

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084235 (2-Diethylamino-benzo[4,5]thieno[2,3-d][1,3]oxazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084232 (2-(diethylamino)-5-isopropyl-4H-thieno[2,3-d][1,3]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084233 (2-Diethylamino-6,7-dihydro-5H-cyclopenta[4,5]thien...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50064308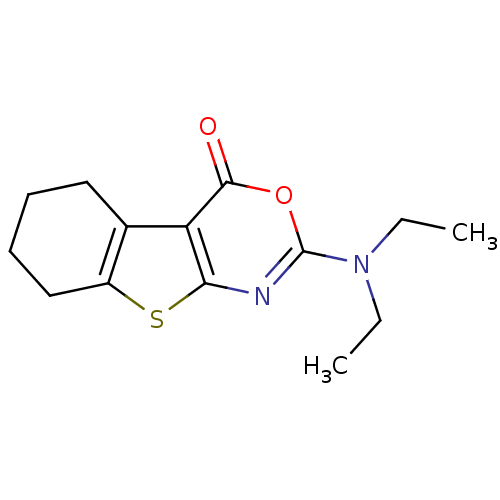 (2-Diethylamino-5,6,7,8-tetrahydro-benzo[4,5]thieno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity AChE from Electrophorus electricus using ATCh substrate and DTNB by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084234 (2-Diethylamino-6,7,8,9-tetrahydro-5H-3-oxa-10-thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084227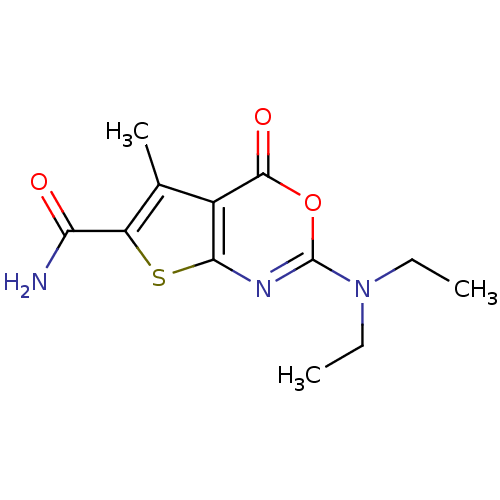 (2-(diethylamino)-5-methyl-4-oxo-4H-thieno[2,3-d][1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084230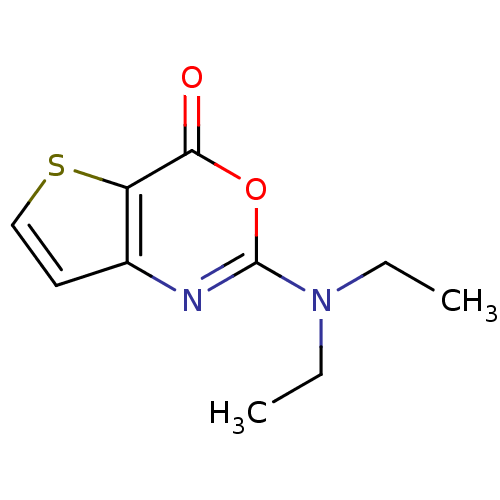 (2-Diethylamino-thieno[3,2-d][1,3]oxazin-4-one | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084229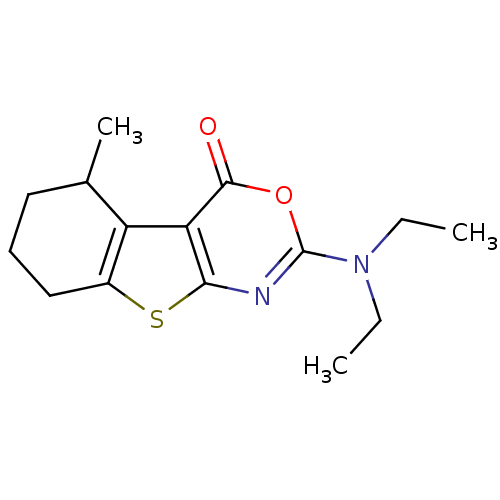 (2-Diethylamino-5-methyl-5,6,7,8-tetrahydro-benzo[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084236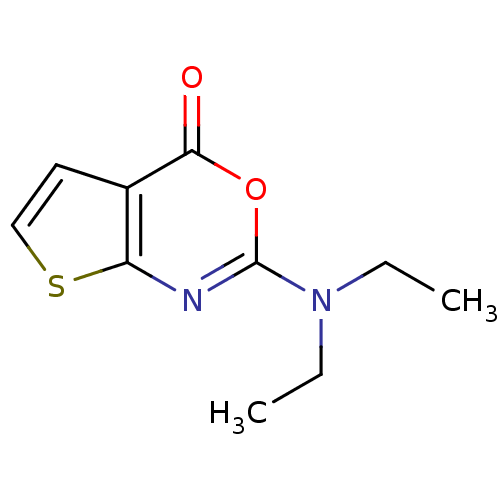 (2-Diethylamino-thieno[2,3-d][1,3]oxazin-4-one | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084231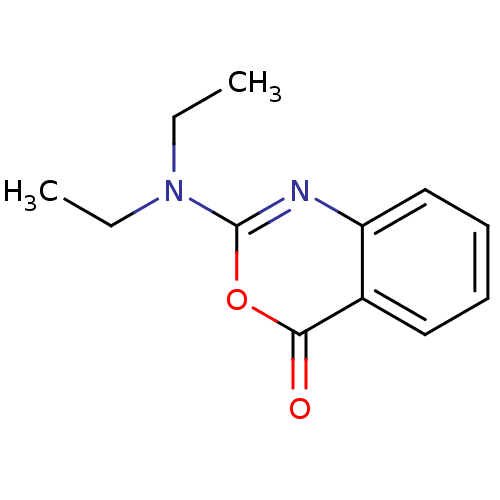 (2-Diethylamino-benzo[d][1,3]oxazin-4-one | CHEMBL3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50084228 (2-(diethylamino)-5-methyl-4H-thieno[2,3-d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against human leukocyte elastase | J Med Chem 42: 5437-47 (2000) BindingDB Entry DOI: 10.7270/Q2K936RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50084233 (2-Diethylamino-6,7-dihydro-5H-cyclopenta[4,5]thien...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50179322 (6,7-dihydro-2-(dimethylamino)-4H,5H-cyclopenta[4,5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50179314 (5,6,7,8-tetrahydro-2-(dimethylamino)-7-(2-methyl-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity AChE from Electrophorus electricus using ATCh substrate and DTNB by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity AChE from Electrophorus electricus using ATCh substrate and DTNB