

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161472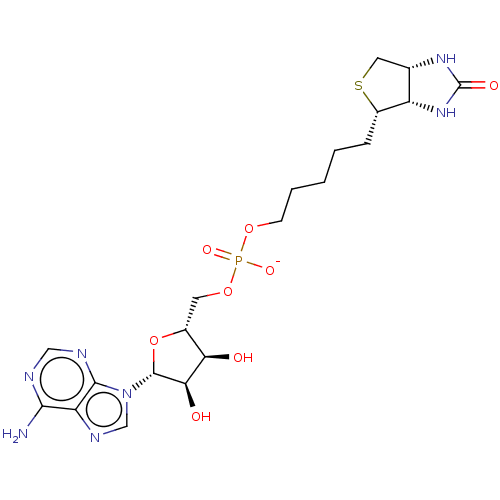 (US9108978, 2.02) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 30 | -44.7 | 203 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161468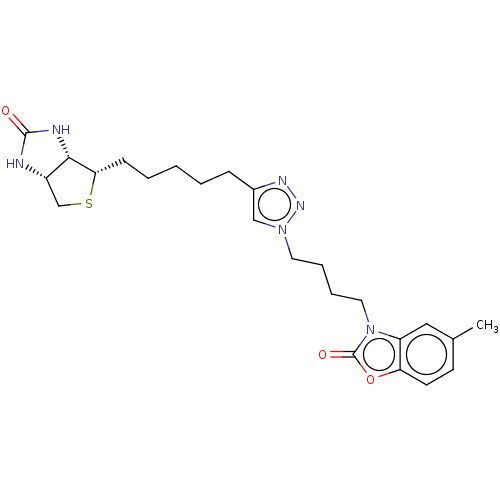 (US9108978, 6.09) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | 90 | -41.8 | 530 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161472 (US9108978, 2.02) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 225 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161472 (US9108978, 2.02) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161470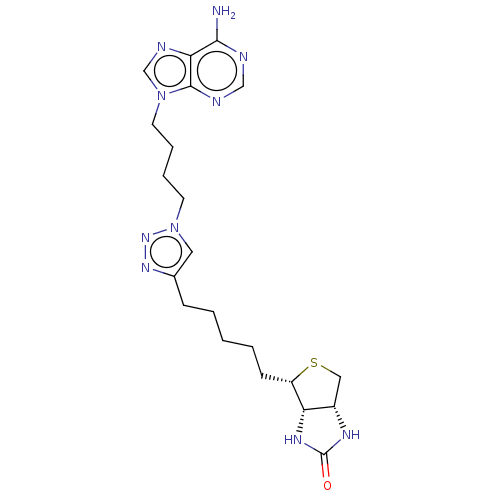 (US9108978, 4.01) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 660 | -36.7 | 4.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161469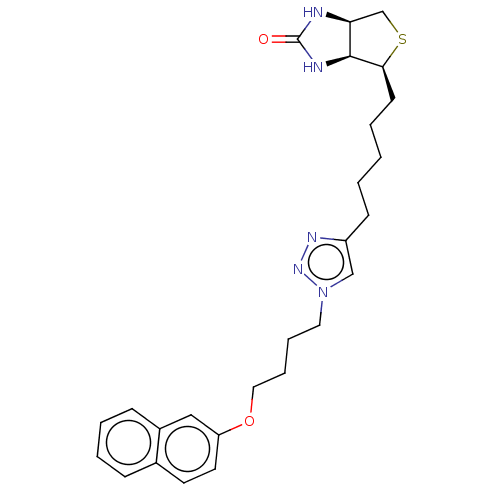 (US9108978, 6.07) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.17E+3 | -35.2 | 7.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161467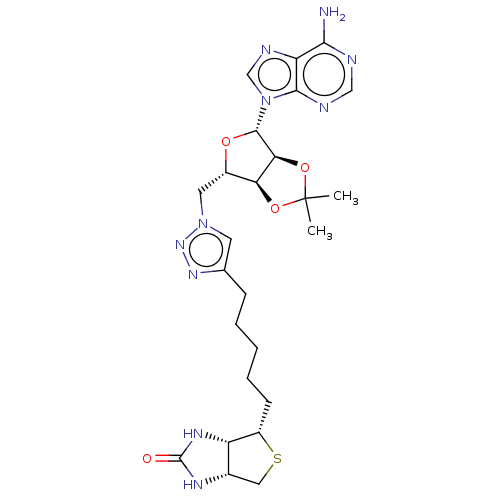 (US9108978, 3.25) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.83E+3 | -34.1 | 1.16E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161471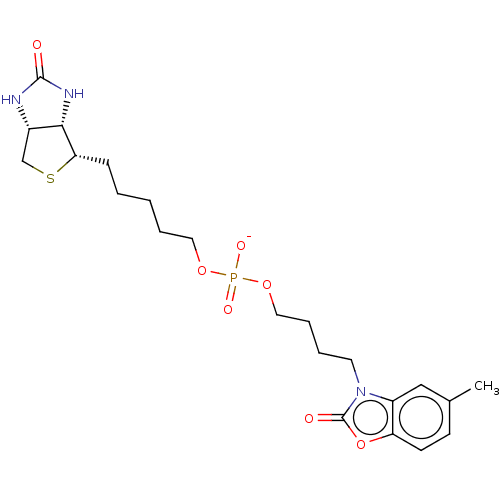 (US9108978, 6.34) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161470 (US9108978, 4.01) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161469 (US9108978, 6.07) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161468 (US9108978, 6.09) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161470 (US9108978, 4.01) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161469 (US9108978, 6.07) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161468 (US9108978, 6.09) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161467 (US9108978, 3.25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161467 (US9108978, 3.25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359672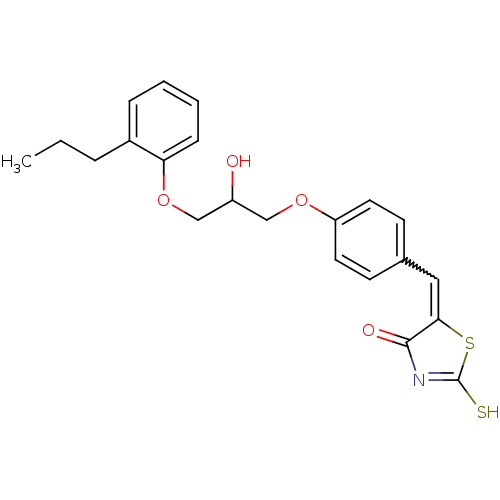 (CHEMBL1929262) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359675 (CHEMBL1929264) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359680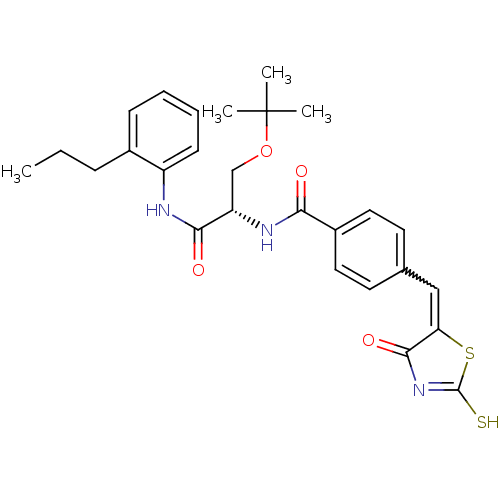 (CHEMBL1929269) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359683 (CHEMBL1929272) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359674 (CHEMBL1929263) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359682 (CHEMBL1929271) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50326793 ((+/-)(1S,2S,3R)-2-[(S)-Amino(carboxy)methyl]-3-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Antagonist activity at mGlu2 receptor expressed in CHO