 Found 402 hits with Last Name = 'pemberton' and Initial = 'n'
Found 402 hits with Last Name = 'pemberton' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155307

(CHEMBL3781796)Show SMILES [H]C1(NC(=O)C(Cc2ccccc2)NC(=O)C([H])(NC(=O)C(CCCCN)NC(=O)C([H])(NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2ccccc2)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(O)=O)NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCC(O)=O)NC(=O)C(CCCNC(N)=N)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(CCSC)NC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(C)NC(=O)C(N)CO)C(C)O)C(C)O)C(C)O Show InChI InChI=1S/C130H204N40O40S3/c1-64(146-106(187)75(34-18-21-44-131)151-108(189)78(37-24-47-142-129(138)139)152-111(192)80(41-42-98(181)182)154-109(190)79(38-25-48-143-130(140)141)155-122(203)93-40-26-49-169(93)126(207)67(4)148-107(188)81(43-51-211-8)150-103(184)66(3)147-121(202)92-39-27-50-170(92)127(208)87(57-96(137)179)162-118(199)88(60-172)163-115(196)85(55-94(135)177)157-104(185)65(2)145-105(186)74(134)59-171)102(183)144-58-97(180)149-90-62-212-213-63-91(128(209)210)165-119(200)89(61-173)164-125(206)101(70(7)176)168-117(198)84(54-73-32-16-11-17-33-73)161-124(205)100(69(6)175)166-112(193)77(36-20-23-46-133)156-123(204)99(68(5)174)167-116(197)83(53-72-30-14-10-15-31-72)159-113(194)82(52-71-28-12-9-13-29-71)158-114(195)86(56-95(136)178)160-110(191)76(153-120(90)201)35-19-22-45-132/h9-17,28-33,64-70,74-93,99-101,171-176H,18-27,34-63,131-134H2,1-8H3,(H2,135,177)(H2,136,178)(H2,137,179)(H,144,183)(H,145,186)(H,146,187)(H,147,202)(H,148,188)(H,149,180)(H,150,184)(H,151,189)(H,152,192)(H,153,201)(H,154,190)(H,155,203)(H,156,204)(H,157,185)(H,158,195)(H,159,194)(H,160,191)(H,161,205)(H,162,199)(H,163,196)(H,164,206)(H,165,200)(H,166,193)(H,167,197)(H,168,198)(H,181,182)(H,209,210)(H4,138,139,142)(H4,140,141,143)/t64-,65-,66-,67-,68+,69+,70+,74-,75-,76+,77-,78-,79-,80-,81-,82-,83-,84-,85?,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155307

