 Found 702 hits with Last Name = 'pearson' and Initial = 's'
Found 702 hits with Last Name = 'pearson' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50304627

((2S,3S,4R,5R,6S)-6-methylpiperidine-2,3,4,5-tetrao...)Show SMILES C[C@@H]1N[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H13NO4/c1-2-3(8)4(9)5(10)6(11)7-2/h2-11H,1H3/t2-,3+,4+,5-,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay |
Bioorg Med Chem 17: 8020-6 (2009)
Article DOI: 10.1016/j.bmc.2009.10.010
BindingDB Entry DOI: 10.7270/Q2FB5307 |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50065258
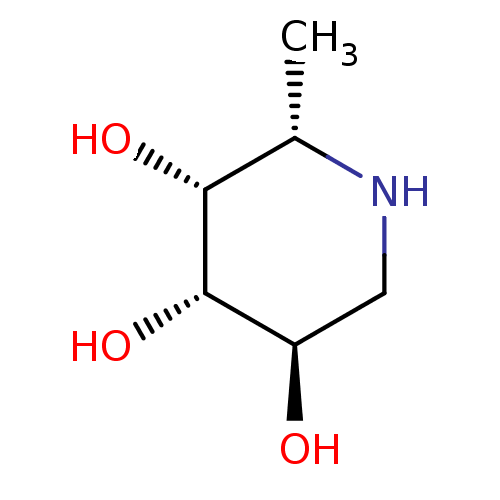
((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...)Show InChI InChI=1S/C6H13NO3/c1-3-5(9)6(10)4(8)2-7-3/h3-10H,2H2,1H3/t3-,4+,5+,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay |
Bioorg Med Chem 17: 8020-6 (2009)
Article DOI: 10.1016/j.bmc.2009.10.010
BindingDB Entry DOI: 10.7270/Q2FB5307 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50304628

