

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-1 (Homo sapiens (Human)) | BDBM50058526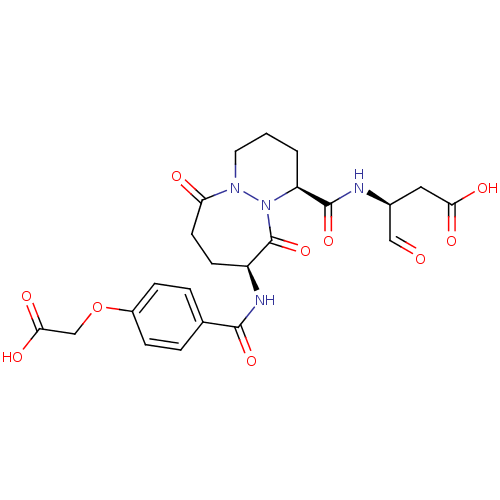 ((S)-3-{[(1S,9S)-9-(4-Carboxymethoxy-benzoylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc. Curated by ChEMBL | Assay Description Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) | J Med Chem 40: 1941-6 (1997) Article DOI: 10.1021/jm9701637 BindingDB Entry DOI: 10.7270/Q2CC0ZSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50058530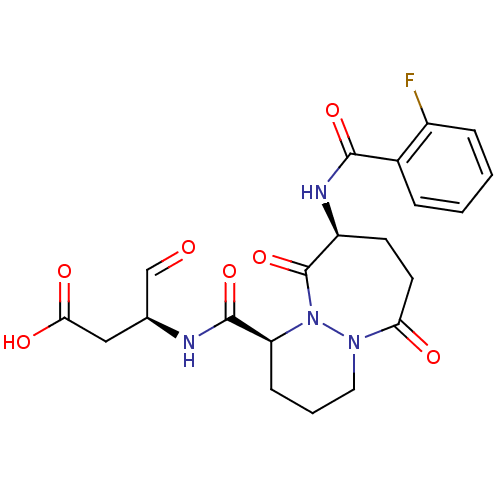 ((S)-3-{[(1S,9S)-9-(2-Fluoro-benzoylamino)-6,10-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc. Curated by ChEMBL | Assay Description Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) | J Med Chem 40: 1941-6 (1997) Article DOI: 10.1021/jm9701637 BindingDB Entry DOI: 10.7270/Q2CC0ZSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50058528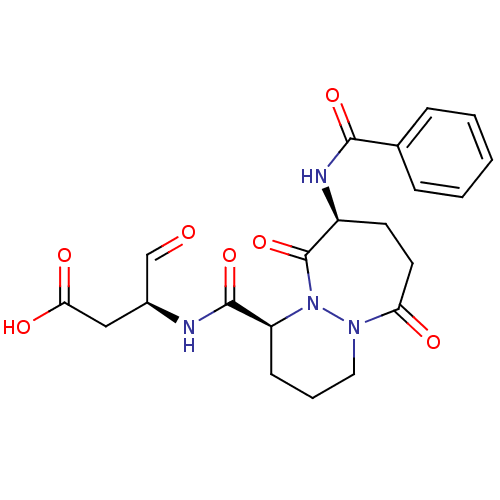 ((S)-3-[((1S,9S)-9-Benzoylamino-6,10-dioxo-octahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc. Curated by ChEMBL | Assay Description Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) | J Med Chem 40: 1941-6 (1997) Article DOI: 10.1021/jm9701637 BindingDB Entry DOI: 10.7270/Q2CC0ZSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50058527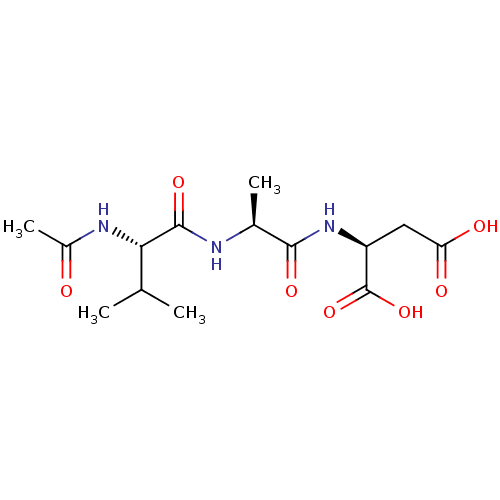 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc. Curated by ChEMBL | Assay Description Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) | J Med Chem 40: 1941-6 (1997) Article DOI: 10.1021/jm9701637 BindingDB Entry DOI: 10.7270/Q2CC0ZSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50058529 ((S)-2-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc. Curated by ChEMBL | Assay Description Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) | J Med Chem 40: 1941-6 (1997) Article DOI: 10.1021/jm9701637 BindingDB Entry DOI: 10.7270/Q2CC0ZSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50058531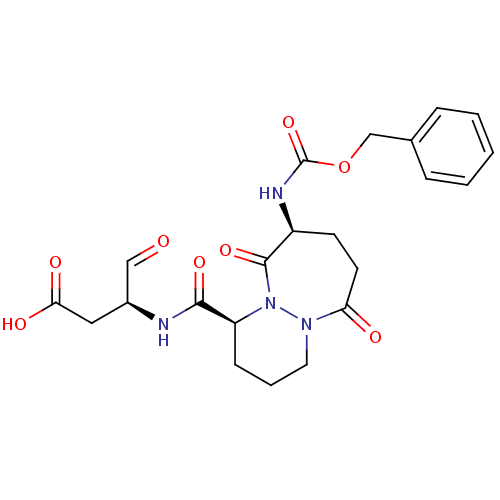 ((S)-3-[((1S,9S)-9-Benzyloxycarbonylamino-6,10-diox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Winthrop Inc. Curated by ChEMBL | Assay Description Reversible Inhibition