cells assessed as increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359681 (CHEMBL1929270) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50092271 ((Z)-5-Benzylidene-2-thioxothiazolidin-4-one | (Z)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359686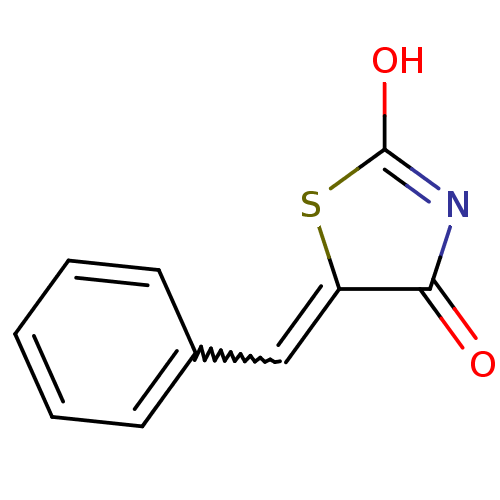 (CHEMBL85105) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359685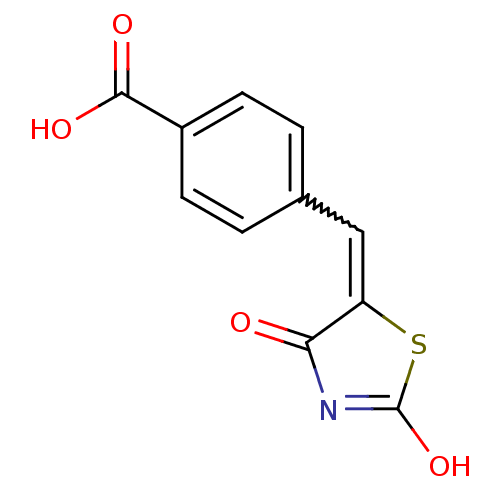 (CHEMBL1929274) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359684 (CHEMBL1929273) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359679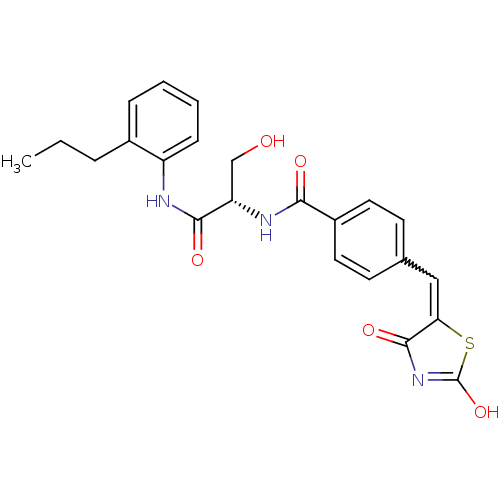 (CHEMBL1929268) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359678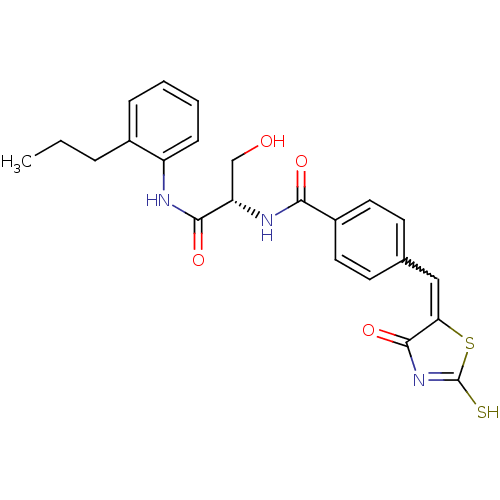 (CHEMBL1929267) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359677 (CHEMBL1929266) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359676 (CHEMBL1929265) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359673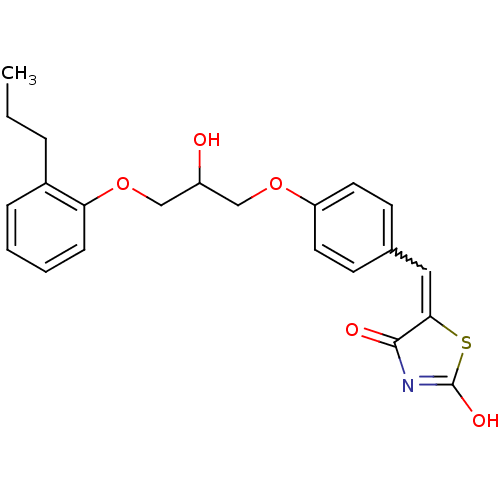 (CHEMBL338214) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometry | Bioorg Med Chem 19: 7453-63 (2011) Article DOI: 10.1016/j.bmc.2011.10.042 BindingDB Entry DOI: 10.7270/Q2WQ0468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50331747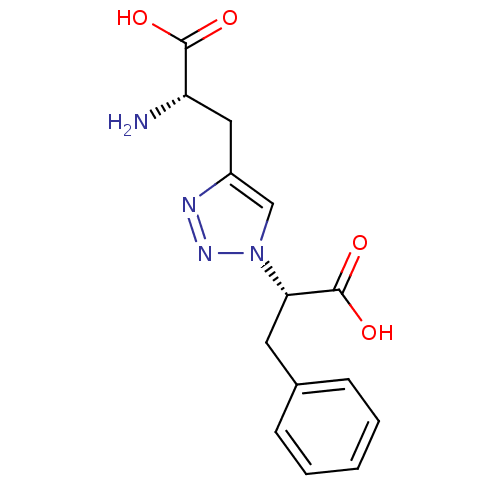 ((S)-2-amino-3-(1-((S)-1-carboxy-2-phenylethyl)-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50326793 ((+/-)(1S,2S,3R)-2-[(S)-Amino(carboxy)methyl]-3-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50326794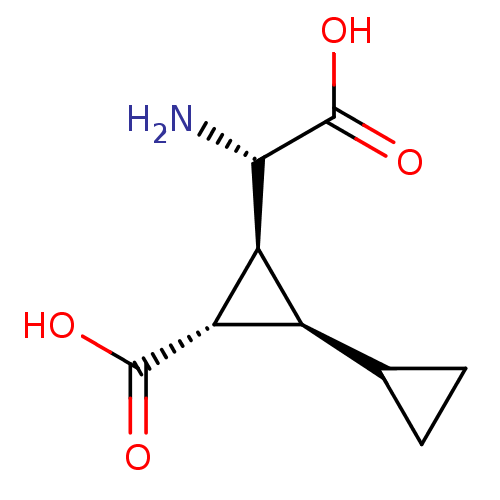 ((+/-)(1R,2S,3S)-3-[(S)-amino(carboxy)-methyl]-1,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50326794 ((+/-)(1R,2S,3S)-3-[(S)-amino(carboxy)-methyl]-1,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation counting | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50138781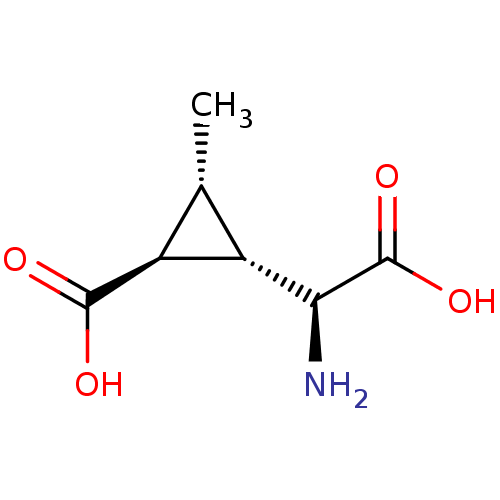 ((1S,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-methyl-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50326794 ((+/-)(1R,2S,3S)-3-[(S)-amino(carboxy)-methyl]-1,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >50 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50138781 ((1S,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-methyl-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >10 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50138781 ((1S,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-methyl-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation counting | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50326793 ((+/-)(1S,2S,3R)-2-[(S)-Amino(carboxy)methyl]-3-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation counting | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50331746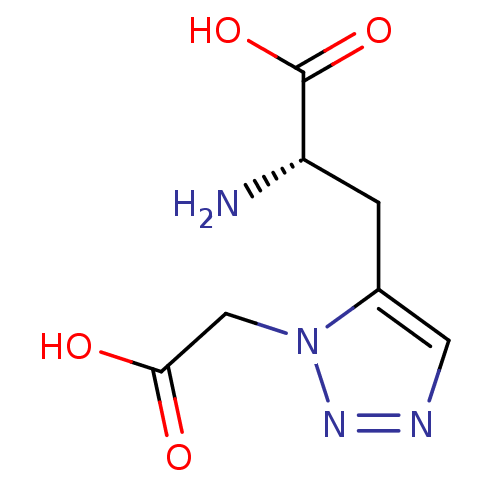 ((S)-2-amino-3-(1-(carboxymethyl)-1H-1,2,3-triazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50331745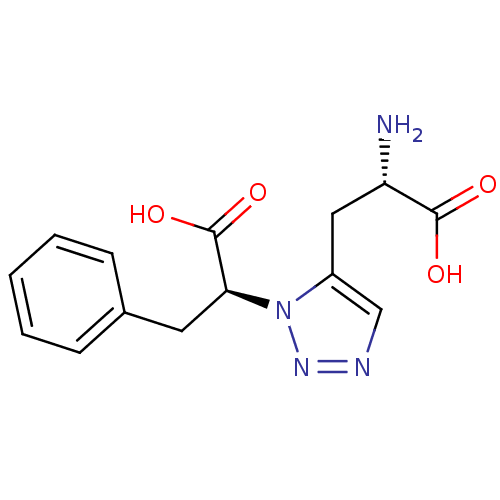 ((S)-2-amino-3-(1-((S)-1-carboxy-2-phenylethyl)-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50331744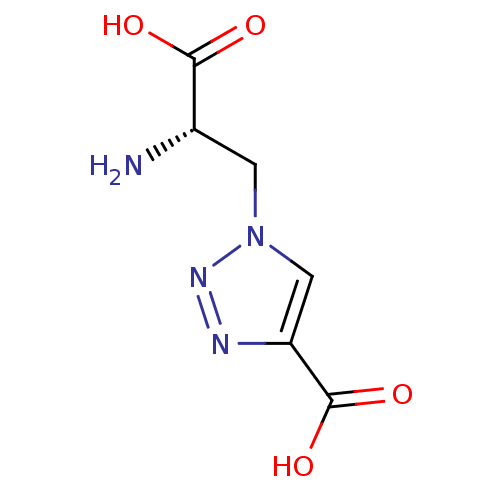 ((S)-1-(2-amino-2-carboxyethyl)-1H-1,2,3-triazole-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50331748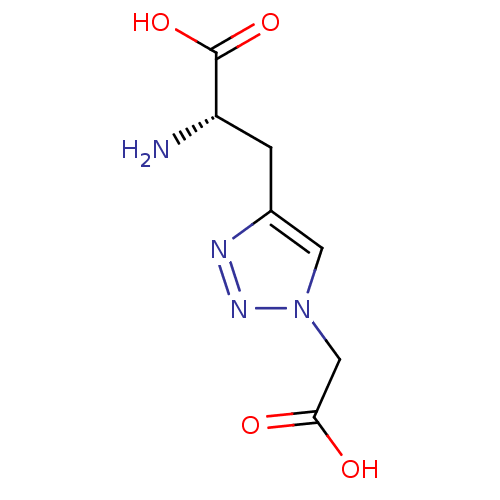 ((S)-2-amino-3-(1-(carboxymethyl)-1H-1,2,3-triazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50331747 ((S)-2-amino-3-(1-((S)-1-carboxy-2-phenylethyl)-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50331746 ((S)-2-amino-3-(1-(carboxymethyl)-1H-1,2,3-triazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50331745 ((S)-2-amino-3-(1-((S)-1-carboxy-2-phenylethyl)-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50331744 ((S)-1-(2-amino-2-carboxyethyl)-1H-1,2,3-triazole-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 mins | Bioorg Med Chem Lett 20: 7512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.09.139 BindingDB Entry DOI: 10.7270/Q2K64J94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |