

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130561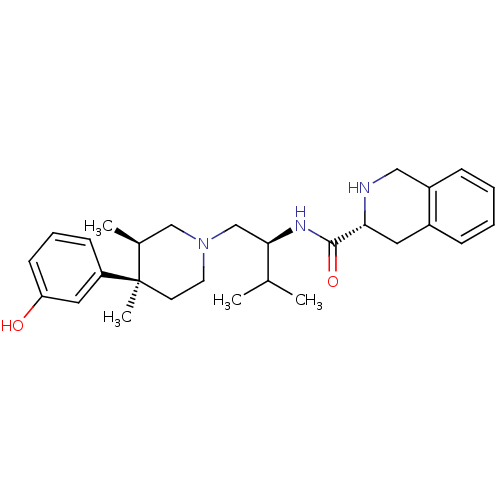 ((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 20: 5847-52 (2010) Article DOI: 10.1016/j.bmcl.2010.07.113 BindingDB Entry DOI: 10.7270/Q23F4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50327257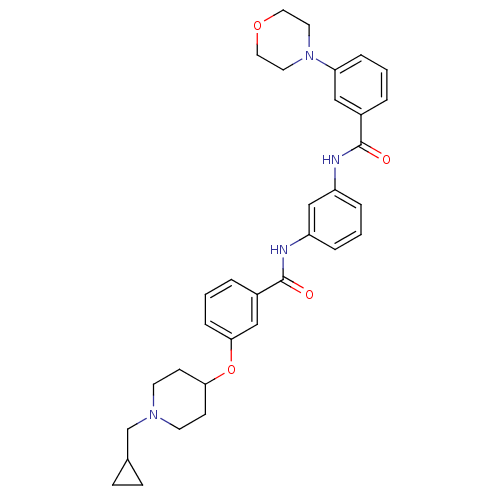 (3-(1-(cyclopropylmethyl)piperidin-4-yloxy)-N-(3-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 20: 5847-52 (2010) Article DOI: 10.1016/j.bmcl.2010.07.113 BindingDB Entry DOI: 10.7270/Q23F4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 20: 5847-52 (2010) Article DOI: 10.1016/j.bmcl.2010.07.113 BindingDB Entry DOI: 10.7270/Q23F4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor... | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005135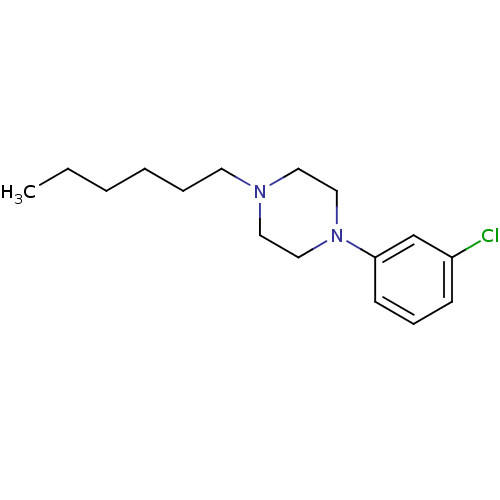 (1-(3-Chloro-phenyl)-4-hexyl-piperazine | CHEMBL279...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005127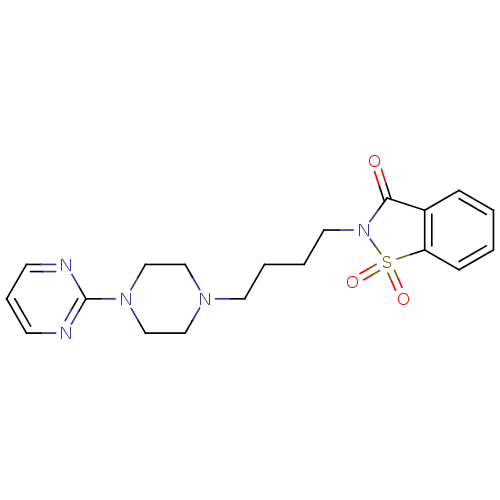 (1,1-Dioxo-2-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005140 (1-(3-Chloro-phenyl)-4-pentyl-piperazine | CHEMBL27...