((2S,3R,4S)-2-methyl-3,4-dihydro-2H-pyrrole-3,4-dio...)Show InChI InChI=1S/C5H9NO2/c1-3-5(8)4(7)2-6-3/h3-4,6-7H,2H2,1H3/t3-,4-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay |
Bioorg Med Chem 17: 8020-6 (2009)
Article DOI: 10.1016/j.bmc.2009.10.010
BindingDB Entry DOI: 10.7270/Q2FB5307 |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50304626
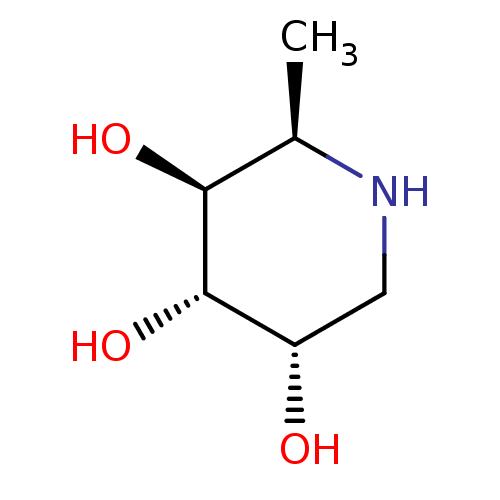
((2R,3S,4S,5S)-2-methylpiperidine-3,4,5-triol | CHE...)Show InChI InChI=1S/C6H13NO3/c1-3-5(9)6(10)4(8)2-7-3/h3-10H,2H2,1H3/t3-,4+,5+,6+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay |
Bioorg Med Chem 17: 8020-6 (2009)
Article DOI: 10.1016/j.bmc.2009.10.010
BindingDB Entry DOI: 10.7270/Q2FB5307 |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50304629
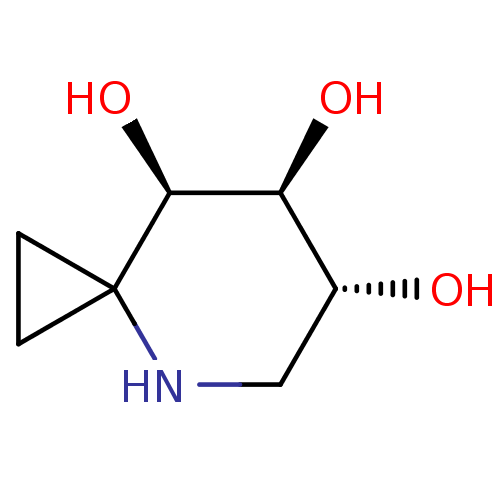
((6R,7S,8R)-4-azaspiro[2.5]octane-6,7,8-triol | CHE...)Show InChI InChI=1S/C7H13NO3/c9-4-3-8-7(1-2-7)6(11)5(4)10/h4-6,8-11H,1-3H2/t4-,5+,6+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay |
Bioorg Med Chem 17: 8020-6 (2009)
Article DOI: 10.1016/j.bmc.2009.10.010
BindingDB Entry DOI: 10.7270/Q2FB5307 |
More data for this
Ligand-Target Pair | |
Beta-glucosidase
(Prunus avium) | BDBM50185227
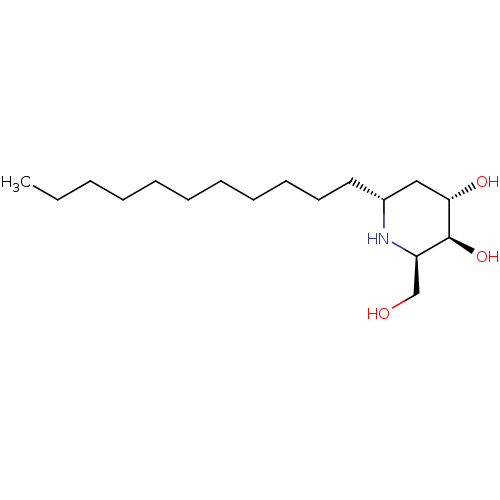
((2R,3S,4S,6R)-2-(hydroxymethyl)-6-undecylpiperidin...)Show SMILES CCCCCCCCCCC[C@@H]1C[C@H](O)[C@@H](O)[C@@H](CO)N1 Show InChI InChI=1S/C17H35NO3/c1-2-3-4-5-6-7-8-9-10-11-14-12-16(20)17(21)15(13-19)18-14/h14-21H,2-13H2,1H3/t14-,15-,16+,17+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of almond beta glucosidase |
Bioorg Med Chem Lett 16: 3262-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.035
BindingDB Entry DOI: 10.7270/Q23F4P7Z |
More data for this
Ligand-Target Pair | |
Beta-glucosidase
(Prunus avium) | BDBM50185229
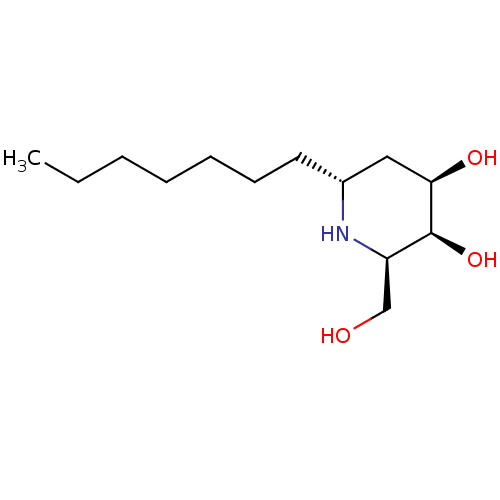
((2R,3S,4R,6R)-6-heptyl-2-(hydroxymethyl)piperidine...)Show SMILES CCCCCCC[C@@H]1C[C@@H](O)[C@@H](O)[C@@H](CO)N1 Show InChI InChI=1S/C13H27NO3/c1-2-3-4-5-6-7-10-8-12(16)13(17)11(9-15)14-10/h10-17H,2-9H2,1H3/t10-,11-,12-,13+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of almond beta glucosidase |
Bioorg Med Chem Lett 16: 3262-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.035
BindingDB Entry DOI: 10.7270/Q23F4P7Z |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase
(Coffea arabica) | BDBM50185229

