

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50532742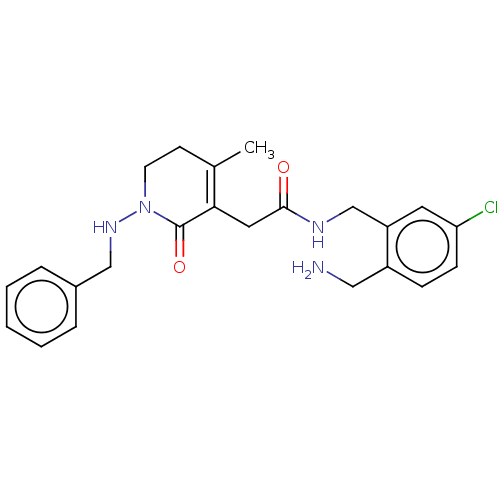 (CHEMBL4455063) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin using S-2366 as substrate after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin using S-2366 as substrate after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin using S-2366 as substrate after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin using S-2366 as substrate after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50532740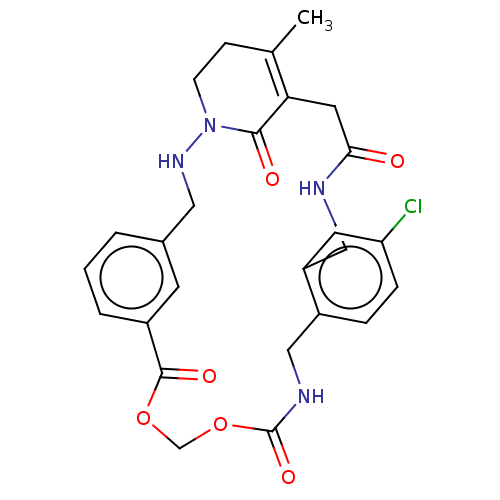 (CHEMBL4518749) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin using S-2366 as substrate after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50532740 (CHEMBL4518749) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin using S-2366 as substrate after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50300802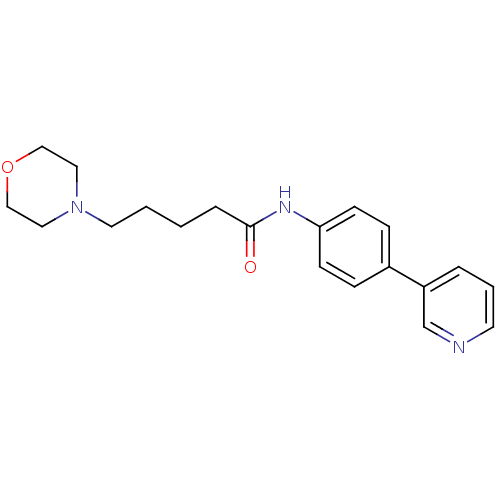 (5-Morpholin-4-ylpentanoic acid(4-pyridin-3-ylpheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat rat alpha7 nAChR expressed in rat GH4C1 cells | Bioorg Med Chem 17: 5247-58 (2009) Article DOI: 10.1016/j.bmc.2009.05.040 BindingDB Entry DOI: 10.7270/Q29K4B8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human trypsin 1 using S-2222 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50532740 (CHEMBL4518749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human trypsin 1 using S-2222 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50532740 (CHEMBL4518749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human trypsin 1 using S-2222 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human trypsin 1 using S-2222 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human trypsin 1 using S-2222 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human trypsin 1 using S-2222 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human plasmin using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human plasmin using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F11a using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F11a using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F11a using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human plasmin using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human plasmin using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F11a using S-2366 as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F9a using pefachrome F9a as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F9a using pefachrome F9a as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50532742 (CHEMBL4455063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F9a using pefachrome F9a as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50532741 (CHEMBL4443619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human F9a using pefachrome F9a as substrate preincubated for 300 secs followed by substrate addition measured after 40 mins | J Med Chem 59: 6658-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01871 BindingDB Entry DOI: 10.7270/Q2183B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8137 (N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Trypanosoma brucei) | BDBM50441529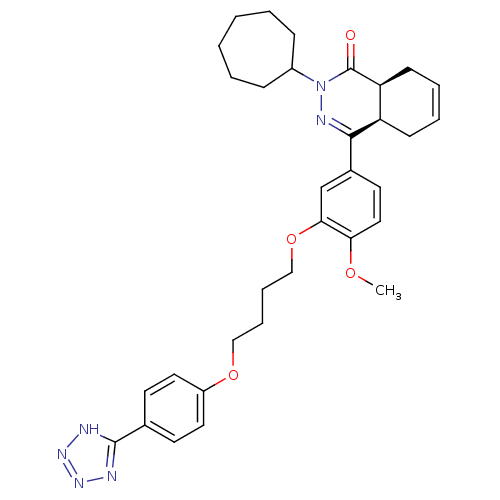 (CHEMBL2436771) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei PDEB1 | Bioorg Med Chem Lett 23: 5971-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.057 BindingDB Entry DOI: 10.7270/Q22B90GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR assessed as inhibition of 4EBP1 phosphorylation after 30 mins by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8126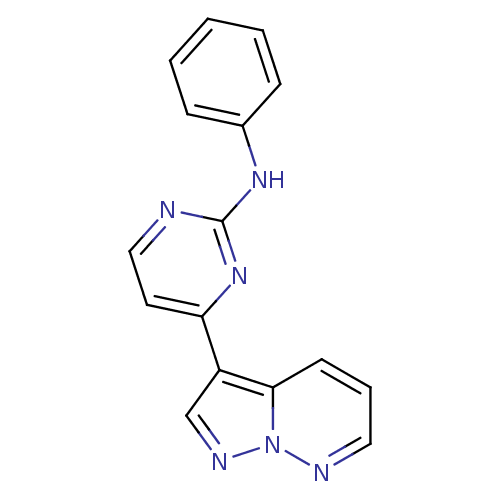 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50008271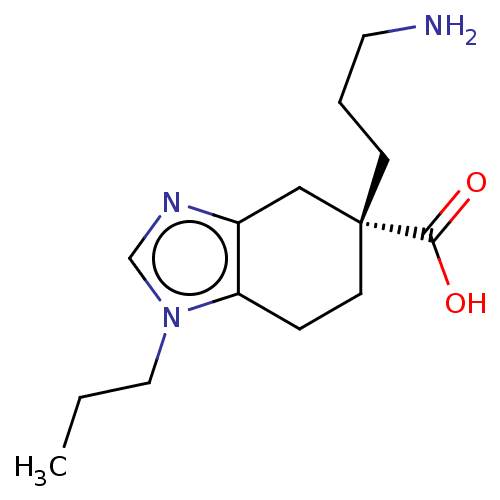 (CHEMBL3235132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human TAF1a using hippuryl-L-arginine/hippuryl-L-lysine as substrate by liquid chromatographic analysis | Bioorg Med Chem 22: 2261-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.010 BindingDB Entry DOI: 10.7270/Q2DZ09TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8137 (N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-delta assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50506295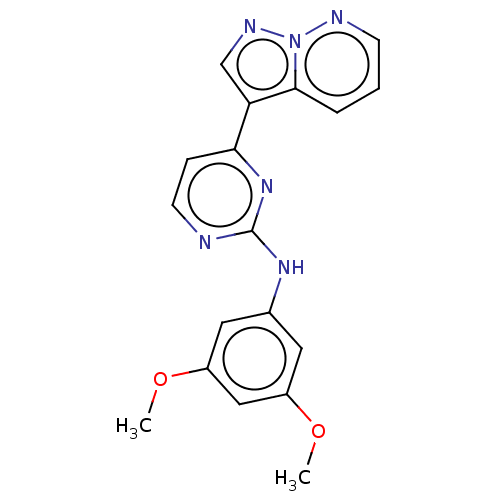 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50506295 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50506295 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-alpha assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8126 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50058062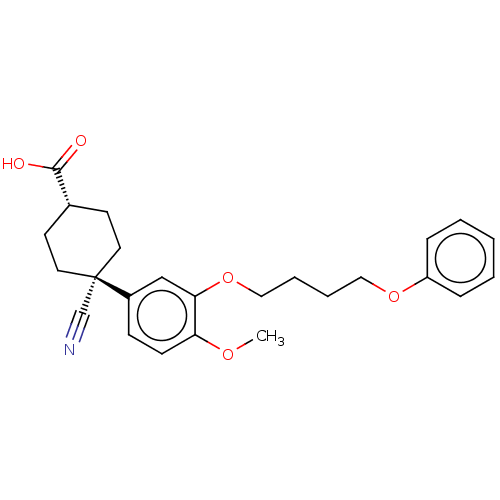 (CHEMBL3323128) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PDE4B expressed in Sf21 cells using cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 24: 4084-9 (2014) Article DOI: 10.1016/j.bmcl.2014.07.063 BindingDB Entry DOI: 10.7270/Q26D5VNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8197 (4-{6-methyl-2-phenylpyrazolo[1,5-a]pyridazin-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50008272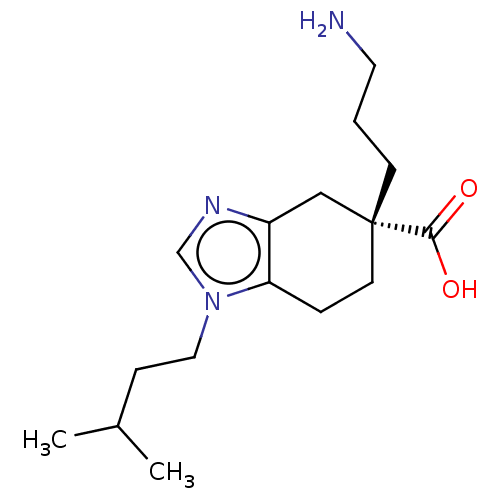 (CHEMBL3235133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human TAF1a | Bioorg Med Chem 22: 2261-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.010 BindingDB Entry DOI: 10.7270/Q2DZ09TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 221 total ) | Next | Last >> |