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005129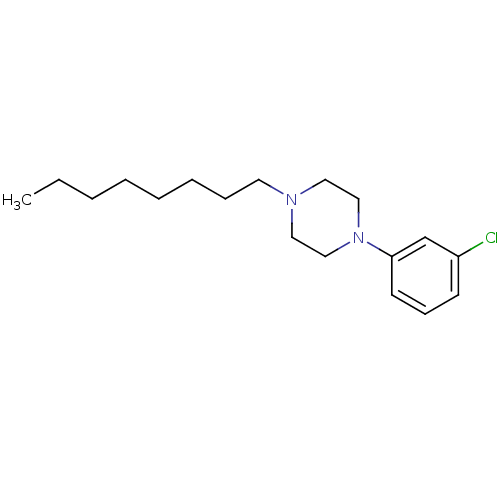 (1-(3-Chloro-phenyl)-4-octyl-piperazine | CHEMBL805...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor... | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005134 (1-Butyl-4-(3-chloro-phenyl)-piperazine | CHEMBL266...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005132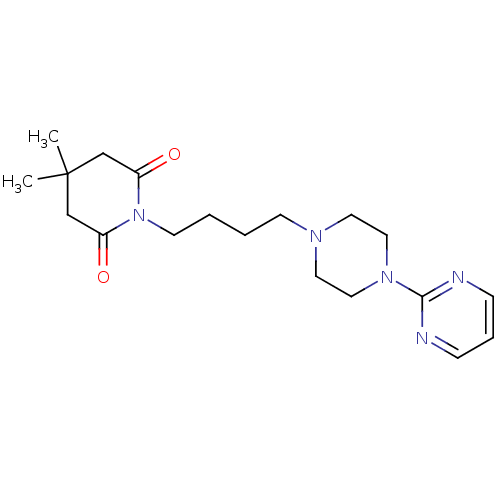 (4,4-Dimethyl-1-[4-(4-pyrimidin-2-yl-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325855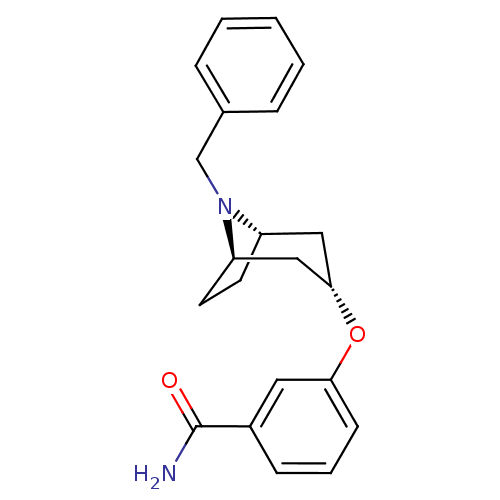 (CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 20: 5847-52 (2010) Article DOI: 10.1016/j.bmcl.2010.07.113 BindingDB Entry DOI: 10.7270/Q23F4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50327259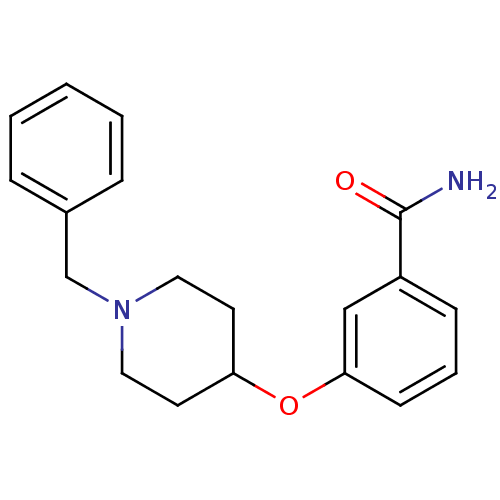 (3-(1-benzylpiperidin-4-yloxy)benzamide | CHEMBL125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 20: 5847-52 (2010) Article DOI: 10.1016/j.bmcl.2010.07.113 BindingDB Entry DOI: 10.7270/Q23F4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005123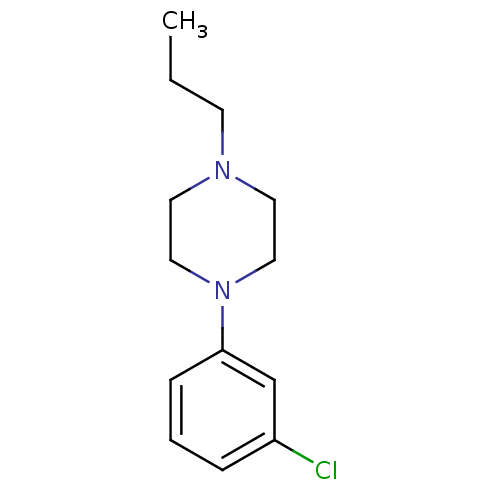 (1-(3-Chloro-phenyl)-4-propyl-piperazine | 1-(3-Chl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005133 (1-sec-Butyl-4-(3-chloro-phenyl)-piperazine | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50001915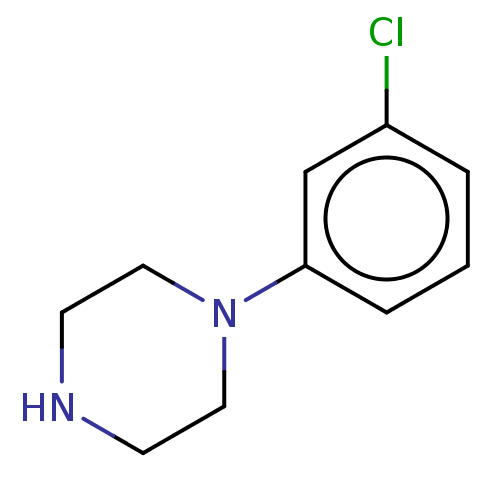 (1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50327258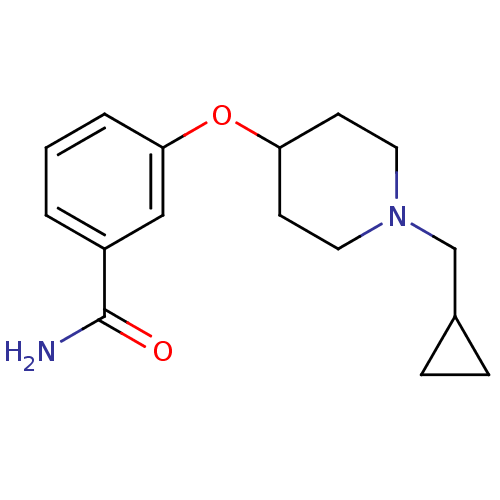 (3-(1-(cyclopropylmethyl)piperidin-4-yloxy)benzamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | Bioorg Med Chem Lett 20: 5847-52 (2010) Article DOI: 10.1016/j.bmcl.2010.07.113 BindingDB Entry DOI: 10.7270/Q23F4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005124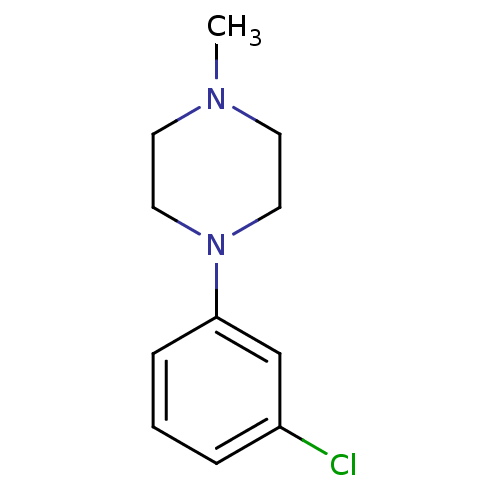 (1-(3-Chloro-phenyl)-4-methyl-piperazine | CHEMBL26...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005142 (1-(3-Chloro-phenyl)-4-isopropyl-piperazine | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005131 (1-Benzyl-4-(3-chloro-phenyl)-piperazine | CHEMBL27...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005143 (2-[4-(3-Chloro-phenyl)-piperazin-1-yl]-ethanol | C...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005139 (1-(3-Chloro-phenyl)-4-ethyl-piperazine | CHEMBL413...