(CHEMBL3781796)Show SMILES [H]C1(NC(=O)C(Cc2ccccc2)NC(=O)C([H])(NC(=O)C(CCCCN)NC(=O)C([H])(NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2ccccc2)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(O)=O)NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCC(O)=O)NC(=O)C(CCCNC(N)=N)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(CCSC)NC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(C)NC(=O)C(N)CO)C(C)O)C(C)O)C(C)O Show InChI InChI=1S/C130H204N40O40S3/c1-64(146-106(187)75(34-18-21-44-131)151-108(189)78(37-24-47-142-129(138)139)152-111(192)80(41-42-98(181)182)154-109(190)79(38-25-48-143-130(140)141)155-122(203)93-40-26-49-169(93)126(207)67(4)148-107(188)81(43-51-211-8)150-103(184)66(3)147-121(202)92-39-27-50-170(92)127(208)87(57-96(137)179)162-118(199)88(60-172)163-115(196)85(55-94(135)177)157-104(185)65(2)145-105(186)74(134)59-171)102(183)144-58-97(180)149-90-62-212-213-63-91(128(209)210)165-119(200)89(61-173)164-125(206)101(70(7)176)168-117(198)84(54-73-32-16-11-17-33-73)161-124(205)100(69(6)175)166-112(193)77(36-20-23-46-133)156-123(204)99(68(5)174)167-116(197)83(53-72-30-14-10-15-31-72)159-113(194)82(52-71-28-12-9-13-29-71)158-114(195)86(56-95(136)178)160-110(191)76(153-120(90)201)35-19-22-45-132/h9-17,28-33,64-70,74-93,99-101,171-176H,18-27,34-63,131-134H2,1-8H3,(H2,135,177)(H2,136,178)(H2,137,179)(H,144,183)(H,145,186)(H,146,187)(H,147,202)(H,148,188)(H,149,180)(H,150,184)(H,151,189)(H,152,192)(H,153,201)(H,154,190)(H,155,203)(H,156,204)(H,157,185)(H,158,195)(H,159,194)(H,160,191)(H,161,205)(H,162,199)(H,163,196)(H,164,206)(H,165,200)(H,166,193)(H,167,197)(H,168,198)(H,181,182)(H,209,210)(H4,138,139,142)(H4,140,141,143)/t64-,65-,66-,67-,68+,69+,70+,74-,75-,76+,77-,78-,79-,80-,81-,82-,83-,84-,85?,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst4 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50274638
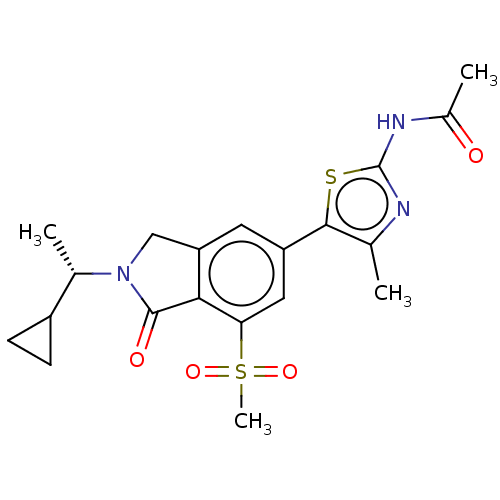
(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human P2Y1 receptor |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155308
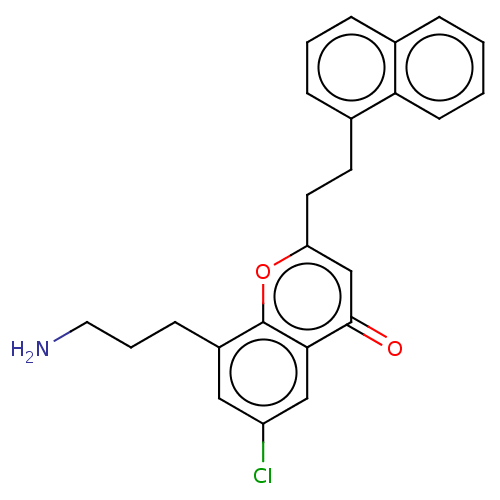
(CHEMBL3780791)Show SMILES Cl.Cl.NCCCc1cc(Cl)cc2c1oc(CCc1cccc3ccccc13)cc2=O Show InChI InChI=1S/C20H26N2O2/c23-19(14-7-2-1-3-8-14)18-11-6-12-22(18)20(24)17-13-15-9-4-5-10-16(15)21-17/h1-3,7-8,15-18,21H,4-6,9-13H2/t15-,16-,17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst4 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50051567
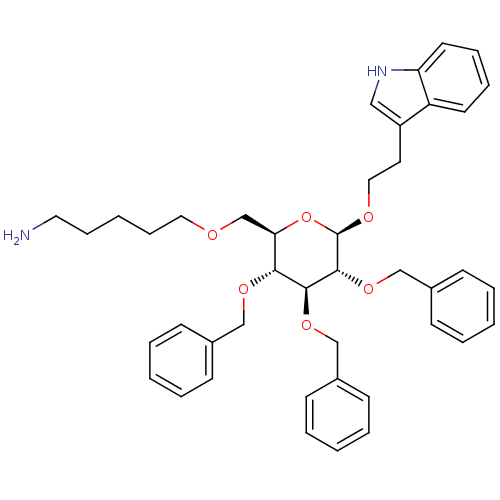
(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to sst4 receptor (unknown origin) |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155306
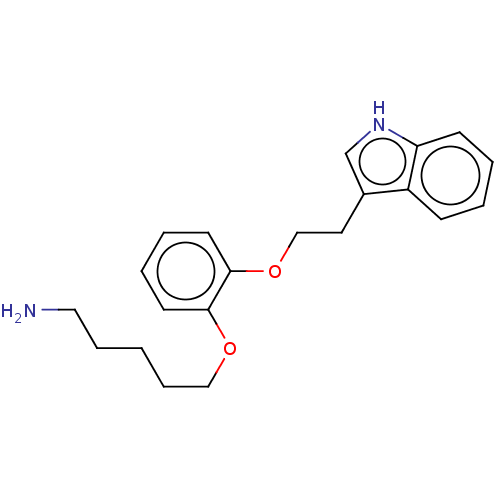
(CHEMBL3781875)Show InChI InChI=1S/C17H21N3O2S/c1-17(2,3)18-10-14(21)20-9-8-12(20)15(22)16-19-11-6-4-5-7-13(11)23-16/h4-7,12,18H,8-10H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to human sst4 receptor |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155305
(CHEMBL3782021)Show SMILES CC(C)CC[C@]1(Cc2ccccc2)NC=C(C1=O)[C@@]1(Cc2ccccc2)NC=C(C1=O)[C@]1(CCCN)NC=C(C1=O)[C@@]1(CC(C)C)NC=C(Cc2ccccc2)C1=O |r,c:15,30,41,t:52| Show InChI InChI=1S/C49H57N5O4/c1-33(2)21-23-46(27-36-17-10-6-11-18-36)43(56)39(30-51-46)49(28-37-19-12-7-13-20-37)45(58)40(31-54-49)47(22-14-24-50)44(57)41(32-53-47)48(26-34(3)4)42(55)38(29-52-48)25-35-15-8-5-9-16-35/h5-13,15-20,29-34,51-54H,14,21-28,50H2,1-4H3/t46-,47+,48-,49-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]-SRIF14 from human sst4 receptor |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155308

