(35(15-7-14-33)29(37)22-11-10-20(3)25(32)16-22)28-34-26-17-23(31)12-13-24(26)30(38)36(28)18-21-8-5-4-6-9-21/h4-6,8-13,16-17,19,27H,7,14-15,18,33H2,1-3H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for wild-type cells |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [D130V]
(Homo sapiens (Human)) | BDBM36350

(6-[4-(Trifluoromethyl)phenyl]-3,4-dihydro-2(1H)-qu...)Show InChI InChI=1S/C16H12F3NO/c17-16(18,19)13-5-1-10(2-6-13)11-3-7-14-12(9-11)4-8-15(21)20-14/h1-3,5-7,9H,4,8H2,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for ispinesib-resistant DCT116-D130V cell |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316497
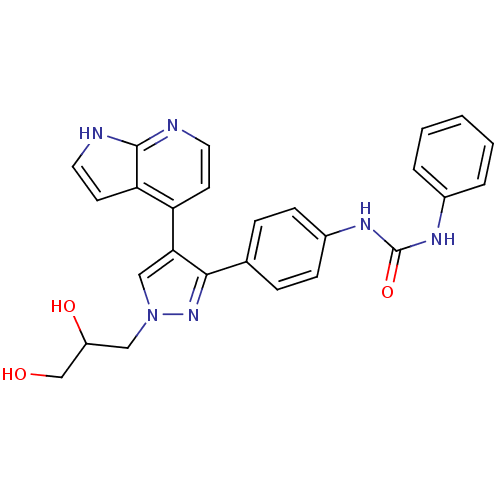
(CHEMBL1097106 | N-{4-[1-(2,3-Dihydroxypropyl)-4-(1...)Show SMILES OCC(O)Cn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O3/c33-16-20(34)14-32-15-23(21-10-12-27-25-22(21)11-13-28-25)24(31-32)17-6-8-19(9-7-17)30-26(35)29-18-4-2-1-3-5-18/h1-13,15,20,33-34H,14,16H2,(H,27,28)(H2,29,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50241089
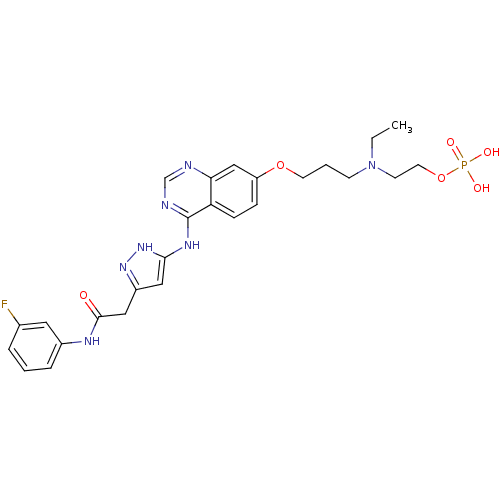
(2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...)Show SMILES CCN(CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1)CCOP(O)(O)=O Show InChI InChI=1S/C26H31FN7O6P/c1-2-34(10-12-40-41(36,37)38)9-4-11-39-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(35)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17H,2,4,9-12,15H2,1H3,(H,30,35)(H2,36,37,38)(H2,28,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220156