((2R,3S,4R,6R)-6-heptyl-2-(hydroxymethyl)piperidine...)Show SMILES CCCCCCC[C@@H]1C[C@@H](O)[C@@H](O)[C@@H](CO)N1 Show InChI InChI=1S/C13H27NO3/c1-2-3-4-5-6-7-10-8-12(16)13(17)11(9-15)14-10/h10-17H,2-9H2,1H3/t10-,11-,12-,13+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of green coffee alpha galactosidase |
Bioorg Med Chem Lett 16: 3262-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.035
BindingDB Entry DOI: 10.7270/Q23F4P7Z |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase
(Coffea arabica) | BDBM50185230
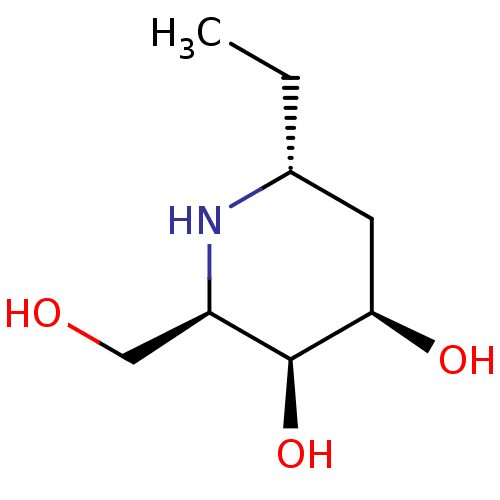
((2R,3S,4R,6R)-6-ethyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C8H17NO3/c1-2-5-3-7(11)8(12)6(4-10)9-5/h5-12H,2-4H2,1H3/t5-,6-,7-,8+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of green coffee alpha galactosidase |
Bioorg Med Chem Lett 16: 3262-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.035
BindingDB Entry DOI: 10.7270/Q23F4P7Z |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743
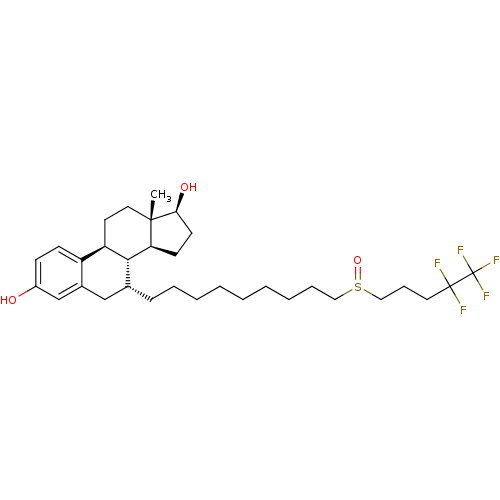
((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
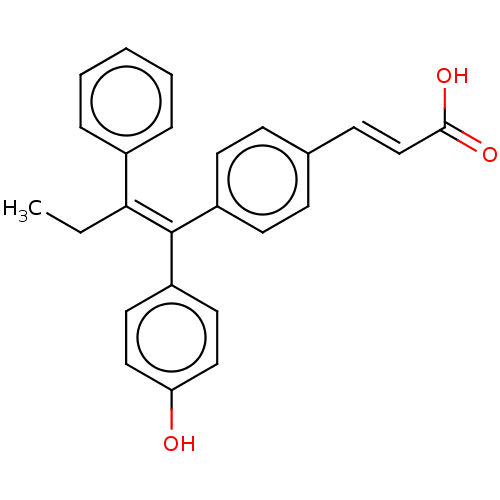
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459004