(CHEMBL3780791)Show SMILES Cl.Cl.NCCCc1cc(Cl)cc2c1oc(CCc1cccc3ccccc13)cc2=O Show InChI InChI=1S/C20H26N2O2/c23-19(14-7-2-1-3-8-14)18-11-6-12-22(18)20(24)17-13-15-9-4-5-10-16(15)21-17/h1-3,7-8,15-18,21H,4-6,9-13H2/t15-,16-,17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to sst2 receptor (unknown origin) |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155306

(CHEMBL3781875)Show InChI InChI=1S/C17H21N3O2S/c1-17(2,3)18-10-14(21)20-9-8-12(20)15(22)16-19-11-6-4-5-7-13(11)23-16/h4-7,12,18H,8-10H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 receptor |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155309
(CHEMBL3780496)Show SMILES NCCCc1cc(Cl)cc2C(=O)CC(CCc3cccc4ccccc34)Oc12 Show InChI InChI=1S/C18H24N2O2S/c21-17(13-7-9-23-11-13)16-6-3-8-20(16)18(22)15-10-12-4-1-2-5-14(12)19-15/h7,9,11-12,14-16,19H,1-6,8,10H2/t12-,14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155309

(CHEMBL3780496)Show SMILES NCCCc1cc(Cl)cc2C(=O)CC(CCc3cccc4ccccc34)Oc12 Show InChI InChI=1S/C18H24N2O2S/c21-17(13-7-9-23-11-13)16-6-3-8-20(16)18(22)15-10-12-4-1-2-5-14(12)19-15/h7,9,11-12,14-16,19H,1-6,8,10H2/t12-,14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst4 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474011

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-({[1-(fluorome...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NCC1(CCF)CC1)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C25H31FN4O4S2/c1-14-22(35-24(28-14)29-16(3)31)18-10-19-12-30(15(2)17-4-5-17)23(32)21(19)20(11-18)36(33,34)27-13-25(6-7-25)8-9-26/h10-11,15,17,27H,4-9,12-13H2,1-3H3,(H,28,29,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474007

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-(ethylsulfamoy...)Show SMILES CCNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H26N4O4S2/c1-5-22-31(28,29)17-9-15(19-11(2)23-21(30-19)24-13(4)26)8-16-10-25(20(27)18(16)17)12(3)14-6-7-14/h8-9,12,14,22H,5-7,10H2,1-4H3,(H,23,24,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239550
(CHEMBL4060741)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1Cc1cccc(CNCCCCOCCc2ccccc2)c1 |r| Show InChI InChI=1S/C35H39N7O2S/c1-24-22-45-32-19-29(25(2)41-34-31(20-36)33(37)39-23-40-34)30(35(43)42(24)32)18-27-11-8-12-28(17-27)21-38-14-6-7-15-44-16-13-26-9-4-3-5-10-26/h3-5,8-12,17,19,22-23,25,38H,6-7,13-16,18,21H2,1-2H3,(H3,37,39,40,41)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239559

(CHEMBL4073594)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1-c1ccccc1 |r| Show InChI InChI=1S/C21H18N6OS/c1-12-10-29-17-8-15(13(2)26-20-16(9-22)19(23)24-11-25-20)18(21(28)27(12)17)14-6-4-3-5-7-14/h3-8,10-11,13H,1-2H3,(H3,23,24,25,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474028

(N-(5-{7-[(3-Cyanophenyl)sulfamoyl]-2-[(1S)-1-cyclo...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)Nc1cccc(c1)C#N)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C26H25N5O4S2/c1-14-24(36-26(28-14)29-16(3)32)19-10-20-13-31(15(2)18-7-8-18)25(33)23(20)22(11-19)37(34,35)30-21-6-4-5-17(9-21)12-27/h4-6,9-11,15,18,30H,7-8,13H2,1-3H3,(H,28,29,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239540
(CHEMBL4086230)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1-c1cccc(CN(C)CCCCN(C)Cc2ccccc2)c1 |r| Show InChI InChI=1S/C35H40N8OS/c1-24-22-45-31-18-29(25(2)40-34-30(19-36)33(37)38-23-39-34)32(35(44)43(24)31)28-14-10-13-27(17-28)21-42(4)16-9-8-15-41(3)20-26-11-6-5-7-12-26/h5-7,10-14,17-18,22-23,25H,8-9,15-16,20-21H2,1-4H3,(H3,37,38,39,40)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50579665

(CHEMBL5082066)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCCCCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50579666
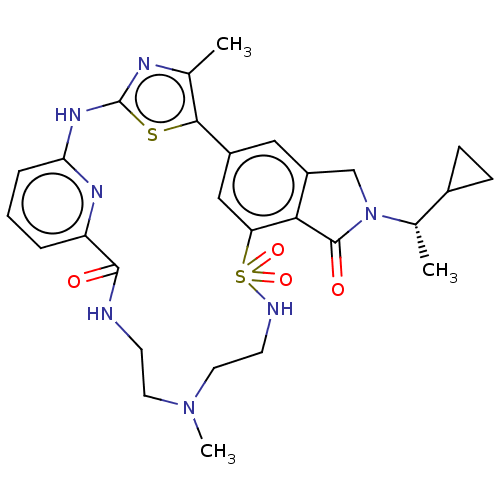
(CHEMBL5081964)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCN(C)CCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489281

(2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(3R)-3-hydro...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CC[C@@H](O)C2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-14-24(39-27(29-14)31-21-5-4-6-22(30-21)32-10-9-19(34)25(32)35)17-11-18-13-33(15(2)16-7-8-16)26(36)23(18)20(12-17)40(37,38)28-3/h4-6,11-12,15-16,19,28,34H,7-10,13H2,1-3H3,(H,29,30,31)/t15-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239549
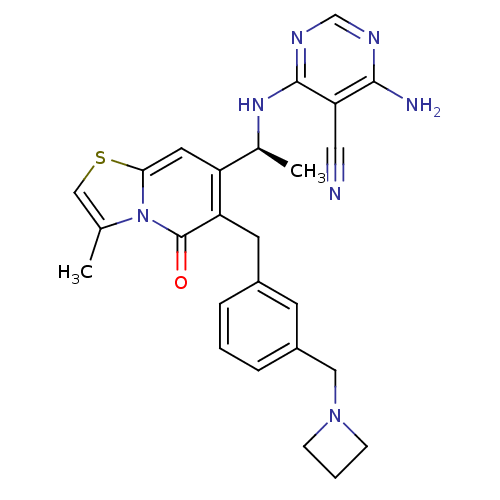
(CHEMBL4096646)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1Cc1cccc(CN2CCC2)c1 |r| Show InChI InChI=1S/C26H27N7OS/c1-16-14-35-23-11-20(17(2)31-25-22(12-27)24(28)29-15-30-25)21(26(34)33(16)23)10-18-5-3-6-19(9-18)13-32-7-4-8-32/h3,5-6,9,11,14-15,17H,4,7-8,10,13H2,1-2H3,(H3,28,29,30,31)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239542

(CHEMBL4069417)Show SMILES CC(C)CN(C)Cc1cccc(Cc2c(cc3scc(C)n3c2=O)[C@H](C)Nc2ncnc(N)c2C#N)c1 |r| Show InChI InChI=1S/C28H33N7OS/c1-17(2)13-34(5)14-21-8-6-7-20(9-21)10-23-22(11-25-35(28(23)36)18(3)15-37-25)19(4)33-27-24(12-29)26(30)31-16-32-27/h6-9,11,15-17,19H,10,13-14H2,1-5H3,(H3,30,31,32,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239552
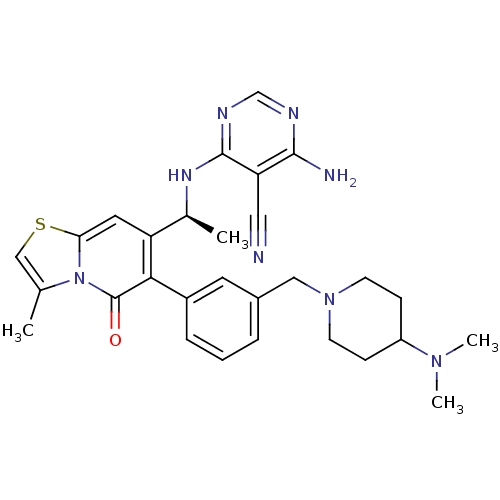
(CHEMBL4070433)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1-c1cccc(CN2CCC(CC2)N(C)C)c1 |r| Show InChI InChI=1S/C29H34N8OS/c1-18-16-39-25-13-23(19(2)34-28-24(14-30)27(31)32-17-33-28)26(29(38)37(18)25)21-7-5-6-20(12-21)15-36-10-8-22(9-11-36)35(3)4/h5-7,12-13,16-17,19,22H,8-11,15H2,1-4H3,(H3,31,32,33,34)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239555
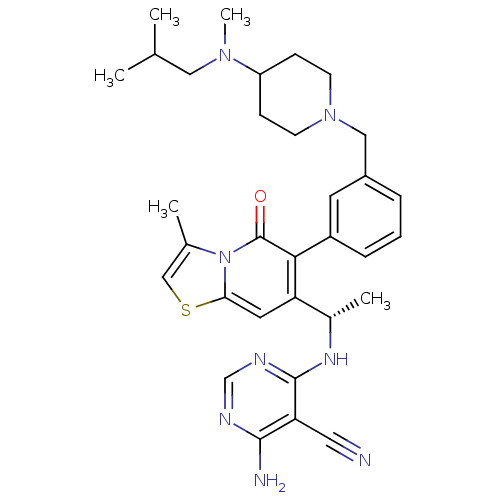
(CHEMBL4094000)Show SMILES CC(C)CN(C)C1CCN(Cc2cccc(c2)-c2c(cc3scc(C)n3c2=O)[C@H](C)Nc2ncnc(N)c2C#N)CC1 |r| Show InChI InChI=1S/C32H40N8OS/c1-20(2)16-38(5)25-9-11-39(12-10-25)17-23-7-6-8-24(13-23)29-26(14-28-40(32(29)41)21(3)18-42-28)22(4)37-31-27(15-33)30(34)35-19-36-31/h6-8,13-14,18-20,22,25H,9-12,16-17H2,1-5H3,(H3,34,35,36,37)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239557
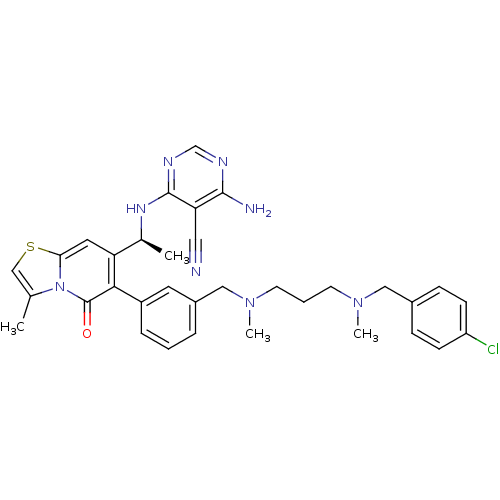
(CHEMBL4102634)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1-c1cccc(CN(C)CCCN(C)Cc2ccc(Cl)cc2)c1 |r| Show InChI InChI=1S/C34H37ClN8OS/c1-22-20-45-30-16-28(23(2)40-33-29(17-36)32(37)38-21-39-33)31(34(44)43(22)30)26-8-5-7-25(15-26)19-42(4)14-6-13-41(3)18-24-9-11-27(35)12-10-24/h5,7-12,15-16,20-21,23H,6,13-14,18-19H2,1-4H3,(H3,37,38,39,40)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239548