((R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-ox...)Show SMILES CC(C)[C@@H](N(CCCN)C(=O)c1ccc(C)c(F)c1)c1nc2cc(Cl)ccc2c(=O)n1Cc1ccccc1 Show InChI InChI=1S/C30H32ClFN4O2/c1-19(2)27(35(15-7-14-33)29(37)22-11-10-20(3)25(32)16-22)28-34-26-17-23(31)12-13-24(26)30(38)36(28)18-21-8-5-4-6-9-21/h4-6,8-13,16-17,19,27H,7,14-15,18,33H2,1-3H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for wild-type cells |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316473
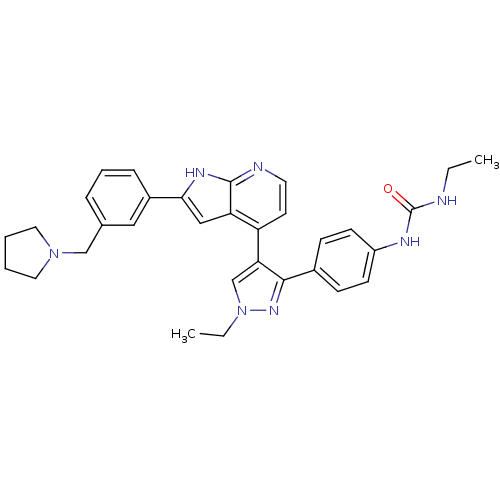
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-12-10-23(11-13-25)30-28(21-39(4-2)37-30)26-14-15-34-31-27(26)19-29(36-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,3-6,16-17,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316475
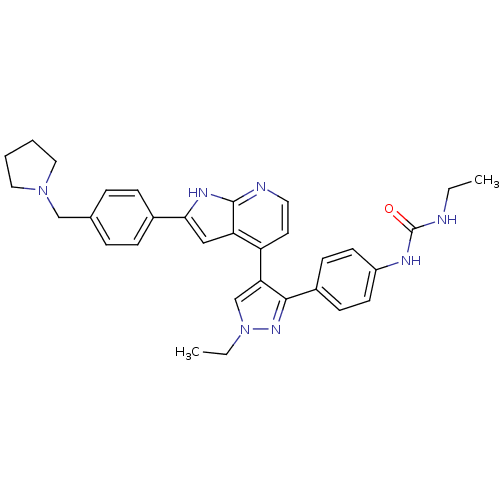
(CHEMBL1097191 | N-Ethyl-N'-[4-(1-ethyl-4-{2-[4-(1-...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C32H35N7O/c1-3-33-32(40)35-25-13-11-24(12-14-25)30-28(21-39(4-2)37-30)26-15-16-34-31-27(26)19-29(36-31)23-9-7-22(8-10-23)20-38-17-5-6-18-38/h7-16,19,21H,3-6,17-18,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316471
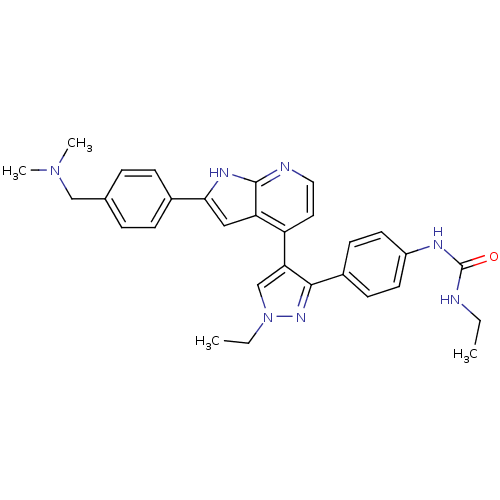
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-13-11-22(12-14-23)28-26(19-37(6-2)35-28)24-15-16-32-29-25(24)17-27(34-29)21-9-7-20(8-10-21)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316498
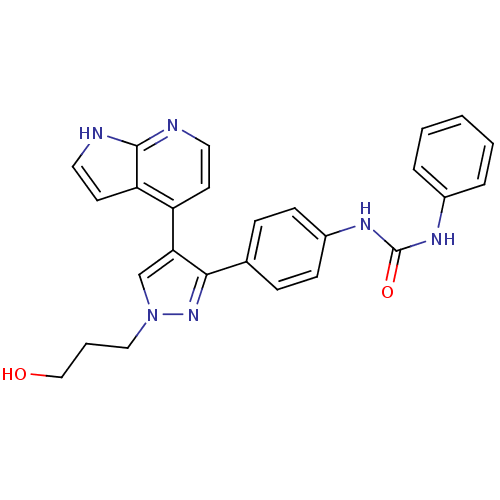
(CHEMBL1097454 | N-{4-[1-(3-Hydroxypropyl)-4-(1H-py...)Show SMILES OCCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C26H24N6O2/c33-16-4-15-32-17-23(21-11-13-27-25-22(21)12-14-28-25)24(31-32)18-7-9-20(10-8-18)30-26(34)29-19-5-2-1-3-6-19/h1-3,5-14,17,33H,4,15-16H2,(H,27,28)(H2,29,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316492
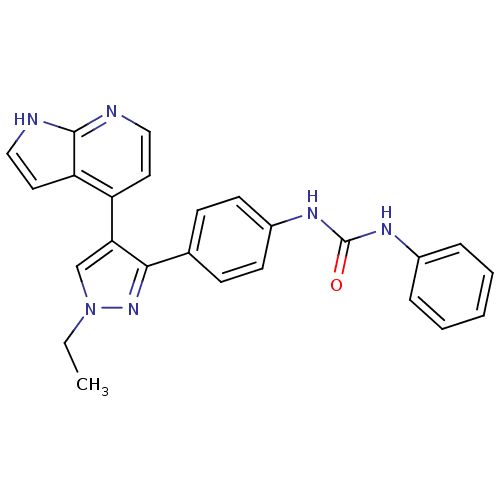
(1-(4-(1-ethyl-4-(1H-pyrrolo[2,3-b]pyridin-4-yl)-1H...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O/c1-2-31-16-22(20-12-14-26-24-21(20)13-15-27-24)23(30-31)17-8-10-19(11-9-17)29-25(32)28-18-6-4-3-5-7-18/h3-16H,2H2,1H3,(H,26,27)(H2,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316474
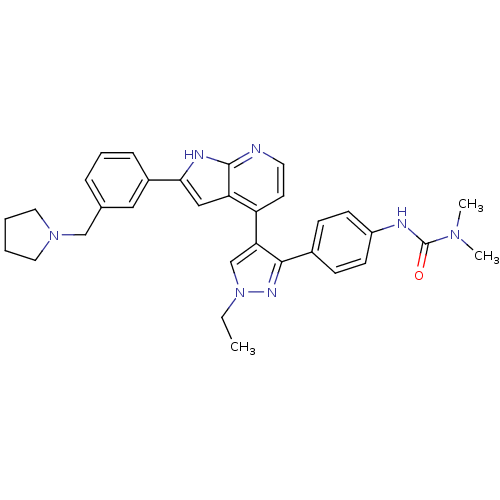
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCCC2)c1 Show InChI InChI=1S/C32H35N7O/c1-4-39-21-28(30(36-39)23-10-12-25(13-11-23)34-32(40)37(2)3)26-14-15-33-31-27(26)19-29(35-31)24-9-7-8-22(18-24)20-38-16-5-6-17-38/h7-15,18-19,21H,4-6,16-17,20H2,1-3H3,(H,33,35)(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316470
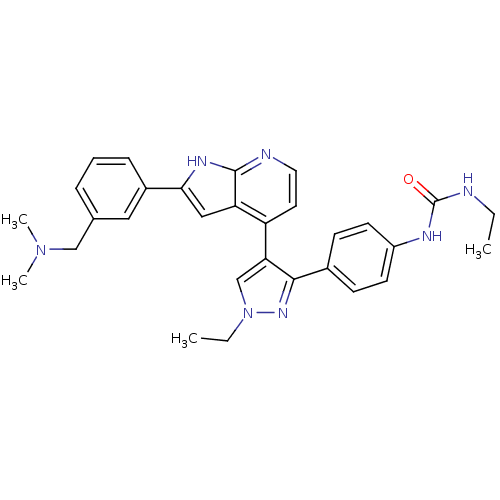
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-5-31-30(38)33-23-12-10-21(11-13-23)28-26(19-37(6-2)35-28)24-14-15-32-29-25(24)17-27(34-29)22-9-7-8-20(16-22)18-36(3)4/h7-17,19H,5-6,18H2,1-4H3,(H,32,34)(H2,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM128366
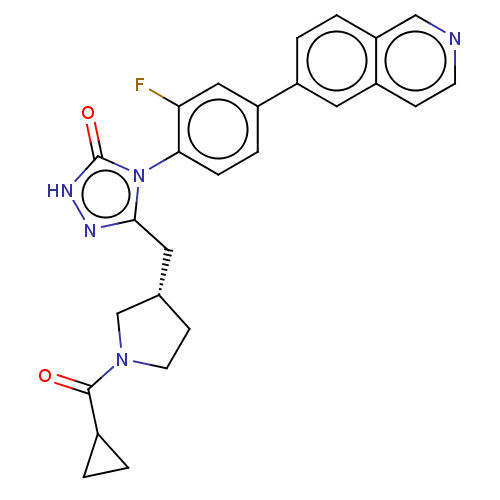
(US8802864, 132)Show SMILES Fc1cc(ccc1-n1c(C[C@@H]2CCN(C2)C(=O)C2CC2)n[nH]c1=O)-c1ccc2cnccc2c1 |r,wU:10.10,(-3.05,-4.92,;-2.28,-3.59,;-.74,-3.59,;.03,-2.25,;-.74,-.92,;-2.28,-.92,;-3.05,-2.25,;-4.59,-2.25,;-5.5,-1.01,;-5.1,.48,;-3.61,.88,;-2.36,-.03,;-1.12,.88,;-1.59,2.34,;-3.13,2.34,;-.5,3.43,;-.9,4.92,;.98,3.03,;2.07,1.95,;2.47,3.43,;-6.96,-1.48,;-6.96,-3.02,;-5.5,-3.5,;-4.73,-4.83,;1.57,-2.25,;2.34,-3.59,;3.88,-3.59,;4.65,-2.25,;6.19,-2.25,;6.96,-.92,;6.19,.41,;4.65,.41,;3.88,-.92,;2.34,-.92,)| Show