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389497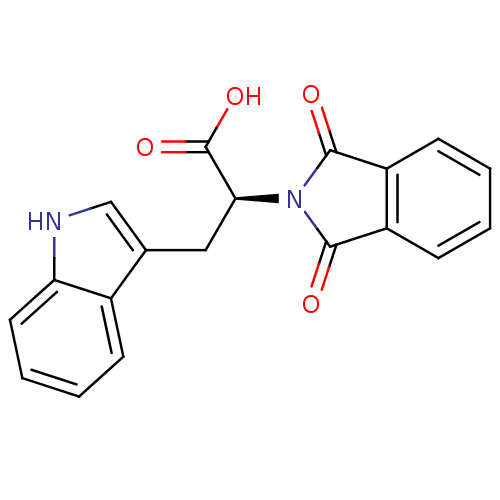 (CHEMBL1564869) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005126 (1-Allyl-4-(3-chloro-phenyl)-piperazine | CHEMBL767...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389487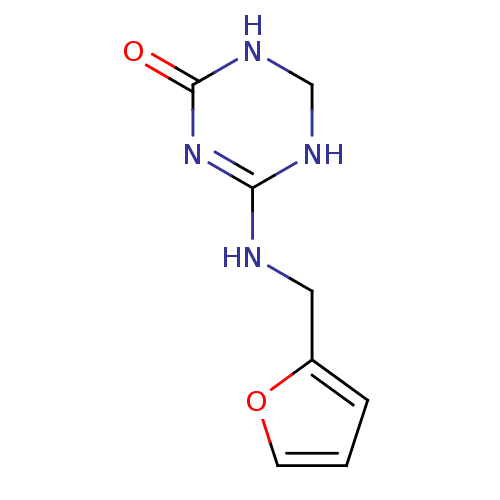 (CHEMBL2063048) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389488 (CHEMBL2063060) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005130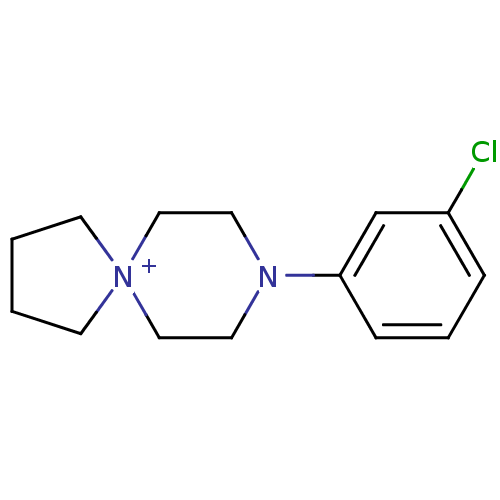 (8-(3-Chloro-phenyl)-8-aza-5-azonia-spiro[4.5]decan...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389495 (CHEMBL2063052) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005138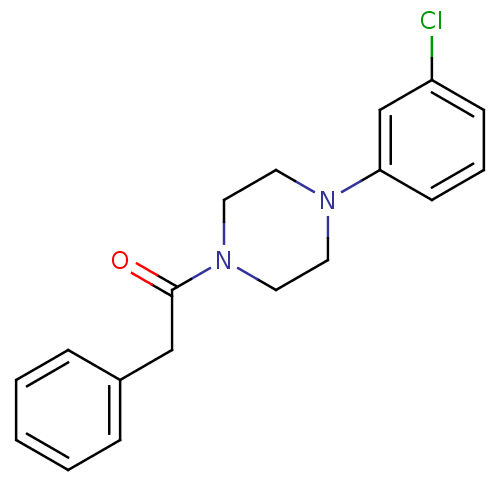 (1-[4-(3-Chloro-phenyl)-piperazin-1-yl]-2-phenyl-et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389498 (CHEMBL2063050) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389489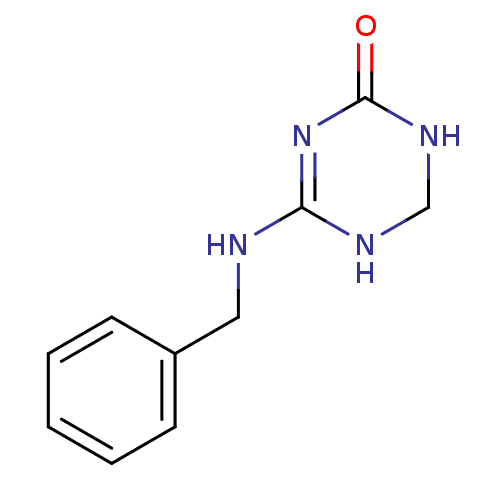 (CHEMBL2063059) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389481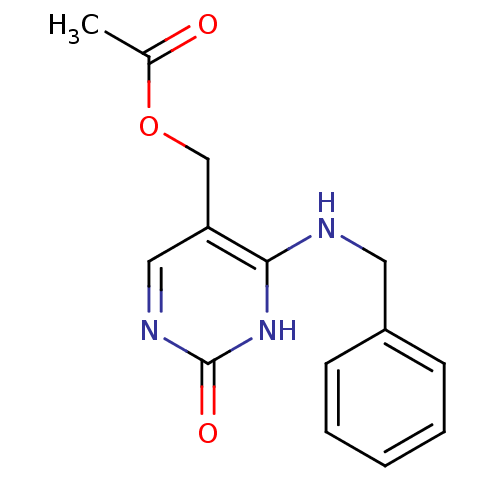 (CHEMBL2063056) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389494 (CHEMBL2063053) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005141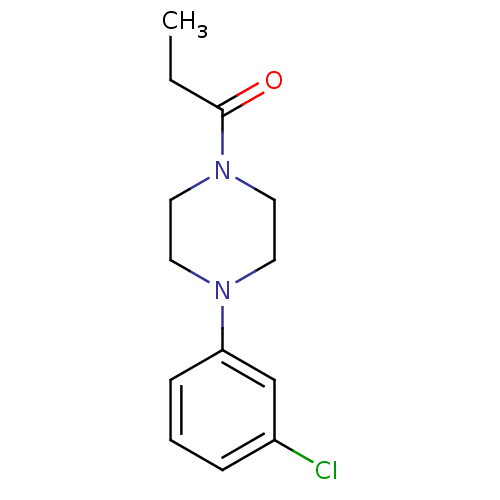 (1-[4-(3-Chloro-phenyl)-piperazin-1-yl]-propan-1-on...