(CHEMBL4217549)Show SMILES C[C@H](Nc1c(nnc2ccc(cc12)-c1ccc(OCCCN(C)C)nc1)C(N)=O)C1CCOCC1 |r| Show InChI InChI=1S/C26H34N6O3/c1-17(18-9-13-34-14-10-18)29-24-21-15-19(5-7-22(21)30-31-25(24)26(27)33)20-6-8-23(28-16-20)35-12-4-11-32(2)3/h5-8,15-18H,4,9-14H2,1-3H3,(H2,27,33)(H,29,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate pretreated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF assa... |
ACS Med Chem Lett 9: 809-814 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00200
BindingDB Entry DOI: 10.7270/Q27D2XRQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
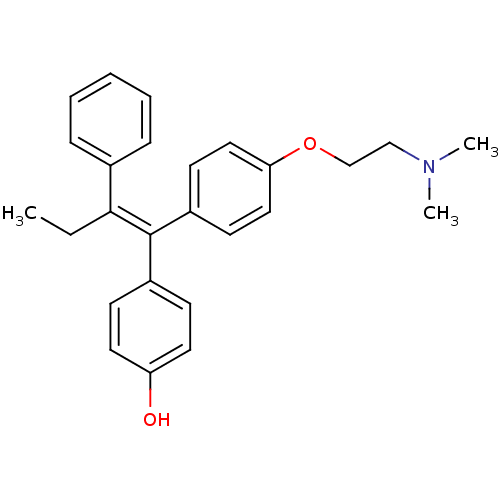
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055
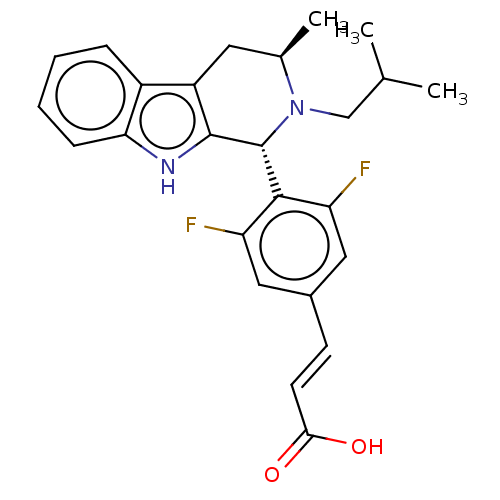
(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459016
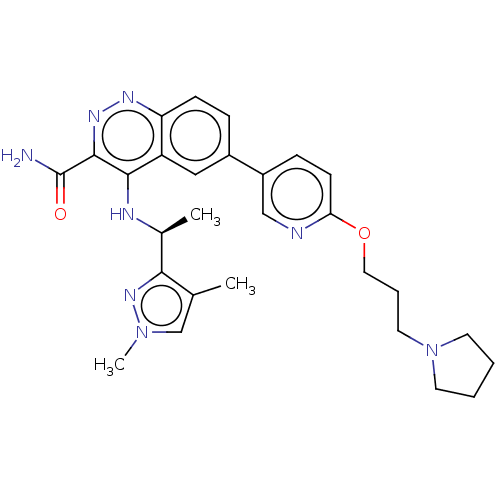
(CHEMBL4210045)Show SMILES C[C@H](Nc1c(nnc2ccc(cc12)-c1ccc(OCCCN2CCCC2)nc1)C(N)=O)c1nn(C)cc1C |r| Show InChI InChI=1S/C28H34N8O2/c1-18-17-35(3)34-25(18)19(2)31-26-22-15-20(7-9-23(22)32-33-27(26)28(29)37)21-8-10-24(30-16-21)38-14-6-13-36-11-4-5-12-36/h7-10,15-17,19H,4-6,11-14H2,1-3H3,(H2,29,37)(H,31,32)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 residue in human HT-29 cells measured after 1 hr by Hoechst staining based fluorescence assay |
ACS Med Chem Lett 9: 809-814 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00200
BindingDB Entry DOI: 10.7270/Q27D2XRQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612201
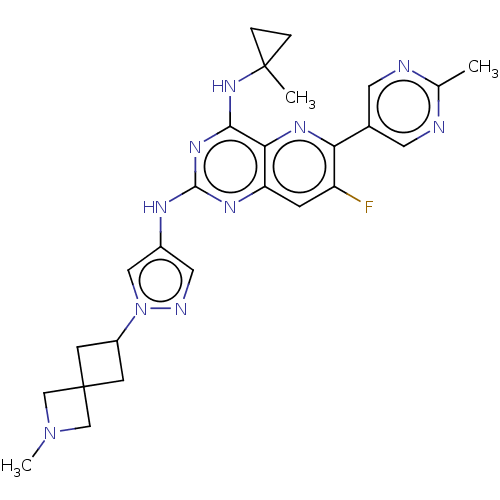
(CHEMBL5270030) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612188