constants of the compound against IL-1 beta converting enzyme (ICE) | J Med Chem 40: 1941-6 (1997) Article DOI: 10.1021/jm9701637 BindingDB Entry DOI: 10.7270/Q2CC0ZSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171438 (2-Acetylamino-3-[4-(2-acetylamino-2-carboxy-ethyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase for 30 minutes pH 7.4 at 25 degree C at 0.2 mM | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4163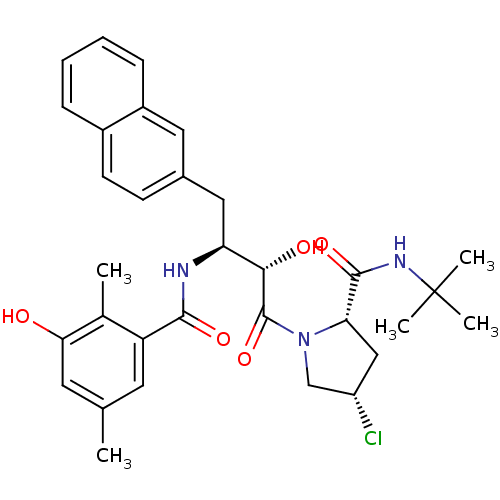 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4124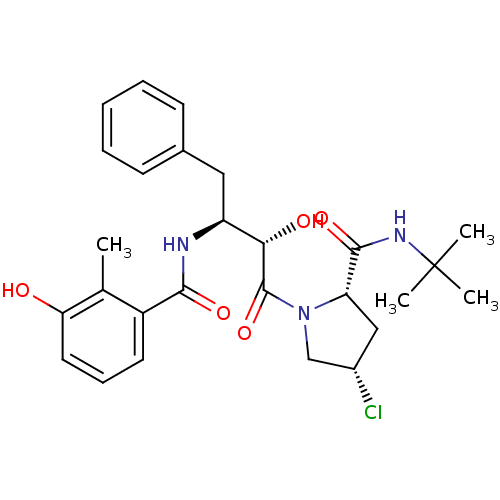 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321697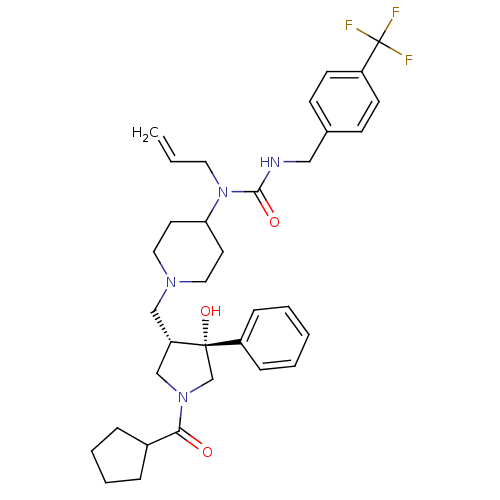 (1-allyl-1-(1-(((3S,4R)-1-(cyclopentanecarbonyl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321683 (4-chlorobenzyl allyl(1-(((3S,4R)-1-(cyclopentaneca...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4165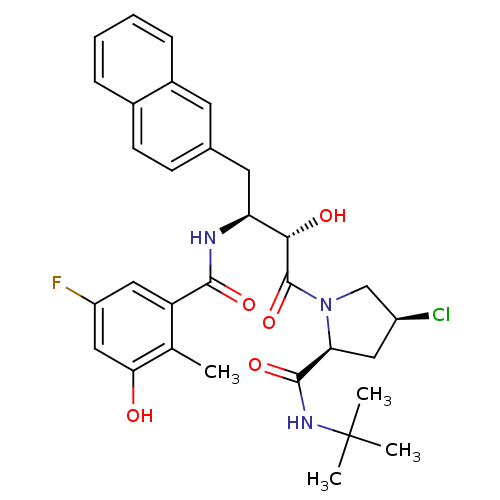 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(5-flu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4161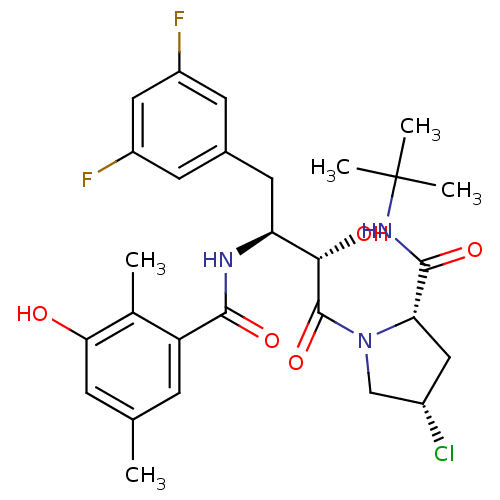 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,5-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4160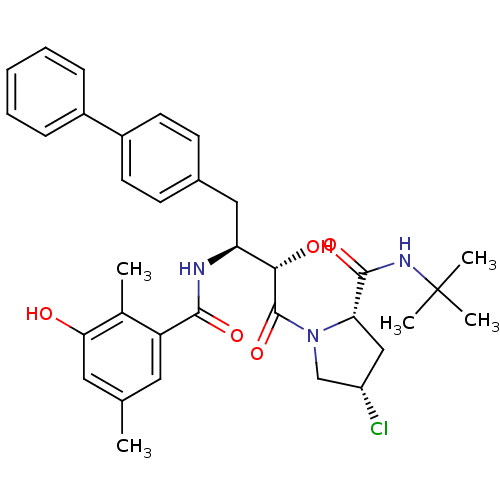 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4154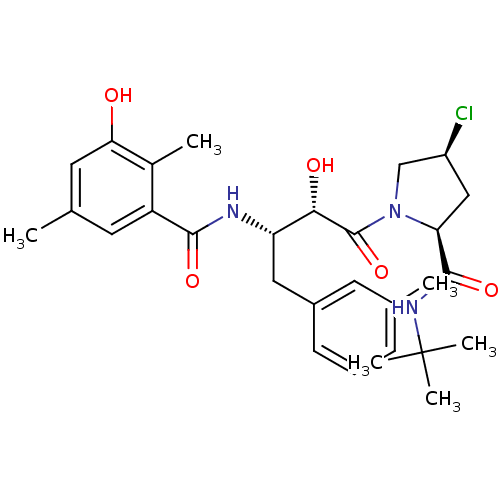 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4144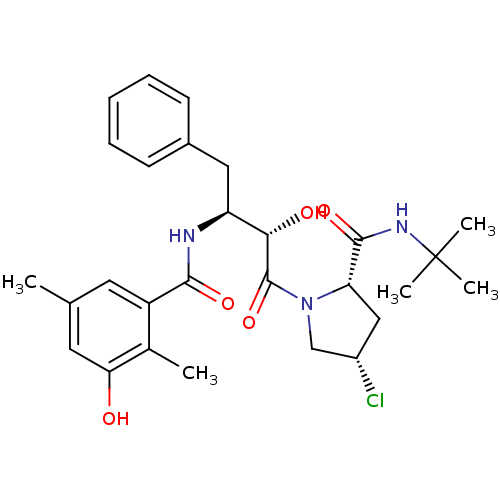 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50325319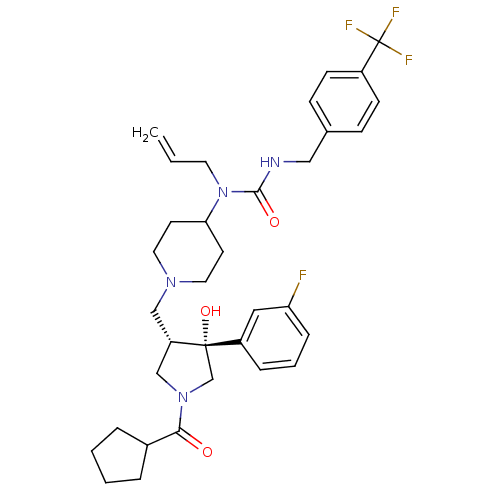 (1-allyl-1-(1-(((3S,4R)-1-(cyclopentanecarbonyl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5334-6 (2010) Article DOI: 10.1016/j.bmcl.2010.05.046 BindingDB Entry DOI: 10.7270/Q2J67H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4264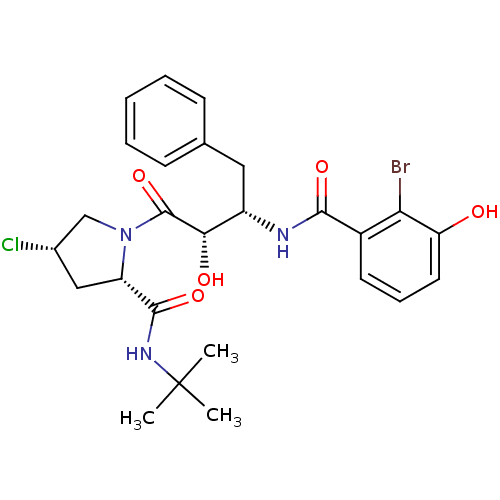 ((2-Bromo-3-hydroxy)benzoyl-(2S,3S)-AHPBA-4(S)-Cl-P...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321712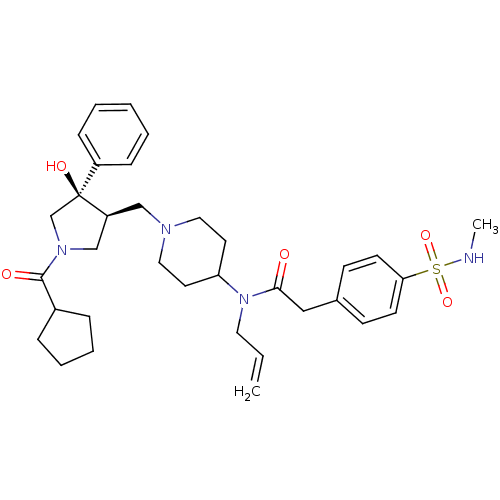 (CHEMBL1172625 | N-allyl-N-(1-(((3S,4R)-1-(cyclopen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321699 (1-allyl-1-(1-(((3S,4R)-1-(cyclopentanecarbonyl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4145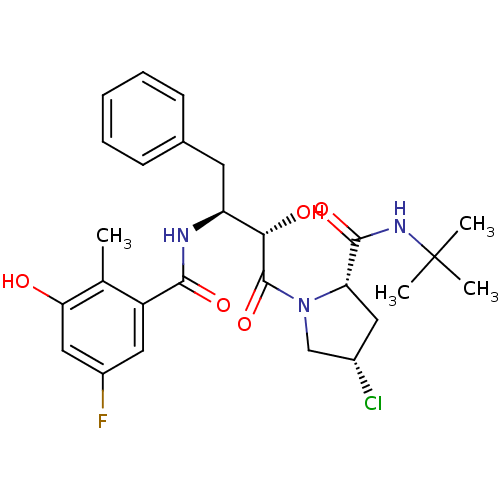 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(5-flu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321700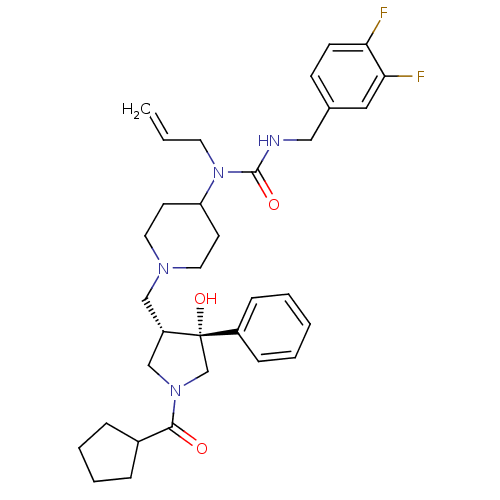 (1-allyl-1-(1-(((3S,4R)-1-(cyclopentanecarbonyl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321694 (1-allyl-1-(1-(((3S,4R)-1-(cyclopentanecarbonyl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4148 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321711 (CHEMBL1172624 | N-allyl-N-(1-(((3S,4R)-1-(cyclopen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4149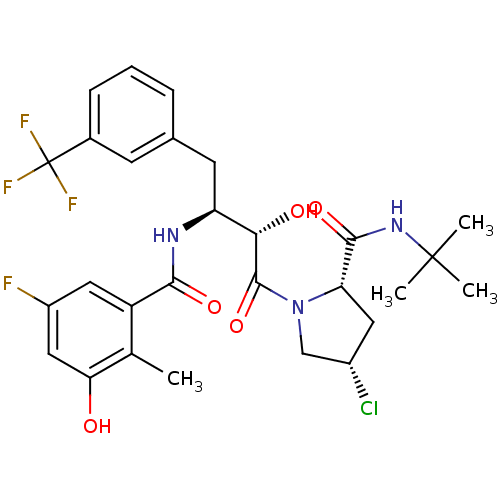 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(5-flu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4153 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4146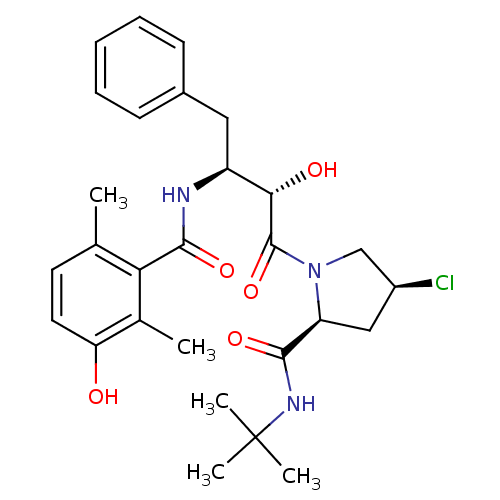 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321695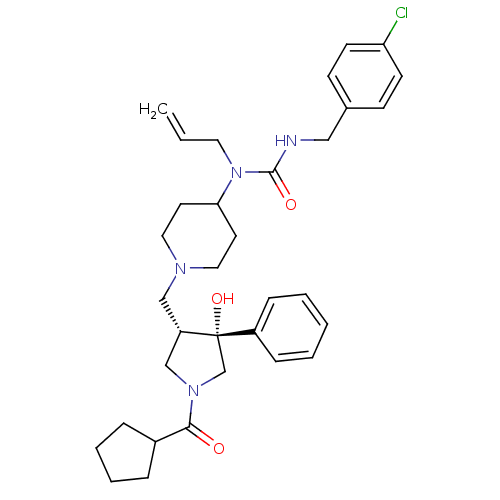 (1-allyl-3-(4-chlorobenzyl)-1-(1-(((3S,4R)-1-(cyclo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4164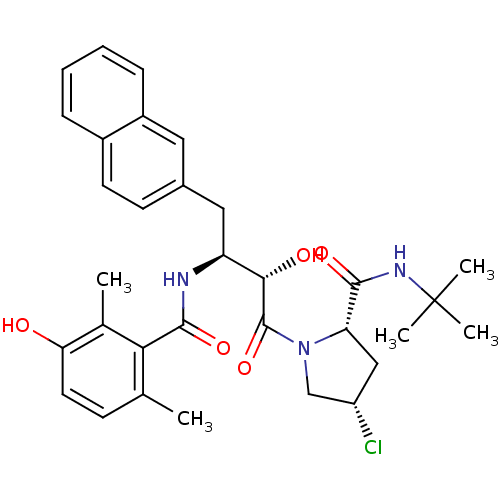 