(CHEMBL4080523)Show SMILES COCCNCc1cccc(Cc2c(cc3scc(C)n3c2=O)[C@H](C)Nc2ncnc(N)c2C#N)c1 |r| Show InChI InChI=1S/C26H29N7O2S/c1-16-14-36-23-11-20(17(2)32-25-22(12-27)24(28)30-15-31-25)21(26(34)33(16)23)10-18-5-4-6-19(9-18)13-29-7-8-35-3/h4-6,9,11,14-15,17,29H,7-8,10,13H2,1-3H3,(H3,28,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274640
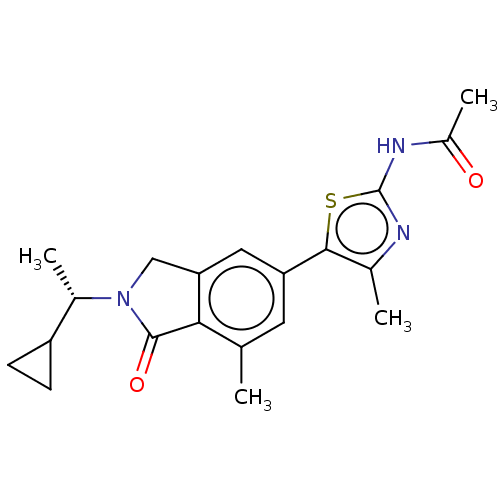
(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239545
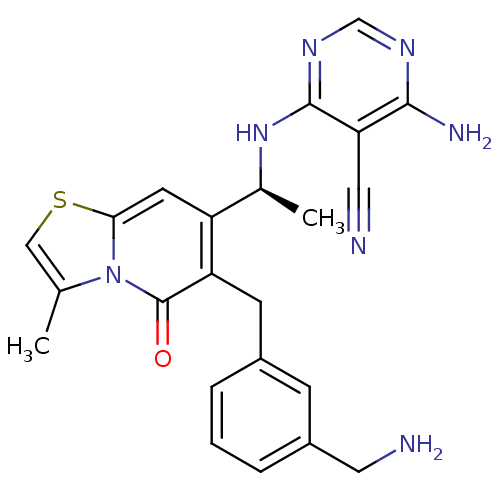
(CHEMBL4092688)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1Cc1cccc(CN)c1 |r| Show InChI InChI=1S/C23H23N7OS/c1-13-11-32-20-8-17(14(2)29-22-19(10-25)21(26)27-12-28-22)18(23(31)30(13)20)7-15-4-3-5-16(6-15)9-24/h3-6,8,11-12,14H,7,9,24H2,1-2H3,(H3,26,27,28,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239560