InChI InChI=1S/C26H24FN5O2/c27-22-13-19(18-3-4-21-14-28-9-7-20(21)12-18)5-6-23(22)32-24(29-30-26(32)34)11-16-8-10-31(15-16)25(33)17-1-2-17/h3-7,9,12-14,16-17H,1-2,8,10-11,15H2,(H,30,34)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC
US Patent
| Assay Description
Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... |
US Patent US8802864 (2014)
BindingDB Entry DOI: 10.7270/Q2N58K26 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316496
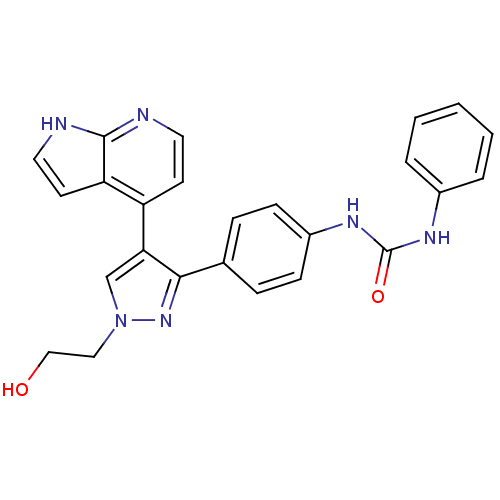
(CHEMBL1099010 | N-{4-[1-(2-Hydroxyethyl)-4-(1H-pyr...)Show SMILES OCCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H22N6O2/c32-15-14-31-16-22(20-10-12-26-24-21(20)11-13-27-24)23(30-31)17-6-8-19(9-7-17)29-25(33)28-18-4-2-1-3-5-18/h1-13,16,32H,14-15H2,(H,26,27)(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11 [D130V]
(Homo sapiens (Human)) | BDBM36350

(6-[4-(Trifluoromethyl)phenyl]-3,4-dihydro-2(1H)-qu...)Show InChI InChI=1S/C16H12F3NO/c17-16(18,19)13-5-1-10(2-6-13)11-3-7-14-12(9-11)4-8-15(21)20-14/h1-3,5-7,9H,4,8H2,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GlaxoSmithKline
| Assay Description
Cell proliferation assay for ispinesib-resistant DCT116-D130V cell |
Nat Chem Biol 3: 722-6 (2007)
Article DOI: 10.1038/nchembio.2007.34
BindingDB Entry DOI: 10.7270/Q2PK0DHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316472
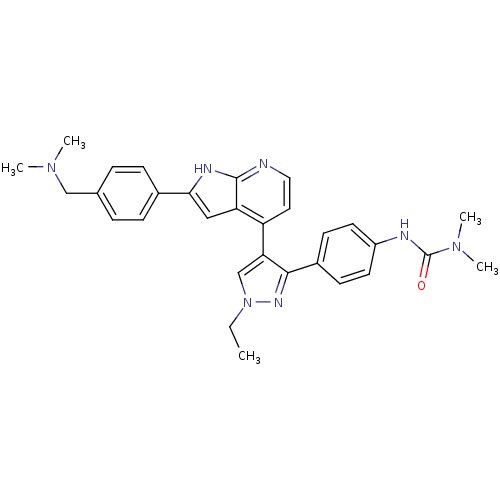
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)22-11-13-23(14-12-22)32-30(38)36(4)5)24-15-16-31-29-25(24)17-27(33-29)21-9-7-20(8-10-21)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093
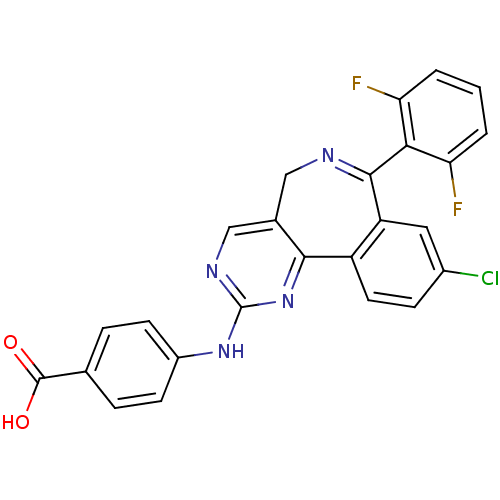
(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316476
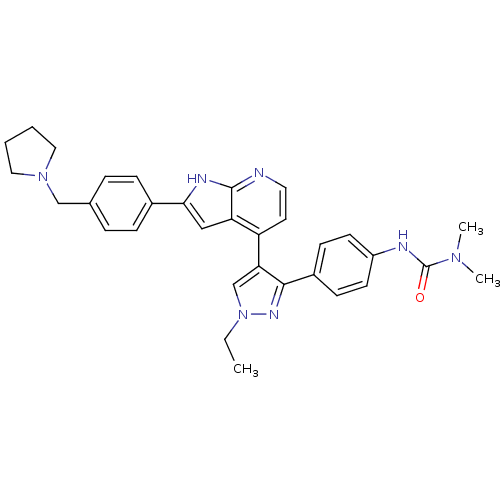
(CHEMBL1097521 | N'-[4-(1-Ethyl-4-{2-[4-(1-pyrrolid...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCCC2)cc1 Show InChI InChI=1S/C32H35N7O/c1-4-39-21-28(30(36-39)24-11-13-25(14-12-24)34-32(40)37(2)3)26-15-16-33-31-27(26)19-29(35-31)23-9-7-22(8-10-23)20-38-17-5-6-18-38/h7-16,19,21H,4-6,17-18,20H2,1-3H3,(H,33,35)(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316478
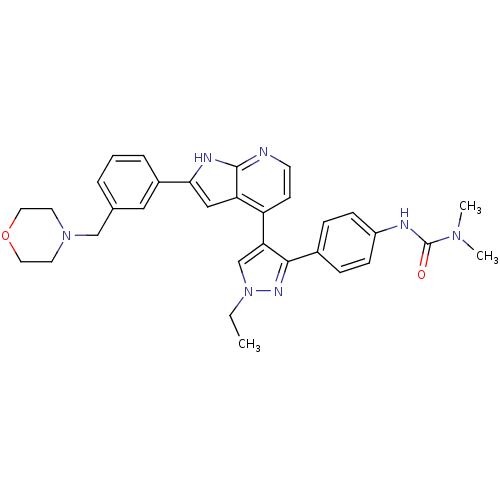
(4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCOCC2)c1 Show InChI InChI=1S/C32H35N7O2/c1-4-39-21-28(30(36-39)23-8-10-25(11-9-23)34-32(40)37(2)3)26-12-13-33-31-27(26)19-29(35-31)24-7-5-6-22(18-24)20-38-14-16-41-17-15-38/h5-13,18-19,21H,4,14-17,20H2,1-3H3,(H,33,35)(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316479
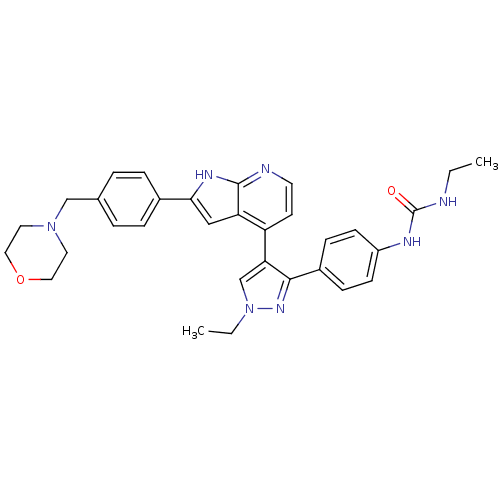
((4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-py...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C32H35N7O2/c1-3-33-32(40)35-25-11-9-24(10-12-25)30-28(21-39(4-2)37-30)26-13-14-34-31-27(26)19-29(36-31)23-7-5-22(6-8-23)20-38-15-17-41-18-16-38/h5-14,19,21H,3-4,15-18,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM128362
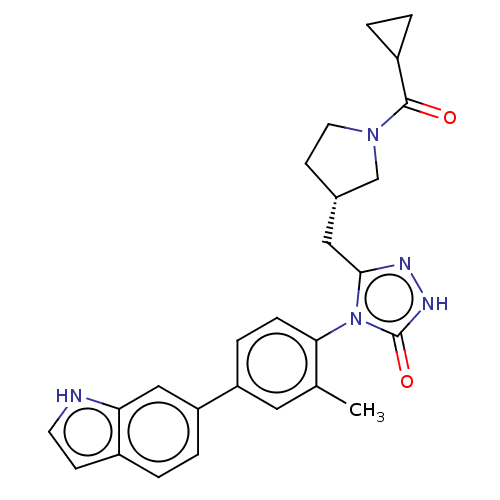
(US8802864, 42)Show SMILES Cc1cc(ccc1-n1c(C[C@@H]2CCN(C2)C(=O)C2CC2)n[nH]c1=O)-c1ccc2cc[nH]c2c1 |r,wU:10.10,(-2.73,-4.92,;-1.96,-3.59,;-.42,-3.59,;.35,-2.25,;-.42,-.92,;-1.96,-.92,;-2.73,-2.25,;-4.27,-2.25,;-5.17,-1.01,;-4.78,.48,;-3.29,.88,;-2.04,-.03,;-.8,.88,;-1.27,2.34,;-2.81,2.34,;-.18,3.43,;-.58,4.92,;1.3,3.03,;2.39,1.95,;2.79,3.43,;-6.64,-1.48,;-6.64,-3.02,;-5.17,-3.5,;-4.4,-4.83,;1.89,-2.25,;2.66,-3.59,;4.2,-3.59,;4.97,-2.25,;6.48,-1.93,;6.64,-.4,;5.23,.23,;4.2,-.92,;2.66,-.92,)| Show InChI InChI=1S/C26H27N5O2/c1-16-12-20(21-5-2-18-8-10-27-22(18)14-21)6-7-23(16)31-24(28-29-26(31)33)13-17-9-11-30(15-17)25(32)19-3-4-19/h2,5-8,10,12,14,17,19,27H,3-4,9,11,13,15H2,1H3,(H,29,33)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC
US Patent
| Assay Description
Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... |
US Patent US8802864 (2014)
BindingDB Entry DOI: 10.7270/Q2N58K26 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220180

(CHEMBL243669 | N-[3-fluoro-4'-(trifluoromethyl)-4-...)Show InChI InChI=1S/C13H10F4N2O2S/c14-11-7-9(3-6-12(11)19-22(18,20)21)8-1-4-10(5-2-8)13(15,16)17/h1-7,19H,(H2,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316477
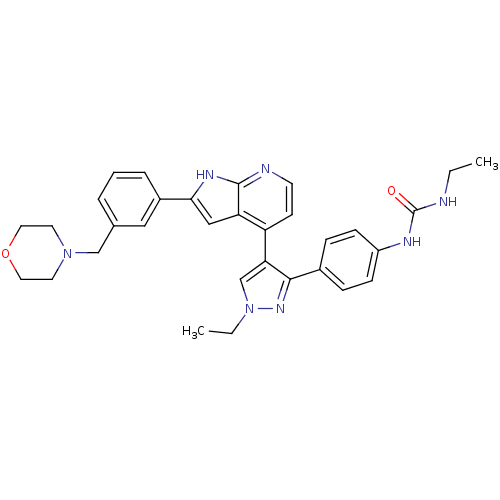
(4-[3-(4-N-Ethylcarbamylaminophenyl)-1-ethyl-1H-pyr...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nn(CC)cc1-c1ccnc2[nH]c(cc12)-c1cccc(CN2CCOCC2)c1 Show InChI InChI=1S/C32H35N7O2/c1-3-33-32(40)35-25-10-8-23(9-11-25)30-28(21-39(4-2)37-30)26-12-13-34-31-27(26)19-29(36-31)24-7-5-6-22(18-24)20-38-14-16-41-17-15-38/h5-13,18-19,21H,3-4,14-17,20H2,1-2H3,(H,34,36)(H2,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316481