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389491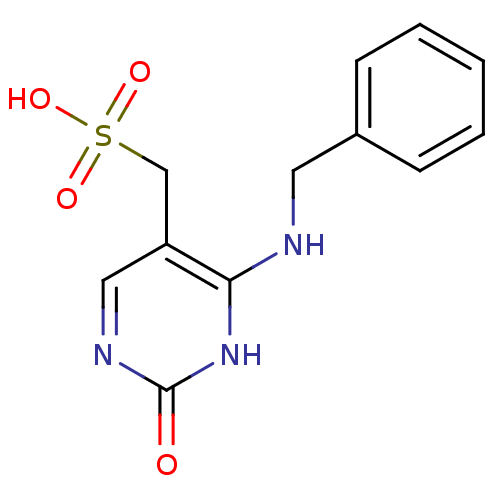 (CHEMBL2063057) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389490 (CHEMBL2063058) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005122 (1-[4-(3-Chloro-phenyl)-piperazin-1-yl]-butan-1-one...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389503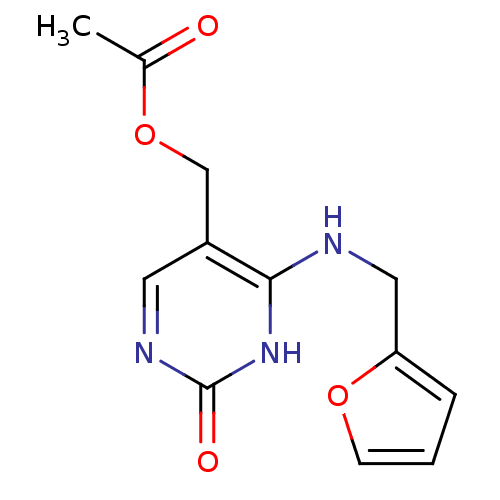 (CHEMBL2063044) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389482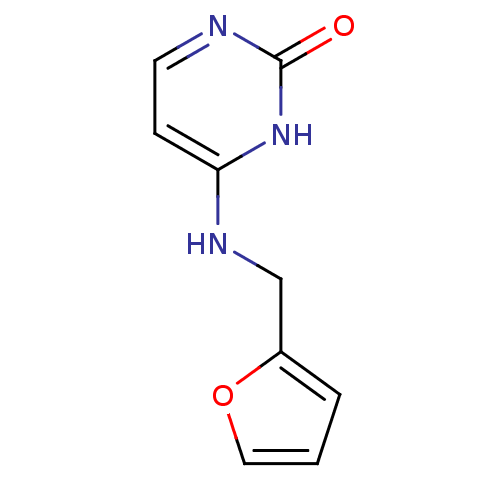 (CHEMBL1699292) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389501 (CHEMBL2063046) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005128 (1-[4-(3-Chloro-phenyl)-piperazin-1-yl]-ethanone | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005136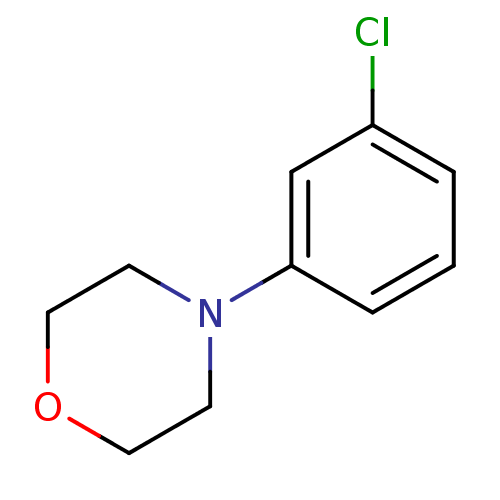 (4-(3-Chloro-phenyl)-morpholine | CHEMBL77181) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005125 (1-[4-(3-Chloro-phenyl)-piperazin-1-yl]-2-methyl-pr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005137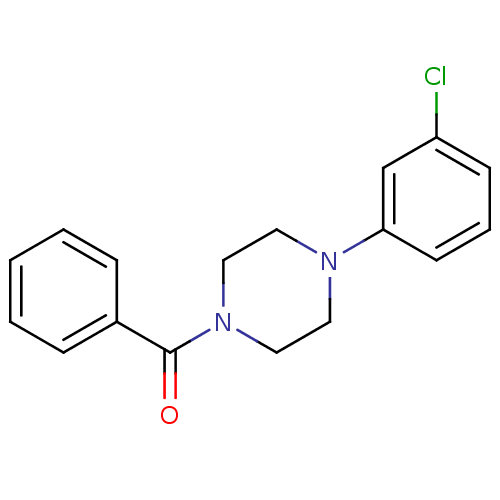 (CHEMBL418386 | [4-(3-Chloro-phenyl)-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-8-hydroxy-2-(di-n-propylamino) tetralin binding from 5-hydroxytryptamine 1A receptor site in rat brain hippocampus | J Med Chem 35: 2369-74 (1992) BindingDB Entry DOI: 10.7270/Q2F18XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389502 (CHEMBL2063045) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389492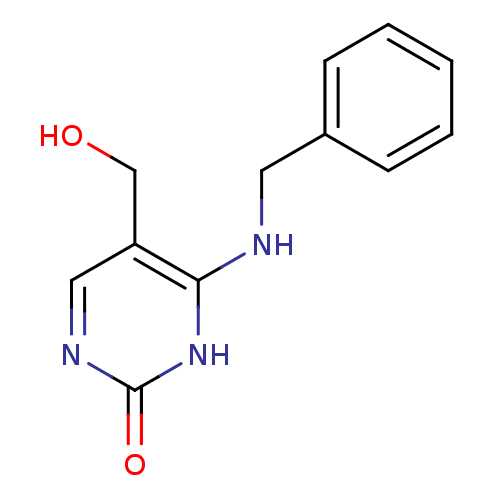 (CHEMBL2063055) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389505 (CHEMBL2063041) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389499 (CHEMBL2063049) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389493 (CHEMBL2063054) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orphan methyltransferase M.SssI (Spiroplasma monobiae strain MQ-1) | BDBM50389504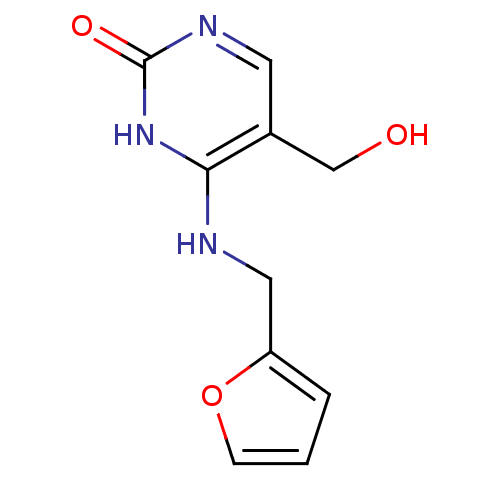 (CHEMBL2063043) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of Spiroplasma sp. MQ1 SssI methyltransferase using pUC18 as substrate measured for 10 mins by Dixon plot analysis | Eur J Med Chem 55: 243-54 (2012) Article DOI: 10.1016/j.ejmech.2012.07.024 BindingDB Entry DOI: 10.7270/Q2GH9K11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 204 total ) | Next | Last >> |