(CHEMBL5283628) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593696
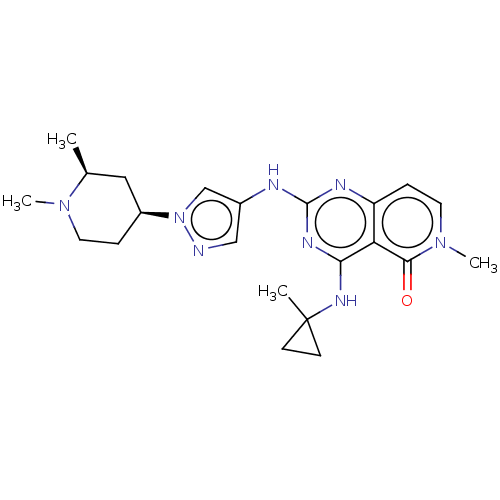
(CHEMBL5200601)Show SMILES C[C@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593694
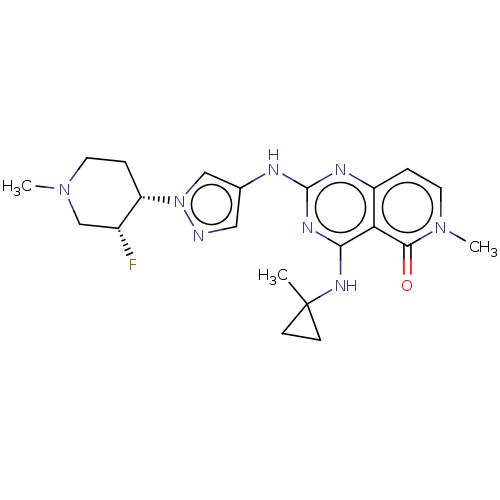
(CHEMBL5193253)Show SMILES CN1CC[C@@H]([C@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459003

(CHEMBL4218854)Show SMILES CNC(=O)c1nnc2ccc(cc2c1N[C@@H](C)C1CCOCC1)-c1ccc(OCCCN(C)C)nc1 |r| Show InChI InChI=1S/C27H36N6O3/c1-18(19-10-14-35-15-11-19)30-25-22-16-20(6-8-23(22)31-32-26(25)27(34)28-2)21-7-9-24(29-17-21)36-13-5-12-33(3)4/h6-9,16-19H,5,10-15H2,1-4H3,(H,28,34)(H,30,31)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate pretreated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF assa... |
ACS Med Chem Lett 9: 809-814 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00200
BindingDB Entry DOI: 10.7270/Q27D2XRQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM20608

(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593693

(CHEMBL5201376)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459014

(CHEMBL4212961)Show SMILES C[C@H](Nc1c(nnc2ccc(cc12)-c1ccc(OCCCN(C)C)nc1)C(N)=O)c1nn(C)cc1C |r| Show InChI InChI=1S/C26H32N8O2/c1-16-15-34(5)32-23(16)17(2)29-24-20-13-18(7-9-21(20)30-31-25(24)26(27)35)19-8-10-22(28-14-19)36-12-6-11-33(3)4/h7-10,13-15,17H,6,11-12H2,1-5H3,(H2,27,35)(H,29,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 residue in human HT-29 cells measured after 1 hr by Hoechst staining based fluorescence assay |
ACS Med Chem Lett 9: 809-814 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00200
BindingDB Entry DOI: 10.7270/Q27D2XRQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593710
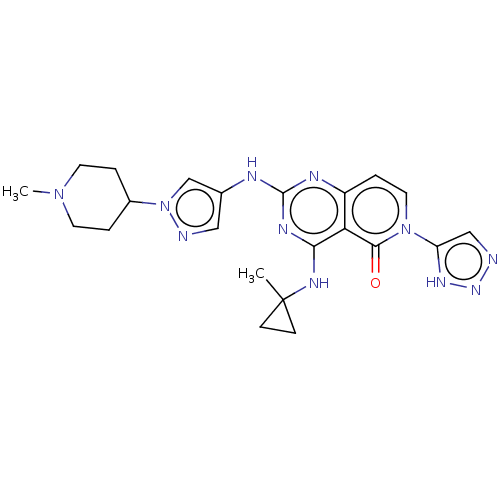
(CHEMBL5193053)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(-c4cnn[nH]4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593695
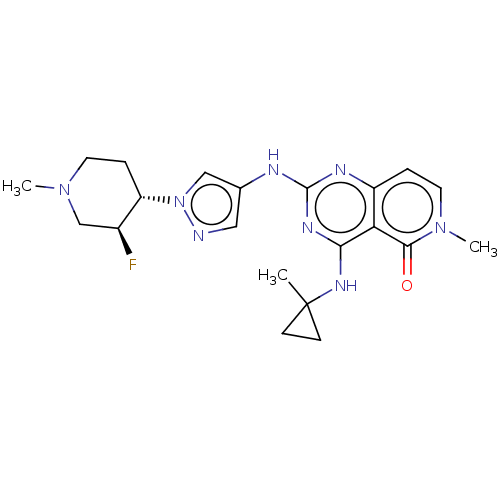
(CHEMBL5209502)Show SMILES CN1CC[C@@H]([C@@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593692