(CHEMBL4081062)Show SMILES CC(C)N1CCC(Cc2cccc(c2)-c2c(cc3scc(C)n3c2=O)[C@H](C)Nc2ncnc(N)c2C#N)CC1 |r| Show InChI InChI=1S/C30H35N7OS/c1-18(2)36-10-8-21(9-11-36)12-22-6-5-7-23(13-22)27-24(14-26-37(30(27)38)19(3)16-39-26)20(4)35-29-25(15-31)28(32)33-17-34-29/h5-7,13-14,16-18,20-21H,8-12H2,1-4H3,(H3,32,33,34,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of photolabeling of 24 kDa polypeptide in adenosine A1 receptor |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239551
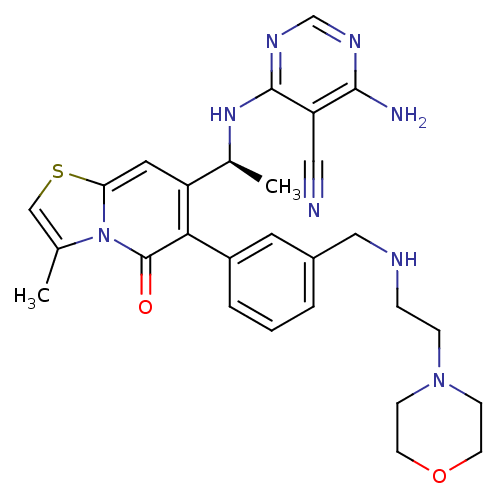
(CHEMBL4091365)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2scc(C)n2c(=O)c1-c1cccc(CNCCN2CCOCC2)c1 |r| Show InChI InChI=1S/C28H32N8O2S/c1-18-16-39-24-13-22(19(2)34-27-23(14-29)26(30)32-17-33-27)25(28(37)36(18)24)21-5-3-4-20(12-21)15-31-6-7-35-8-10-38-11-9-35/h3-5,12-13,16-17,19,31H,6-11,15H2,1-2H3,(H3,30,32,33,34)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239553
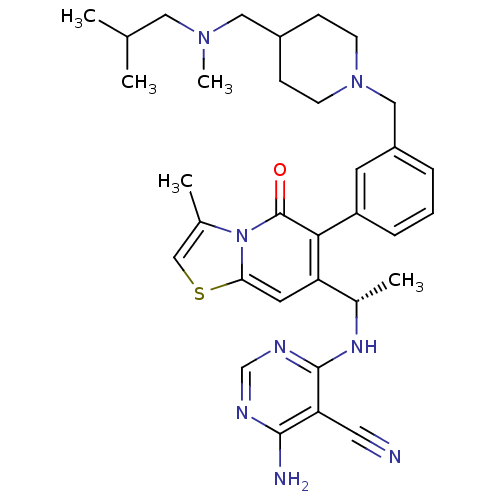
(CHEMBL4083582)Show SMILES CC(C)CN(C)CC1CCN(Cc2cccc(c2)-c2c(cc3scc(C)n3c2=O)[C@H](C)Nc2ncnc(N)c2C#N)CC1 |r| Show InChI InChI=1S/C33H42N8OS/c1-21(2)16-39(5)17-24-9-11-40(12-10-24)18-25-7-6-8-26(13-25)30-27(14-29-41(33(30)42)22(3)19-43-29)23(4)38-32-28(15-34)31(35)36-20-37-32/h6-8,13-14,19-21,23-24H,9-12,16-18H2,1-5H3,(H3,35,36,37,38)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 15 mins followed by substrate addit... |
J Med Chem 60: 5057-5071 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00401
BindingDB Entry DOI: 10.7270/Q2T43W8X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489248
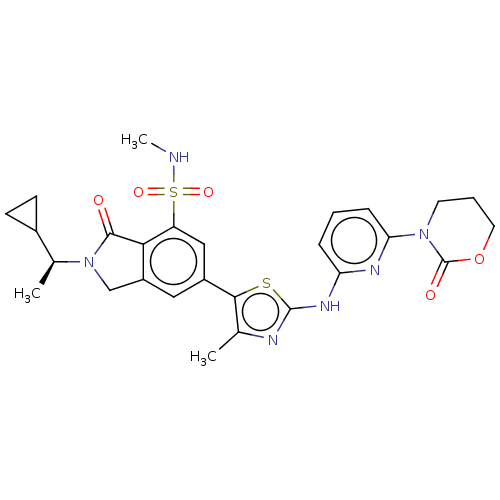
(2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCCOC2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-15-24(39-26(29-15)31-21-6-4-7-22(30-21)32-10-5-11-38-27(32)35)18-12-19-14-33(16(2)17-8-9-17)25(34)23(19)20(13-18)40(36,37)28-3/h4,6-7,12-13,16-17,28H,5,8-11,14H2,1-3H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489247
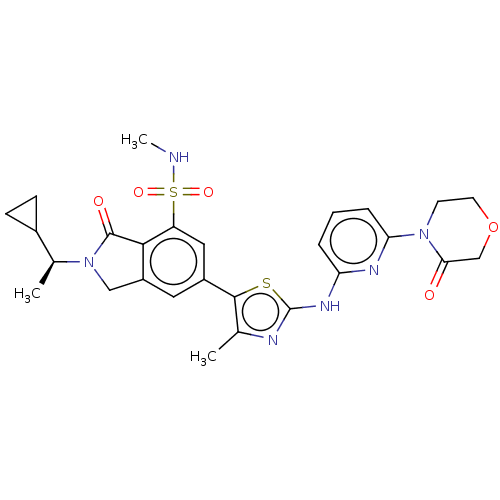