by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50084229 (2-Diethylamino-5-methyl-5,6,7,8-tetrahydro-benzo[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50179312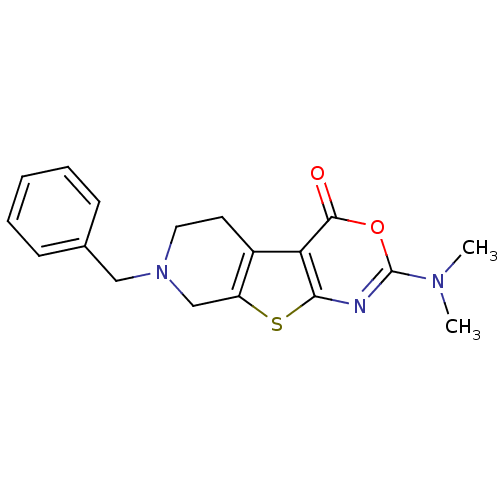 (7-benzyl-5,6,7,8-tetrahydro-2-(dimethylamino)-4H-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity AChE from Electrophorus electricus using ATCh substrate and DTNB by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50064308 (2-Diethylamino-5,6,7,8-tetrahydro-benzo[4,5]thieno...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461625 (CHEMBL4228924) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50179331 (5,6,7,8-tetrahydro-2-(dimethylamino)-4H-benzo[4,5]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461612 (CHEMBL4227301) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461615 (CHEMBL4228875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461619 (CHEMBL4224727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461617 (CHEMBL4229073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461633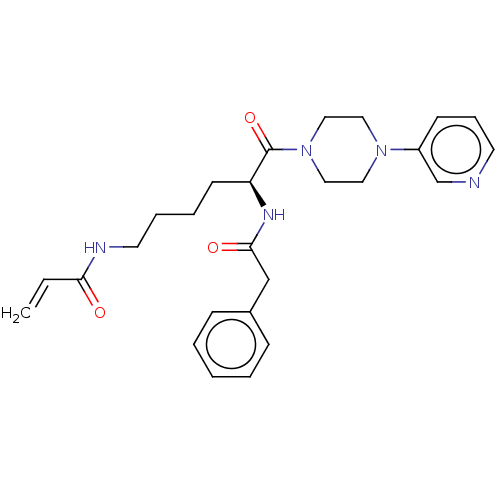 (CHEMBL4227896) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461610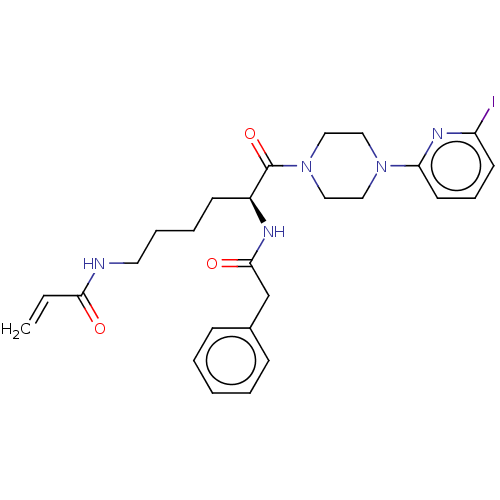 (CHEMBL4227835) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461626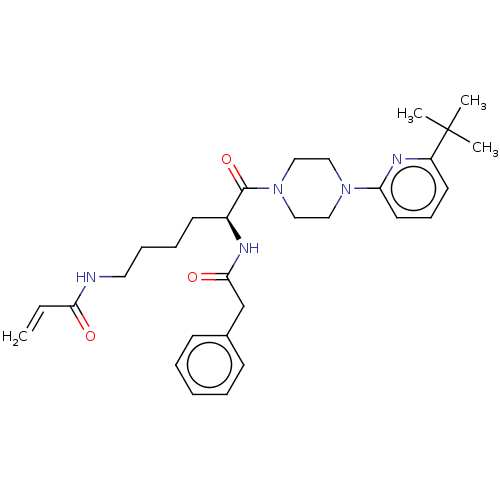 (CHEMBL4228542) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50179326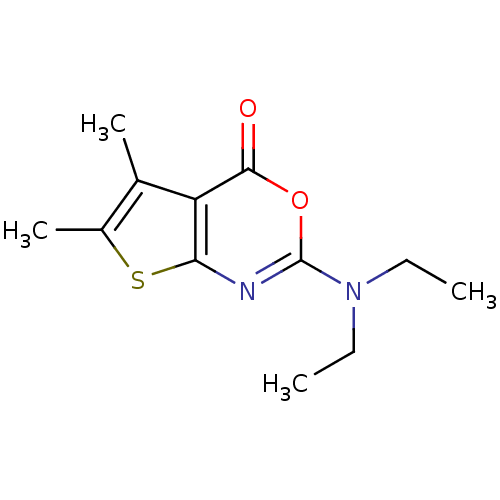 (2-(diethylamino)-5,6-dimethyl-4H-thieno[2,3-d][1,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461609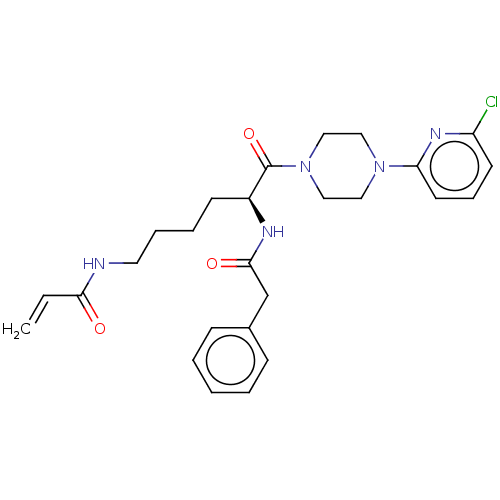 (CHEMBL4227328) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461621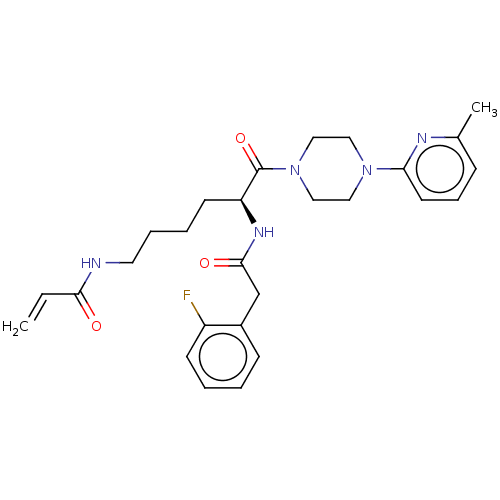 (CHEMBL4226871) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461622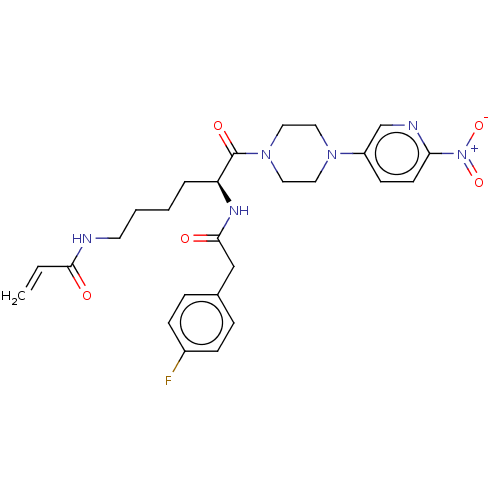 (CHEMBL4228726) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461620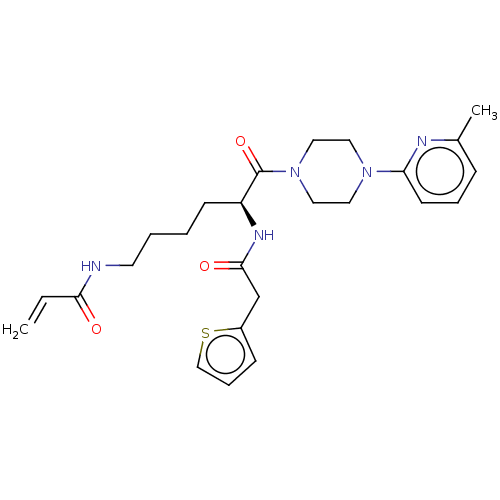 (CHEMBL4228541) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461608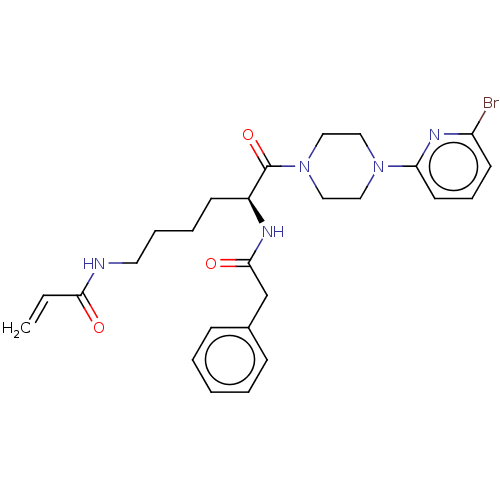 (CHEMBL4228671) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50179329 (2-morpholin-4-yl-5,6,7,8-tetrahydro-benzo[4,5]thie...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461635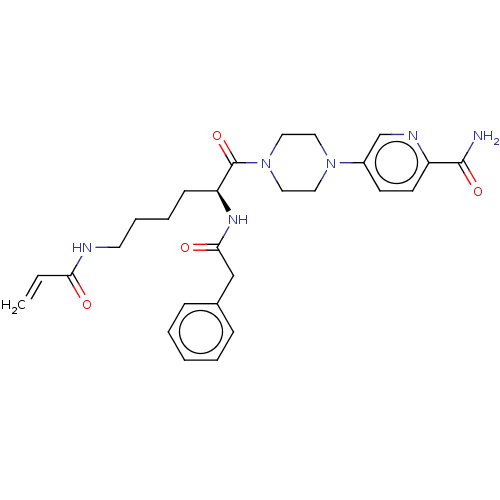 (CHEMBL4228810) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50400621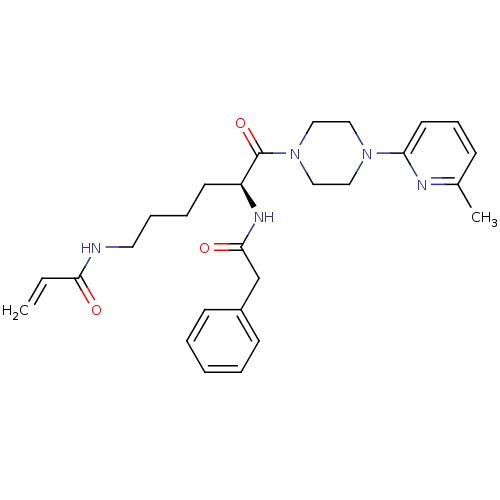 (CHEMBL2203451) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50179318 (2-dimethylamino-5,8-dihydro-4H,6H-thiopyrano[4',3'...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50084234 (2-Diethylamino-6,7,8,9-tetrahydro-5H-3-oxa-10-thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461607 (CHEMBL4228168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50084232 (2-(diethylamino)-5-isopropyl-4H-thieno[2,3-d][1,3]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461630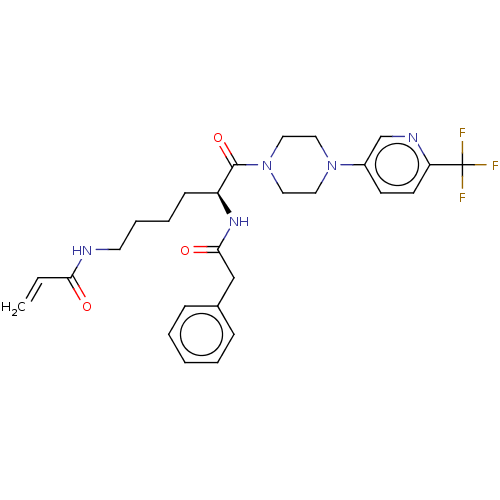 (CHEMBL4225325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461614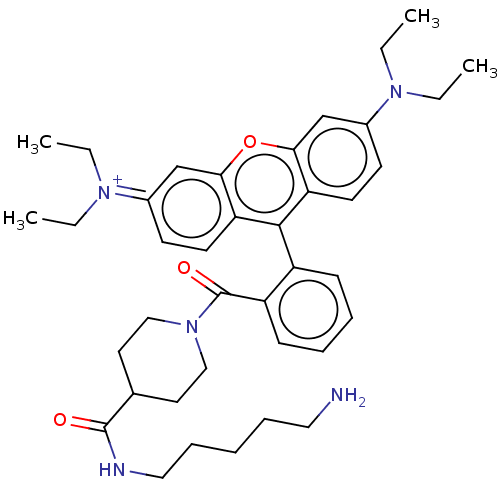 (CHEMBL4226594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Inhibition of human TGase 2 using R-I-Cad/DMC as substrate preincubated for 5 mins followed by substrate addition measured at 30 secs interval over 9... | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461616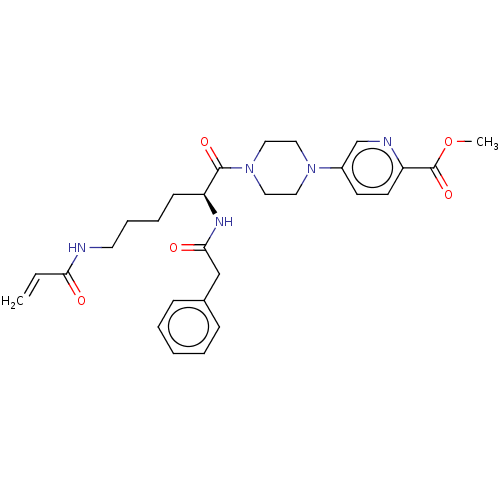 (CHEMBL4224787) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461629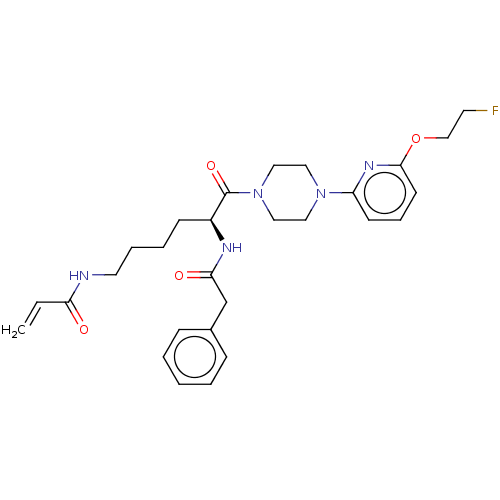 (CHEMBL4228146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50084235 (2-Diethylamino-benzo[4,5]thieno[2,3-d][1,3]oxazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic CEase in presence of pNPB chromogenic substrate by spectrophotometry | J Med Chem 48: 8270-88 (2005) Article DOI: 10.1021/jm0508639 BindingDB Entry DOI: 10.7270/Q2GQ6XB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461618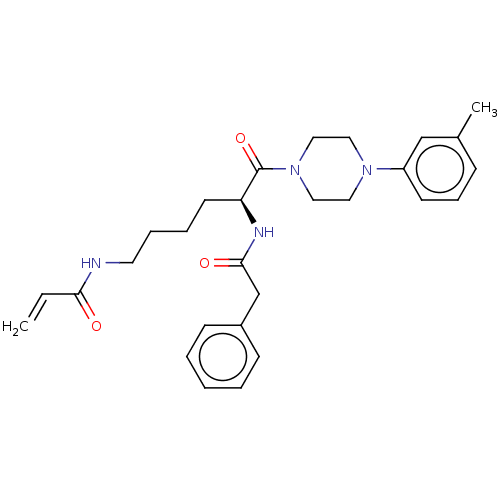 (CHEMBL4225321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461636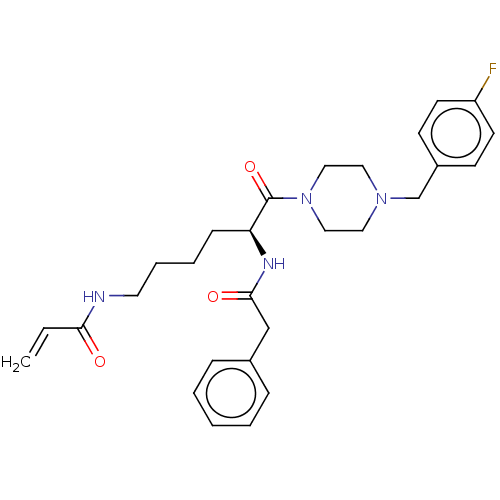 (CHEMBL4228357) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461624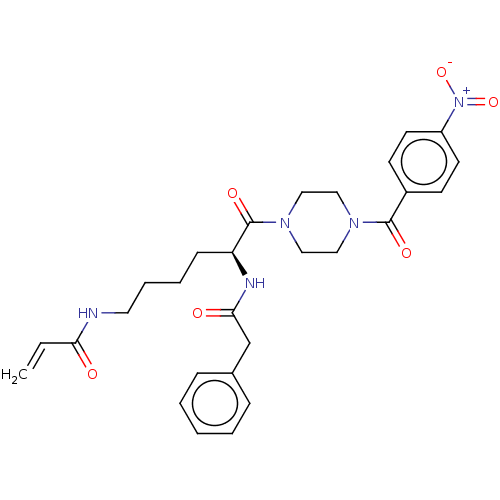 (CHEMBL4226385) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Irreversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50461611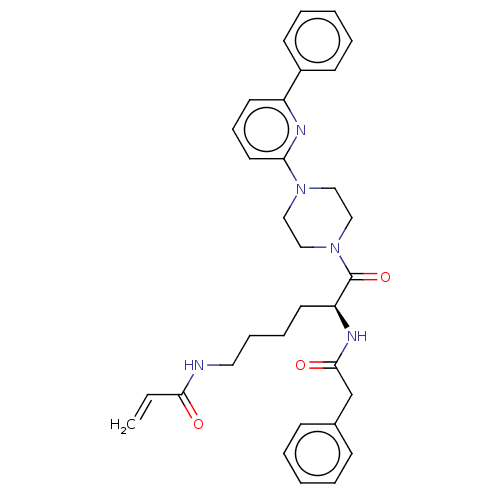 (CHEMBL4226785) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf Curated by ChEMBL | Assay Description Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay | J Med Chem 61: 4528-4560 (2018) Article DOI: 10.1021/acs.jmedchem.8b00286 BindingDB Entry DOI: 10.7270/Q2W66PC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 159 total ) | Next | Last >> |