(CHEMBL5200432)Show SMILES CN(C)CC(=O)N1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593689
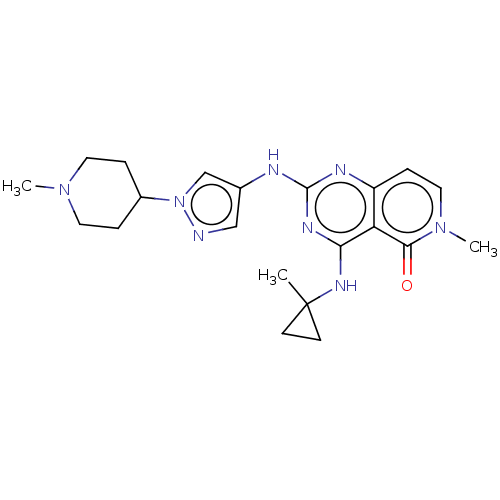
(CHEMBL5174529)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593700

(CHEMBL5193024)Show SMILES CN1CC2(CC(C2)n2cc(Nc3nc(NC4(C)CC4)c4c(ccn(C)c4=O)n3)cn2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593697

(CHEMBL5196755)Show SMILES C[C@@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612187
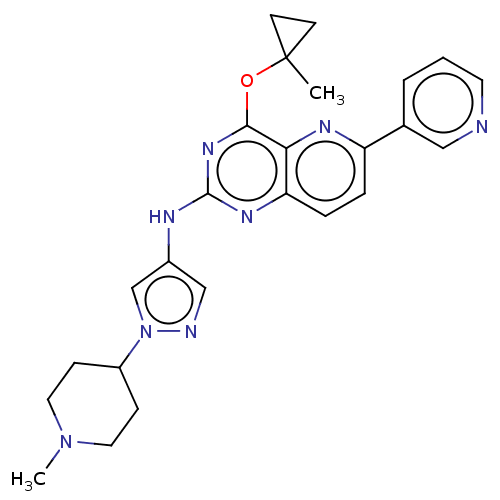
(CHEMBL5288696) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612189

(CHEMBL5281595) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612193

(CHEMBL5290090) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190001
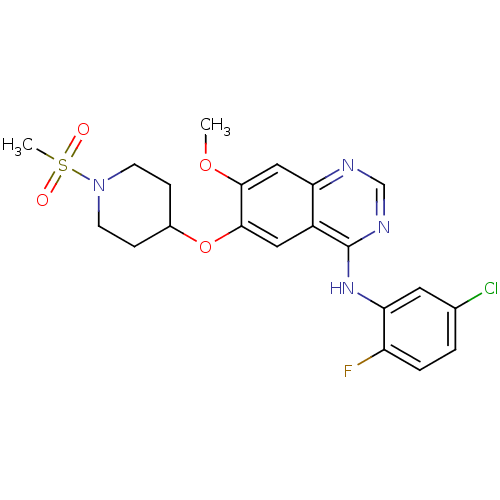
(CHEMBL213007 | N-(5-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cc(Cl)ccc3F)c2cc1OC1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C21H22ClFN4O4S/c1-30-19-11-17-15(10-20(19)31-14-5-7-27(8-6-14)32(2,28)29)21(25-12-24-17)26-18-9-13(22)3-4-16(18)23/h3-4,9-12,14H,5-8H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459002