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321682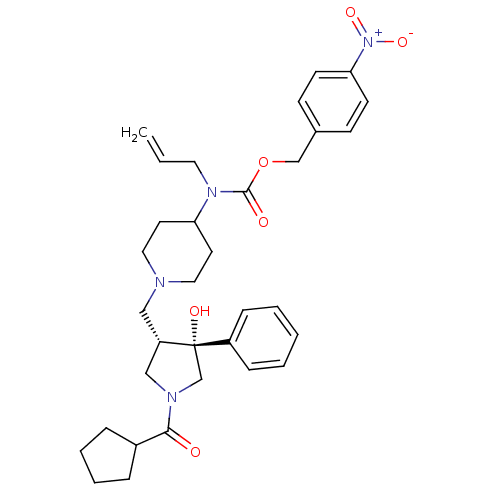 (CHEMBL1172035 | nifeviroc) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321682 (CHEMBL1172035 | nifeviroc) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5334-6 (2010) Article DOI: 10.1016/j.bmcl.2010.05.046 BindingDB Entry DOI: 10.7270/Q2J67H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321701 (4-((3-allyl-3-(1-(((3S,4R)-1-(cyclopentanecarbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50325321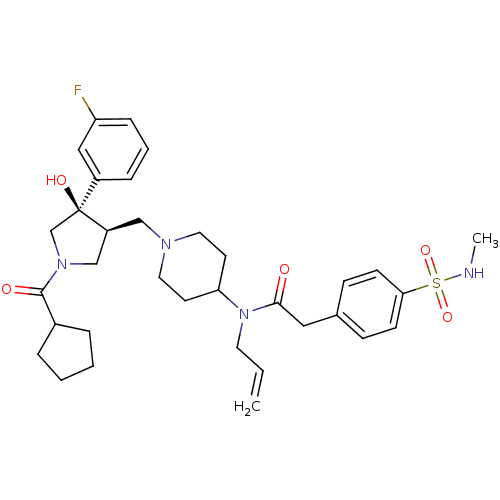 (CHEMBL1222771 | N-allyl-N-(1-(((3S,4R)-1-(cyclopen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5334-6 (2010) Article DOI: 10.1016/j.bmcl.2010.05.046 BindingDB Entry DOI: 10.7270/Q2J67H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321696 (1-allyl-1-(1-(((3S,4R)-1-(cyclopentanecarbonyl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4162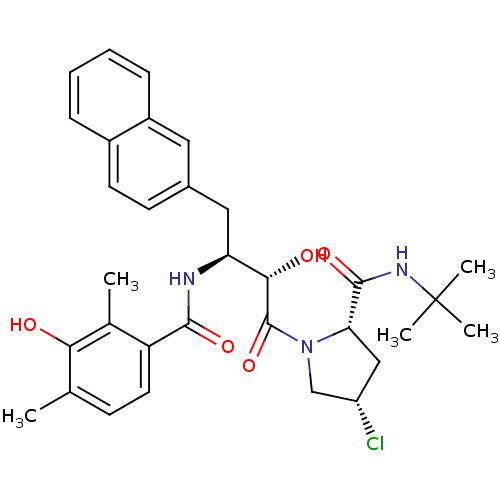 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321713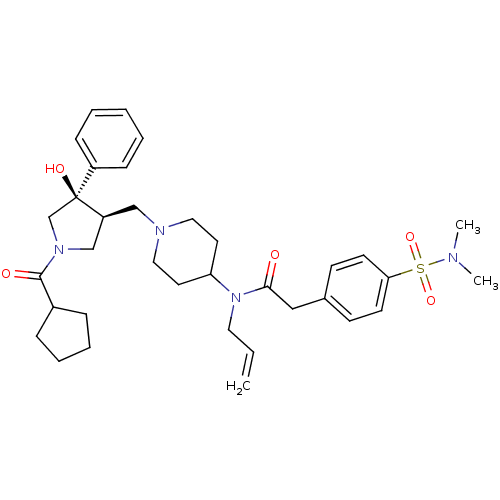 (CHEMBL1172626 | N-allyl-N-(1-(((3S,4R)-1-(cyclopen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4155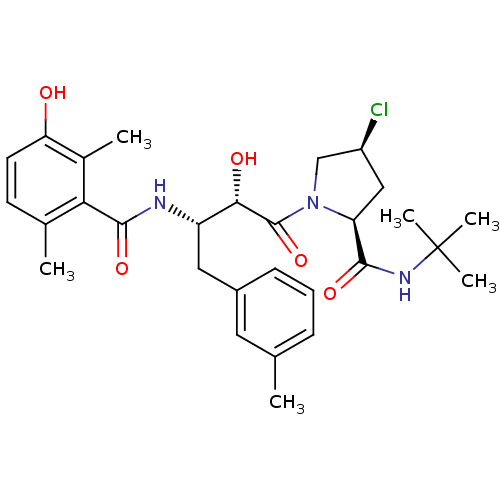 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321682 (CHEMBL1172035 | nifeviroc) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced calcium elevation | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4147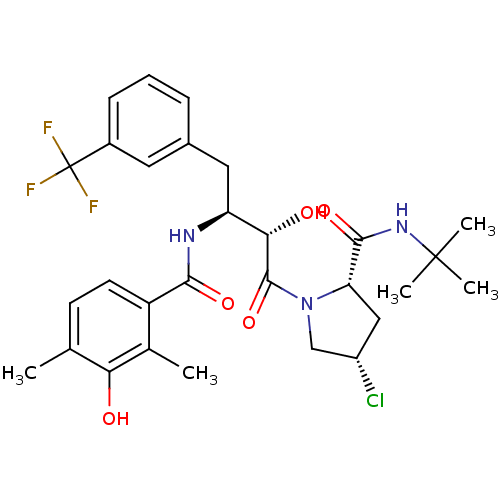 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321703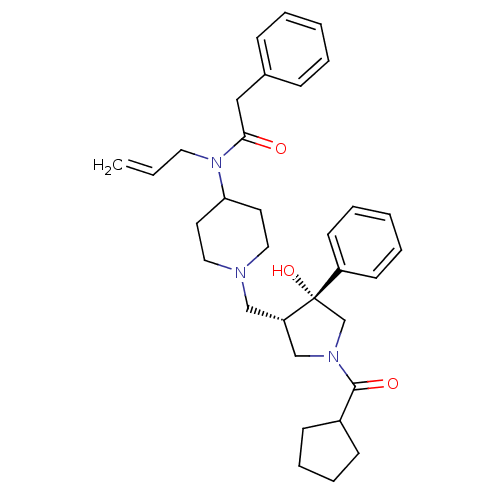 (CHEMBL1172157 | N-allyl-N-(1-(((3S,4R)-1-(cyclopen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321707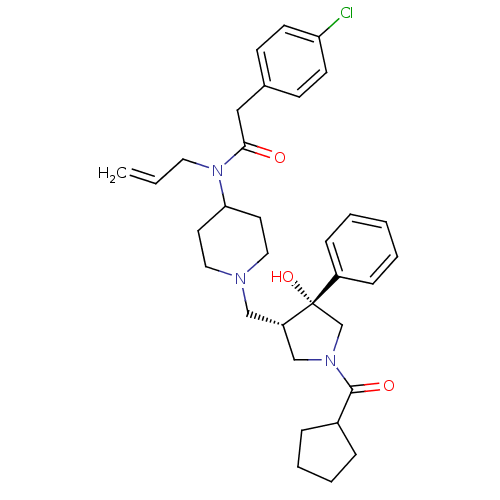 (CHEMBL1171030 | N-allyl-2-(4-chlorophenyl)-N-(1-((...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4157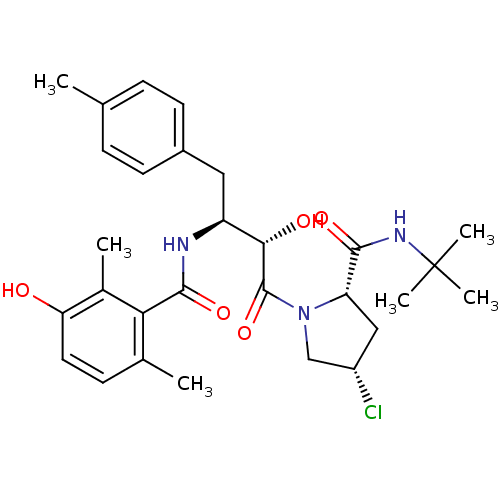 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4256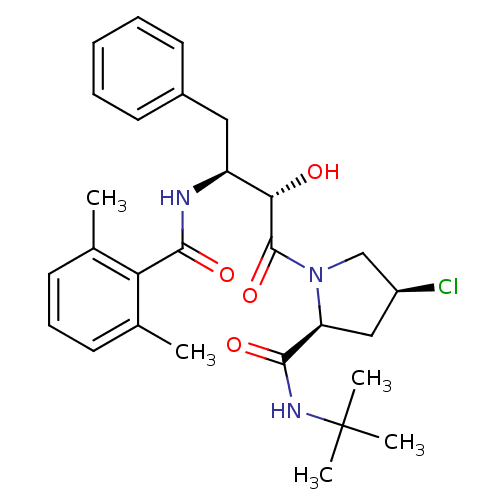 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(2,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4152 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50325325 (4-((3-allyl-3-((1R,3S,5S)-8-(((3S,4R)-1-(cyclopent...