(2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCOCC2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-15-25(39-27(29-15)31-21-5-4-6-22(30-21)32-9-10-38-14-23(32)34)18-11-19-13-33(16(2)17-7-8-17)26(35)24(19)20(12-18)40(36,37)28-3/h4-6,11-12,16-17,28H,7-10,13-14H2,1-3H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489261
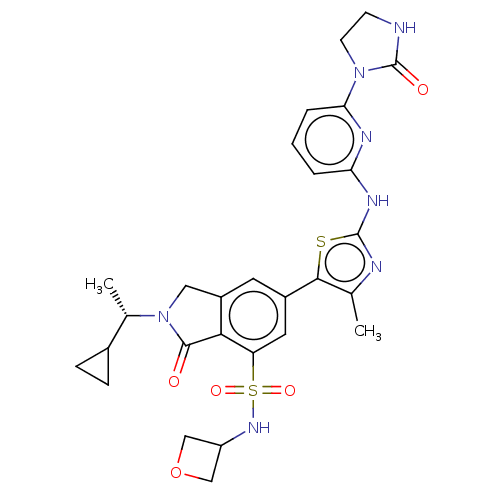
(2-[(1S)-1-Cyclopropylethyl]-6-(4-methyl-2-{[6-(2-o...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(Nc2cccc(n2)N2CCNC2=O)nc1C |r| Show InChI InChI=1S/C28H31N7O5S2/c1-15-25(41-27(30-15)32-22-4-3-5-23(31-22)34-9-8-29-28(34)37)18-10-19-12-35(16(2)17-6-7-17)26(36)24(19)21(11-18)42(38,39)33-20-13-40-14-20/h3-5,10-11,16-17,20,33H,6-9,12-14H2,1-2H3,(H,29,37)(H,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512861
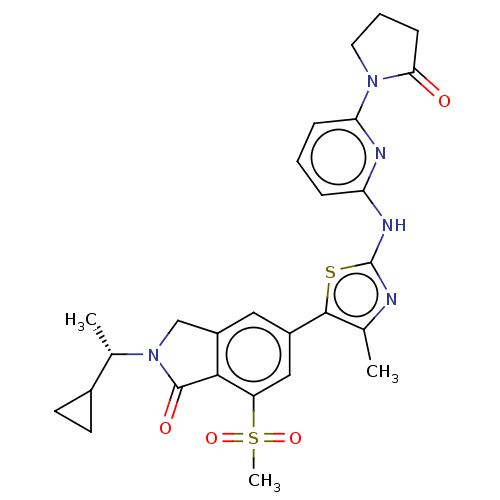
(CHEMBL4558527)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)N2CCCC2=O)nc1C |r| Show InChI InChI=1S/C27H29N5O4S2/c1-15-25(37-27(28-15)30-21-6-4-7-22(29-21)31-11-5-8-23(31)33)18-12-19-14-32(16(2)17-9-10-17)26(34)24(19)20(13-18)38(3,35)36/h4,6-7,12-13,16-17H,5,8-11,14H2,1-3H3,(H,28,29,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50579668
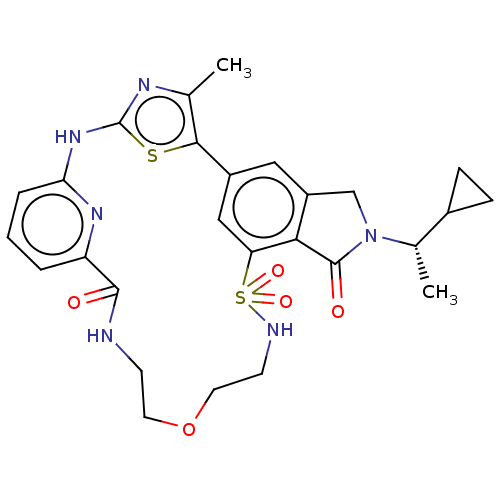
(CHEMBL5091438)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCOCCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50579667
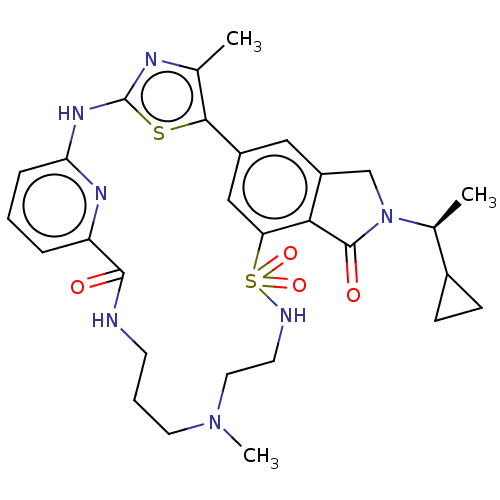
(CHEMBL5090799)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCN(C)CCCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274660
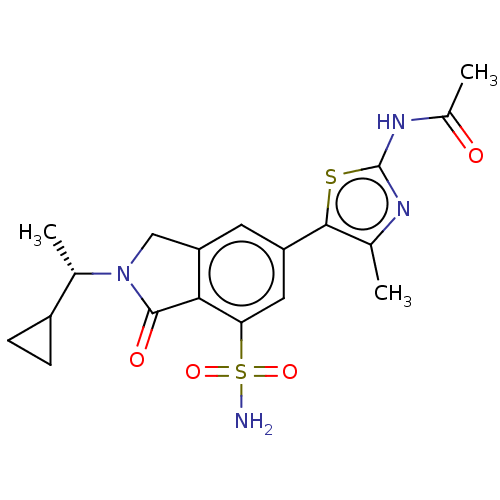
(CHEMBL4128537 | US10858355, Example 12)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(N)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H22N4O4S2/c1-9-17(28-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(7-13)29(20,26)27/h6-7,10,12H,4-5,8H2,1-3H3,(H2,20,26,27)(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648
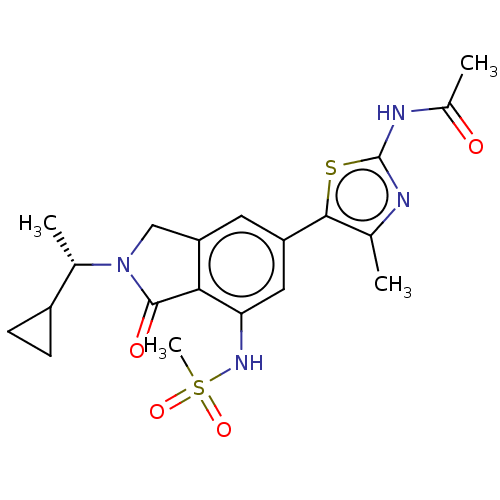
(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50579671
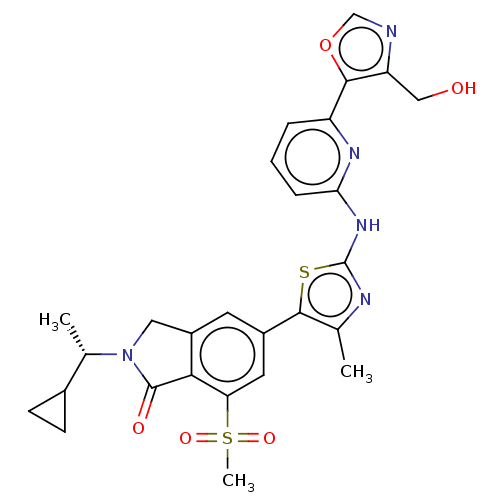
(CHEMBL5090959)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)-c2ocnc2CO)nc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474008

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-(oxetan-3-ylsu...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H26N4O5S2/c1-11-20(32-22(23-11)24-13(3)27)15-6-16-8-26(12(2)14-4-5-14)21(28)19(16)18(7-15)33(29,30)25-17-9-31-10-17/h6-7,12,14,17,25H,4-5,8-10H2,1-3H3,(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474012

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-1-oxo-7-[(2,2,2-...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NCC(F)(F)F)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H23F3N4O4S2/c1-10-18(33-20(26-10)27-12(3)29)14-6-15-8-28(11(2)13-4-5-13)19(30)17(15)16(7-14)34(31,32)25-9-21(22,23)24/h6-7,11,13,25H,4-5,8-9H2,1-3H3,(H,26,27,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474015

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-[(cyclopropyls...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(=O)(=O)C3CC3)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H26N4O4S2/c1-11-20(31-22(23-11)24-13(3)27)15-8-16-10-26(12(2)14-4-5-14)21(28)19(16)18(9-15)25-32(29,30)17-6-7-17/h8-9,12,14,17,25H,4-7,10H2,1-3H3,(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474020

(US10858355, Example 28)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(=O)(=O)CC3CC3)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C23H28N4O4S2/c1-12-21(32-23(24-12)25-14(3)28)17-8-18-10-27(13(2)16-6-7-16)22(29)20(18)19(9-17)26-33(30,31)11-15-4-5-15/h8-9,13,15-16,26H,4-7,10-11H2,1-3H3,(H,24,25,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474017

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-[(ethylsulfony...)Show SMILES CCS(=O)(=O)Nc1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H26N4O4S2/c1-5-31(28,29)24-17-9-15(19-11(2)22-21(30-19)23-13(4)26)8-16-10-25(20(27)18(16)17)12(3)14-6-7-14/h8-9,12,14,24H,5-7,10H2,1-4H3,(H,22,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data