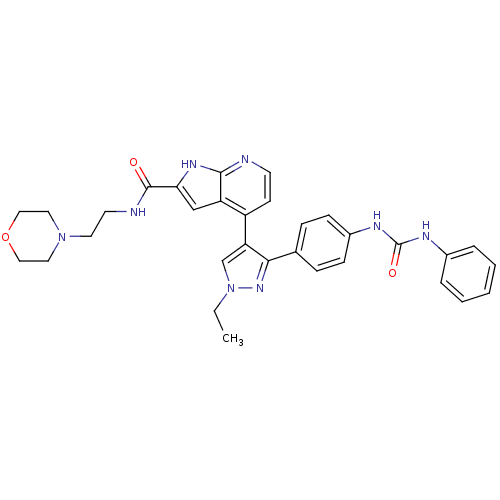
(4-[1-Ethyl-3-(4-{[(phenylamino)carbonyl]amino}phen...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]c(cc12)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C32H34N8O3/c1-2-40-21-27(29(38-40)22-8-10-24(11-9-22)36-32(42)35-23-6-4-3-5-7-23)25-12-13-33-30-26(25)20-28(37-30)31(41)34-14-15-39-16-18-43-19-17-39/h3-13,20-21H,2,14-19H2,1H3,(H,33,37)(H,34,41)(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50316483
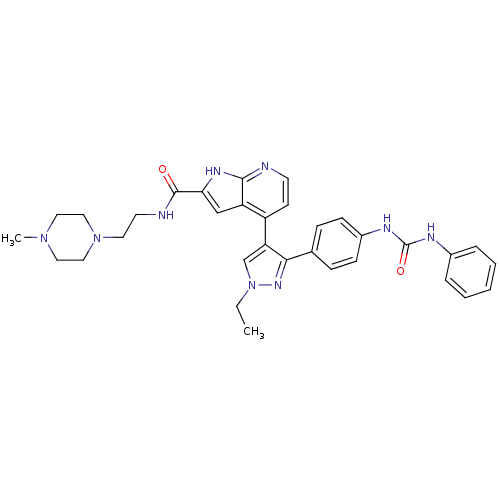
(4-[1-Ethyl-3-(4-{[(phenylamino)carbonyl]amino}phen...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)Nc2ccccc2)cc1)-c1ccnc2[nH]c(cc12)C(=O)NCCN1CCN(C)CC1 Show InChI InChI=1S/C33H37N9O2/c1-3-42-22-28(30(39-42)23-9-11-25(12-10-23)37-33(44)36-24-7-5-4-6-8-24)26-13-14-34-31-27(26)21-29(38-31)32(43)35-15-16-41-19-17-40(2)18-20-41/h4-14,21-22H,3,15-20H2,1-2H3,(H,34,38)(H,35,43)(H2,36,37,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM128359
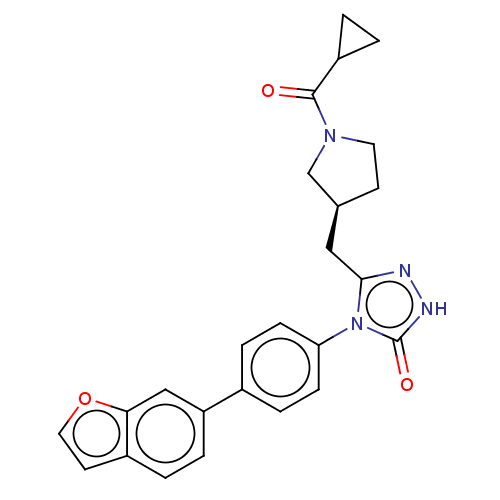
(US8802864, 14)Show SMILES O=C(C1CC1)N1CC[C@@H](Cc2n[nH]c(=O)n2-c2ccc(cc2)-c2ccc3ccoc3c2)C1 |r| Show InChI InChI=1S/C25H24N4O3/c30-24(19-2-3-19)28-11-9-16(15-28)13-23-26-27-25(31)29(23)21-7-5-17(6-8-21)20-4-1-18-10-12-32-22(18)14-20/h1,4-8,10,12,14,16,19H,2-3,9,11,13,15H2,(H,27,31)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC
US Patent
| Assay Description
Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... |
US Patent US8802864 (2014)
BindingDB Entry DOI: 10.7270/Q2N58K26 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data