(CHEMBL4208569)Show SMILES C[C@H](Nc1c(nnc2ccc(cc12)-c1ccc(OCCCN2CCCC2)nc1)C(N)=O)c1ccn(C)n1 |r| Show InChI InChI=1S/C27H32N8O2/c1-18(22-10-14-34(2)33-22)30-25-21-16-19(6-8-23(21)31-32-26(25)27(28)36)20-7-9-24(29-17-20)37-15-5-13-35-11-3-4-12-35/h6-10,14,16-18H,3-5,11-13,15H2,1-2H3,(H2,28,36)(H,30,31)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 residue in human HT-29 cells measured after 1 hr by Hoechst staining based fluorescence assay |
ACS Med Chem Lett 9: 809-814 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00200
BindingDB Entry DOI: 10.7270/Q27D2XRQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50072961
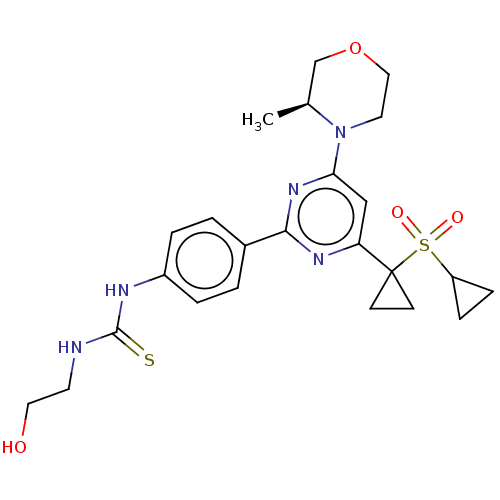
(CHEMBL3410672)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C24H31N5O4S2/c1-16-15-33-13-11-29(16)21-14-20(24(8-9-24)35(31,32)19-6-7-19)27-22(28-21)17-2-4-18(5-3-17)26-23(34)25-10-12-30/h2-5,14,16,19,30H,6-13,15H2,1H3,(H2,25,26,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459015

(CHEMBL4213503)Show SMILES C[C@H](Nc1c(nnc2ccc(cc12)-c1ccc(OCCCN2CCCC2)nc1)C(N)=O)C1CCOCC1 |r| Show InChI InChI=1S/C28H36N6O3/c1-19(20-9-15-36-16-10-20)31-26-23-17-21(5-7-24(23)32-33-27(26)28(29)35)22-6-8-25(30-18-22)37-14-4-13-34-11-2-3-12-34/h5-8,17-20H,2-4,9-16H2,1H3,(H2,29,35)(H,31,32)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 residue in human HT-29 cells measured after 1 hr by Hoechst staining based fluorescence assay |
ACS Med Chem Lett 9: 809-814 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00200
BindingDB Entry DOI: 10.7270/Q27D2XRQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612191

(CHEMBL5273100) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50459004

(CHEMBL4217549)Show SMILES C[C@H](Nc1c(nnc2ccc(cc12)-c1ccc(OCCCN(C)C)nc1)C(N)=O)C1CCOCC1 |r| Show InChI InChI=1S/C26H34N6O3/c1-17(18-9-13-34-14-10-18)29-24-21-15-19(5-7-22(21)30-31-25(24)26(27)33)20-6-8-23(28-16-20)35-12-4-11-32(2)3/h5-8,15-18H,4,9-14H2,1-3H3,(H2,27,33)(H,29,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 residue in human HT-29 cells measured after 1 hr by Hoechst staining based fluorescence assay |
ACS Med Chem Lett 9: 809-814 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00200
BindingDB Entry DOI: 10.7270/Q27D2XRQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50612188

(CHEMBL5283628) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data