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5334-6 (2010) Article DOI: 10.1016/j.bmcl.2010.05.046 BindingDB Entry DOI: 10.7270/Q2J67H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321710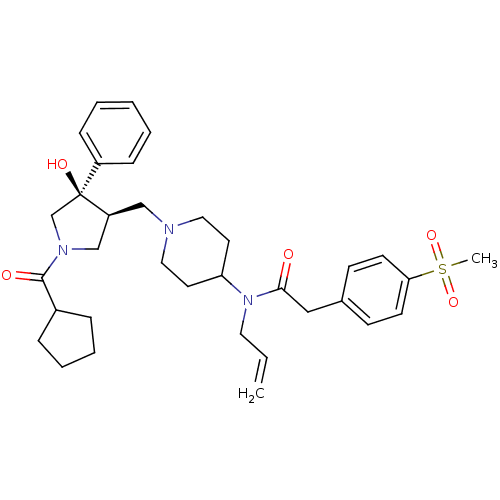 (CHEMBL1172623 | N-allyl-N-(1-(((3S,4R)-1-(cyclopen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4150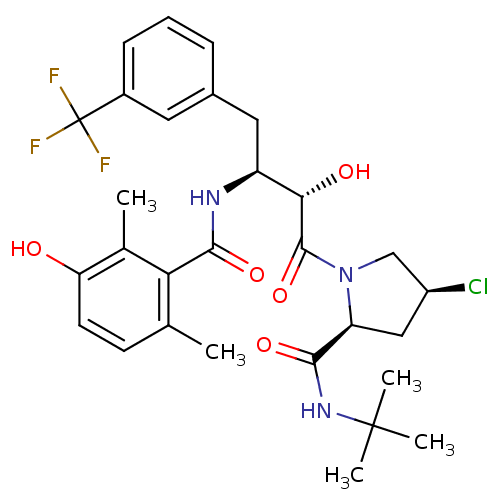 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321702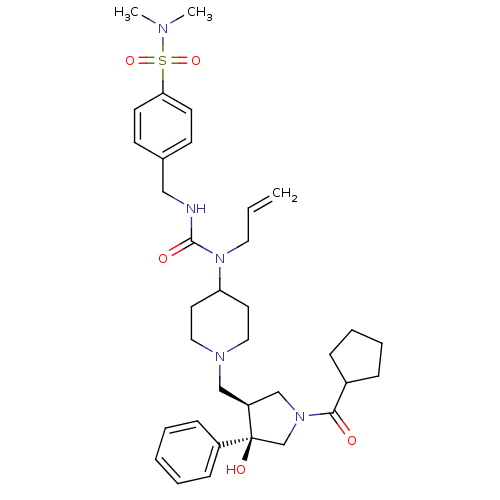 (4-((3-allyl-3-(1-(((3S,4R)-1-(cyclopentanecarbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321705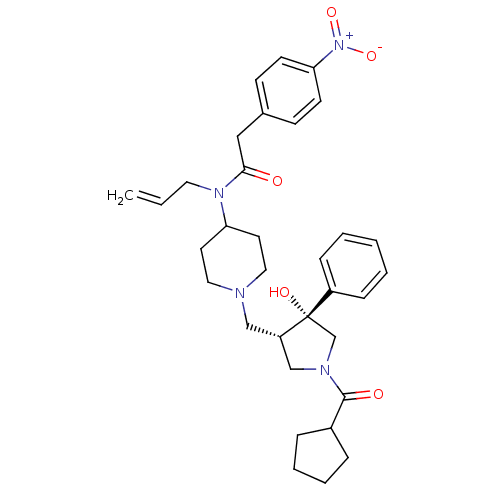 (CHEMBL1172439 | N-allyl-N-(1-(((3S,4R)-1-(cyclopen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-induced [35S]GTPgamma binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 4012-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.102 BindingDB Entry DOI: 10.7270/Q2H132Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |